Published online Aug 28, 2010. doi: 10.3748/wjg.v16.i32.4084
Revised: May 31, 2010
Accepted: June 7, 2010
Published online: August 28, 2010
AIM: To investigate the serological diagnostic factors for liver metastasis in patients with colorectal cancer.
METHODS: One hundred and six adult in-patients with colorectal cancer were studied and divided into patients with liver metastasis (n = 56) and patients without liver metastasis (n = 50). Serum levels of tumor and biochemical markers for liver were measured at the time of diagnosis.
RESULTS: The mean survival time was 55.9 mo, 36.8 mo and 68.3 mo for the overall patients, patients with liver metastasis and patients without liver metastasis, respectively. Lactate dehydrogenase (LDH) level was significantly correlated with the survival time of colorectal cancer patients. The levels of alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase (GGT), LDH and carcinoembryonic antigen (CEA) were significantly higher in patients with liver metastasis than in those without liver metastasis. Patients with lymph node metastasis had a higher risk of liver metastasis than those without lymph node metastasis. The cut points of LDH, GGT and CEA for screening liver metastasis were 180 U/L, 30 U/L and 5.0 μg/L, respectively. The sensitivity was 64.3%, 69.6% and 70.4%, and the specificity was 64.0%, 60.0% and 52.4%, respectively. The sensitivity of parallel test was 85.2% for LDH and CEA, and 92.6% for GGT and CEA, respectively. The specificity of serial test was 85.7% for LDH (or GGT) and CEA.
CONCLUSION: Early diagnosis of liver metastasis is of great significance. The sensitivity and specificity of combined tumor and biochemical markers are rather good in screening colorectal liver metastasis.
- Citation: Wu XZ, Ma F, Wang XL. Serological diagnostic factors for liver metastasis in patients with colorectal cancer. World J Gastroenterol 2010; 16(32): 4084-4088
- URL: https://www.wjgnet.com/1007-9327/full/v16/i32/4084.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i32.4084
Colorectal cancer is the 3rd most common malignancy worldwide and the second most lethal cancer type in the developed world[1]. Most patients with colorectal cancer succumb to the effects of distant metastatic lesions, especially liver metastasis rather than the primary colorectal cancer itself[2]. The liver is a primary target organ of metastatic lesions and the main cause of death. About 25% of patients with colorectal cancer have liver metastases at the time of diagnosis and another 25%-30% of them will present with liver metastases in the following 2-3 years[3]. Without treatment, the life expectancy for patients with colorectal metastases is poor and ranges from 5 to 9 mo[2,4]. Thus early diagnosis of liver metastases of colorectal caner leads to timely treatment, which favors a better prognosis.
Laparoscopy has not been advocated as a screening test for colorectal liver metastases due to its invasiveness. Fine needle aspiration cytology also has not been advocated as a screening test, because of its high risk of complications[5]. It has been shown that the incidence of needle tract metastases is 0.4%-5.1% after fine needle aspiration and use of the procedure in abdominal tumors is fatal in 0.006%-0.031% of cases[6,7]. Most deaths are due to hemorrhage of liver tumors[3]. Imaging modalities, such as contrast enhanced computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography CT (PET-CT), may establish the diagnosis of liver metastasis of colorectal cancer[8]. However, it is more difficult to make the clinical diagnosis of early liver metastases of colorectal cancer due to the absence of typical symptoms or signs. Serological examination including tumor and biochemical markers for liver function evaluation is routinely performed, though its accuracy is not high[9]. The level of carcinoembryonic antigen (CEA) is elevated in 63% of patients, while the activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) is increased in about 30% of patients with liver metastases of colorectal cancer[10]. To reduce metastases-related mortality, the development of new methods for diagnosis of liver metastases of colorectal cancer is of great significance.
The purpose of the present study was to determine whether CEA and biochemical hepatic tests can be used in assessing liver metastasis in patients with colorectal cancer.
One hundred and six in-patients with colorectal cancer admitted to Cancer Institute and Hospital, Tianjin Medical University, from December 1996 to January 2004, were included in this study. Pathological test was performed to confirm their colorectal cancer and contrast enhanced CT, MRI or PET-CT was performed to confirm their liver metastasis. Moreover, liver metastasis was confirmed by operation, biopsy or progression of the disease. The patients who had a history of liver disease and did not undergo contrast enhanced CT and MRI were excluded from this study.
Blind tests were performed for total bilirubin (TB), direct bilirubin (DB), ALT, AST, serum total protein (TP), globulin (GLOB), γ-glutamyltransferase (GGT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and CEA. Liver biochemical test was performed within 1 wk after liver metastasis was diagnosed by contrast enhanced CT and MRI in our hospital. The methods to determine biomarkers and upper or lower limits of normality used in our laboratory are shown in Table 1.
| Diagnostic factors | Methods | Lower and upper limits |
| Total bilirubin (μmol/L) | Jendrassik-Grof | 2-20 |
| Direct bilirubin (μmol/L) | Jendrassik-Grof | 0-10 |
| Alanine aminotransferase (U/L) | Rate | 0-40 |
| Aspartate aminotransferase (U/L) | Rate | 0-42 |
| Serum total protein (g/L) | Biuret | 60-80 |
| Globulin (g/L) | 27-35 | |
| γ-glutamyltransferase (U/L) | Nitrophenol rate | 0-50 |
| Alkaline phosphatase (U/L) | P-nitrophenol phosphate rate | 45-132 |
| Lactate dehydrogenase (U/L) | Rate | 80-240 |
| Carcinoembryonic antigen (μg/L) | Elisa | 0-5 |
One-sample Kolmogorov-Smirnov test was used to determine the distribution of ALP, TP, ALB, GLOB, GGT, ALT, AST, TBIL, DBIL, LDH and CEA. Data with the skewed distribution were presented as median (Quartile interval). Two-independent-sample test and χ2 test were respectively used to determine whether there is any significant difference between patients with and without liver metastasis. Cox regression analysis was performed for GGT, ALP, LDH, TB, DB, ALT, AST, TP, GLB, CEA, lymph node metastasis in order to find the characteristic factors for survival time. Screening test for LDH, GGT and CEA, parallel test and serial test for GGT and LDH, CEA and LDH, CEA and GGT were used to determine the diagnostic factors for liver metastasis in patients with colorectal cancer. Statistical analysis was performed by SPSS (Version: 16.0, Chicago, USA).
The screening tests were evaluated by calculating their sensitivity (SE), specificity (SP), diagnostic index (DI), false positive rate (α), false negative rate (β), crude accuracy (CA), positive predictive value (PV+), negative predictive value (PV-).
SE was defined as the proportion of patients with LM testing positive (A/A + C) where C is the number of false negative cases. SP was defined as the proportion of patients without LM testing negative (D/B + D) where B is the number of false positive cases. DI was defined as the (SE + SP)-1. α was defined as the proportion of negative cases that were erroneously reported as positive while β was defined as the proportion of positive cases that were erroneously reported as negative. CA was defined as the proportion of cases correctly diagnosed by the test (A + D/A + B + C + D) where B + C is the number of cases erroneously diagnosed by the test. PV+ was defined as the proportion of patients testing positive with LM confirmed by pathology (A/A + B) while PV- was defined as the proportion of patients testing negative proved to be free of LM by pathology (D/C + D).
Serial test was defined as positive only if all the results were positive when we considered two or more tests together in the single patient. Parallel test was defined as positive if one of the results was positive when two or more tests were considered in the single patient.
The age of our patients ranged 26-80 years with a median of 56 years. Males constituted 68 of patients with a male to female ratio of 1.788 to 1. No significant difference was found in age and sex of the patients with and without liver metastasis. The characteristics of patients with primary tumor and lymph node metastasis are shown in Table 2.
| With liver metastasis | Without liver metastasis | |
| Age (yr) | 55.69 ± 11.773 | 55.21 ± 13.225 |
| Sex | ||
| Male | 32 | 36 |
| Female | 18 | 20 |
| Primary tumor (UICC stage) | ||
| T1 | 1 | 0 |
| T2 | 4 | 0 |
| T3 | 12 | 10 |
| T4 | 22 | 23 |
| Lymph node metastasis (UICC stage) | ||
| N0 | 29 | 12 |
| N1 | 8 | 13 |
| N2 | 2 | 6 |
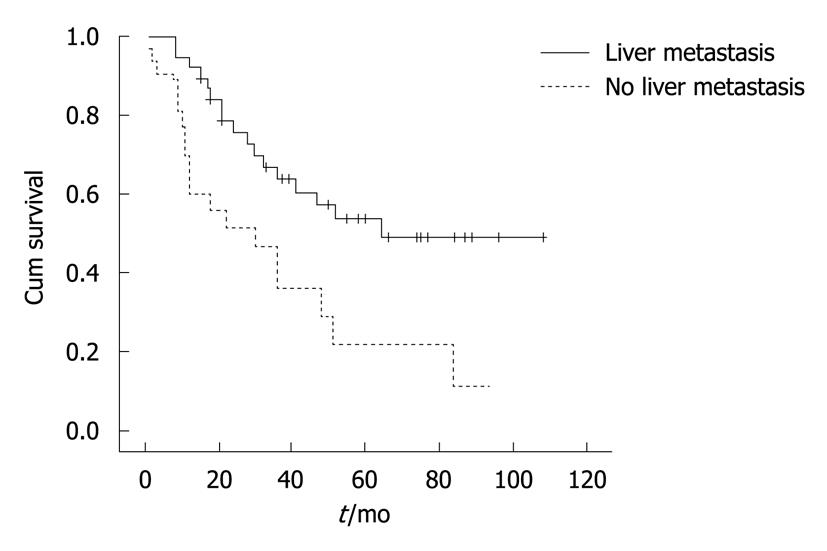
The median survival time was 47, 30 and 64 mo for the overall patients, patients with liver metastasis and patients without liver metastasis, respectively. Their mean survival time was 55.9 ± 5.5 mo, 36.8 ± 6.5 mo and 68.3 ± 7.0 mo, respectively. The survival curves for patients with and without liver metastasis were significantly different (P = 0.005) (Figure 1). Cox regression analysis showed that LDH was significantly correlated with the survival time of colorectal cancer patients with an increased risk of liver metastasis (P = 0.005) (Table 3).
| χ2 | P | |
| Serum total protein (g/L) | 0.093 | 0.761 |
| Globulin (g/L) | < 0.000 | 0.994 |
| Alanine aminotransferase (U/L) | 1.943 | 0.163 |
| Aspartate aminotransferase (U/L) | 0.143 | 0.705 |
| Total bilirubin (μmol/L) | 0.122 | 0.726 |
| Direct bilirubin (μmol/L) | 0.063 | 0.801 |
| γ-glutamyltransferase (U/L) | 1.126 | 0.289 |
| Alkaline phosphatase (U/L) | 1.006 | 0.316 |
| Lactate dehydrogenase (U/L) | 11.254 | 0.001 |
| Carcinoembryonic antigen (μg/L) | 0.159 | 0.690 |
| Lymph node metastasis | 1.601 | 0.206 |
One-sample Kolmogorov-Smirnov test showed that the distribution of ALP, TP, ALB, GLOB and ALB/GLOB was normal, while that of GGT, ALT, AST, TBIL, DBIL, LDH and CEA was skewed. The levels of GGT, ALT, AST, LDH and CEA were significantly higher in patients with liver metastasis than in those without liver metastasis (P < 0.05) (Table 4). Patients with lymph node metastasis had a higher risk of liver metastasis than those without lymph node metastasis (χ2 = 9.046, P = 0.003). No significant difference was found in ALP, TP, ALB, GLOB, ALB/GLOB, TBIL, and DBIL levels between patients with and without liver metastasis (data not shown).
| With liver metastasis | Without liver metastasis | P | |
| GGT (U/L) | 43.00 (64.75) | 24.00 (35) | 0.001 |
| ALT (U/L) | 22.00 (22.75) | 13.00 (12.50) | < 0.001 |
| AST (U/L) | 22.00 (17.50) | 16.00 (10.00) | < 0.001 |
| LDH (U/L) | 201.5 (169.50) | 164.50 (70.75) | 0.003 |
| CEA (μg/L) | 13.70 (93.8) | 4.87 (12.82) | 0.039 |
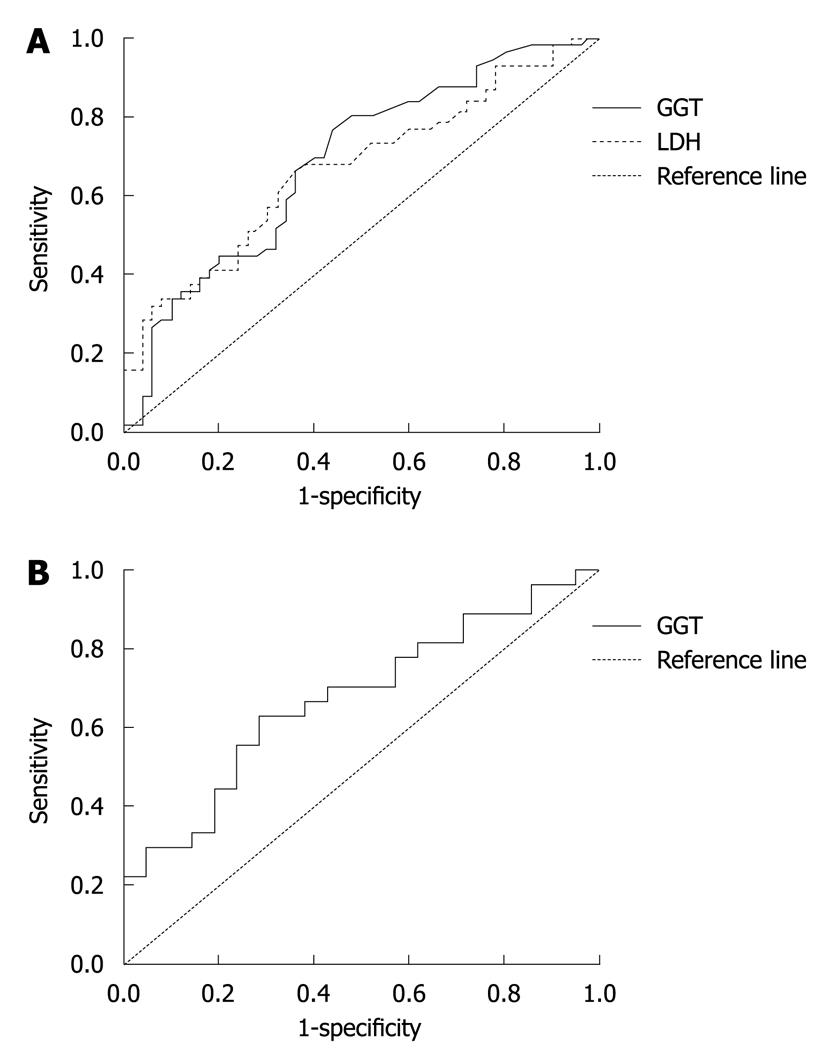
Because the diagnostic indices of LDH, GGT and CEA at 180, 30 and 5.0 for screening liver metastasis were the greatest, the cut off points were selected at 180, 30 and 5.0, respectively (Figure 2). The area under the curves of LDH, GGT and CEA was 0.671, 0. 687 and 0.675, respectively (P = 0.05). The κ of parallel test and serial test for CEA and LDH, CEA and GGT was 0.293, 0.326, and 0.357, 0.284, respectively (P = 0.05). The SE, SP, DI, false positive rate (α), false negative rate (β), CA, adjusted agreement, PV+ and PV- are shown in Table 5.
| Sen (%) | Spe (%) | DI | CA | AA | PV+ | PV- | P | α | β | |
| LDH | 64.3 | 64.0 | 1.283 | 0.642 | 0.641 | 0.667 | 0.615 | 0.003 | 0.360 | 0.356 |
| GGT | 69.6 | 60.0 | 1.296 | 0.651 | 0.649 | 0.661 | 0.638 | 0.001 | 0.400 | 0.304 |
| CEA | 70.4 | 52.4 | 1.228 | 0.625 | 0.623 | 0.655 | 0.579 | 0.039 | 0.476 | 0.296 |
| LDH and CEA (serial test) | 51.9 | 85.7 | 1.376 | 0.667 | 0.695 | 0.823 | 0.581 | 0.007 | 0.143 | 0.481 |
| LDH and CEA (parallel test) | 85.2 | 42.9 | 1.281 | 0.667 | 0.657 | 0.657 | 0.692 | 0.030 | 0.571 | 0.148 |
| GGT and CEA (serial test) | 44.4 | 85.7 | 1.301 | 0.625 | 0.662 | 0.800 | 0.545 | 0.025 | 0.143 | 0.556 |
| GGT and CEA (parallel test) | 92.6 | 38.1 | 1.307 | 0.687 | 0.691 | 0.658 | 0.800 | 0.009 | 0.619 | 0.074 |
Colorectal cancer metastasis occurs in various organs, most frequent in lymph nodes and liver[1]. In this study, the patients with lymph node metastasis had a higher risk of liver metastasis than those without lymph node metastasis, indicating that regular imaging modalities, such as contrast enhanced CT and MRI, may be performed every 3 or 6 mo after surgery for patients with colorectal cancer to establish an early diagnosis of liver metastasis.
The overall life expectancy of patients with colorectal cancer is mainly determined by the progression of liver metastasis rather than by the primary carcinoma itself[3]. The median survival time was 47, 30 and 64 mo for the overall patients, patients with liver metastasis and patients without liver metastasis, respectively, with a mean survival time of 55.9, 36.8 and 68.3 mo, respectively. A significant difference was observed in survival curves for patients with and without liver metastasis. The levels of GGT, ALT, AST, LDH and CEA were significantly higher in patients with liver metastasis than in those without liver metastasis. Cox regression analysis showed that LDH was significantly correlated with the survival time of colorectal cancer patients, indicating that LDH may be used to predict the life expectancy of patients with liver metastasis of colorectal cancer.
CEA was demonstrated in fetal gut tissue and gastrointestinal tract tumor four decades ago, and subsequently detected in the circulation of patients and recognized as a serum marker for colorectal cancer. Expression of carbohydrate antigen (CA) 19-9 has been described in colorectal cancer, but its sensitivity is lower than CEA[11]. Lack of sensitivity and specificity precludes the use of any available serum markers, such as CEA, CA 19-9, CA 242, CA 72-4, tissue polypeptide antigen or tissue polypeptide-specific antigen, for the early detection of colorectal cancer[12]. However, a preoperative CEA serum level can predict the prognosis of recurrence and survival time of colorectal cancer patients[11,13]. Moreover, circulating levels of LDH, ALP, and GGT in malignant tissues can directly contribute to liver replacement[14,15]. In patients with metastatic colorectal cancer, CEA, ALP and LDH have been reported as prognostic factors[16-19].
A screening test was performed to show whether LDH, GGT and CEA can be used to screen liver metastasis in patients with colorectal cancer. Because the diagnostic indices of LDH, GGT and CEA at 180 U/L, 30 U/L and 5.0 μg/L for screening liver metastasis were the greatest, the cut off points were selected at 180 U/L, at 30 U/L, and at 5.0 μg/L, respectively. The sensitivity were 64.3%, 69.6% and 70.4%, respectively. The sensitivity were 64.0%, 60.0% and 52.4%, respectively. As a tumor marker, CEA test had a moderate sensitivity and a low specificity for liver metastasis in patients with colorectal cancer. Thus, tumor markers in combination with biochemical markers for liver function may improve the sensitivity and specificity for screening liver metastases in patients with colorectal cancer.
Couples of tests, usually CEA and another, would demonstrate a better accuracy than a single test[20,21], which is consistent with the findings in our study. In the present study, the sensitivity of parallel test for LDH and CEA, GGT and CEA was 85.2% and 92.6%, respectively. The specificity of serial test for LDH and CEA was 85.7% and the specificity of serial test for GGT and CEA was 85.7% too, indicating that its sensitivity and specificity of tumor marker (CEA) in combination with biochemical markers including LDH and GGT are rather good in patients with colorectal cancer, if LDH > 180 U/L and CEA > 5.0 μg/L, or GGT > 30 U/L and CEA > 5.0 μg/L. Contrast enhanced CT, MRI or PET-CT may be performed immediately to confirm liver metastasis and timely treatment may improve the survival of patients with liver metastasis of colorectal cancer. Thus metastatic liver disease may be diagnosed before symptoms occur and liver metastases of colorectal cancer can be diagnosed more rapidly and accurately.
The overall life expectancy of patients with colorectal cancer is mainly determined by the progression of liver metastasis rather than by the primary carcinoma. Improved early screening modalities are still needed and molecular beacons may be sufficiently sensitive, specific, and cost-effective for screening of colorectal liver metastases.
Although various diagnostic modalities, such as ultrasonography, computed tomography scan and magnetic resonance imaging have been used in demonstrating metastases, but their accuracy is low, particularly when the lesions are small. The present study demonstrated the value of carcinoembryonic antigen (CEA) and some biochemical hepatic tests in detection of hepatic metastases in patients with primary colorectal cancer.
Laboratory tests have limits in detecting LM but they can rapidly and accurately evaluate liver metastasis in patients with primary colorectal cancer. This study showed that measurements of plasma biomarkers increase the sensitivity and selectivity of liver metastasis diagnosis.
The results of this study can improve early screening modalities. Furthermore, combination of markers and even modalities with imaging or endoscopic ultrasound will be needed to achieve a sufficient reliability.
It is a very interesting paper describing the diagnosis of liver metastases of colorectal cancer. The authors studied 106 patients with colorectal cancer, showing that the alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase, lactate dehydrogenase and CEA levels are increased in patients with liver metastasis of colorectal cancer.
Peer reviewer: Omar Vergara-Fernandez, MD, Department of Surgery, National Institute for Medical Sciences and Nutrition Salvador Zubirán, Vasco de Quiroga No. 15, Col. Seccion XVI. Deleg. Tlalpan, CP 14000, Mexico
S- Editor Wang YR L- Editor Wang XL E- Editor Zheng XM
| 1. | Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia: Elsevier Saunders 2005; . |
| 2. | Rothbarth J, van de Velde CJ. Treatment of liver metastases of colorectal cancer. Ann Oncol. 2005;16 Suppl 2:ii144-ii149. |
| 3. | Paschos KA, Bird N. Current diagnostic and therapeutic approaches for colorectal cancer liver metastasis. Hippokratia. 2008;12:132-138. |
| 4. | McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16:3-5. |
| 5. | Metcalfe MS, Bridgewater FH, Mullin EJ, Maddern GJ. Useless and dangerous--fine needle aspiration of hepatic colorectal metastases. BMJ. 2004;328:507-508. |
| 6. | Fornari F, Civardi G, Cavanna L, Di Stasi M, Rossi S, Sbolli G, Buscarini L. Complications of ultrasonically guided fine-needle abdominal biopsy. Results of a multicenter Italian study and review of the literature. The Cooperative Italian Study Group. Scand J Gastroenterol. 1989;24:949-955. |
| 7. | Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology. 1991;178:253-258. |
| 8. | Bipat S, van Leeuwen MS, Ijzermans JN, Comans EF, Planting AS, Bossuyt PM, Greve JW, Stoker J. Evidence-base guideline on management of colorectal liver metastases in the Netherlands. Neth J Med. 2007;65:5-14. |
| 9. | Ottmar MD, Gonda RL Jr, Leithauser KJ, Gutierrez OH. Liver function tests in patients with computed tomography demonstrated hepatic metastases. Gastrointest Radiol. 1989;14:55-58. |
| 10. | Mielczarek M, Chrzanowska A, Scibior D, Skwarek A, Ashamiss F, Lewandowska K, Barańczyk-Kuźma A. Arginase as a useful factor for the diagnosis of colorectal cancer liver metastases. Int J Biol Markers. 2006;21:40-44. |
| 11. | Grotowski M. Antigens (CEA and CA 19-9) in diagnosis and prognosis colorectal cancer]. Pol Merkur Lekarski. 2002;12:77-80. |
| 12. | Duffy MJ, van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R, Nilsson O, Sturgeon C, Topolcan O. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer. 2003;39:718-727. |
| 13. | Takagawa R, Fujii S, Ohta M, Nagano Y, Kunisaki C, Yamagishi S, Osada S, Ichikawa Y, Shimada H. Preoperative serum carcinoembryonic antigen level as a predictive factor of recurrence after curative resection of colorectal cancer. Ann Surg Oncol. 2008;15:3433-3439. |
| 14. | Munjal D, Chawla PL, Lokich JJ, Zamcheck N. Carcinoembryonic antigen and phosphohexose isomerase, gammaglutamyl transpeptidase and lactate dehydorgenase levels in patients with and without liver metastases. Cancer. 1976;37:1800-1807. |
| 15. | Baur M, Schlappack O, Havelec L, Wrba F, Dittrich C. Prognostic significance of liver metastases as first site of generalisation in patients with breast cancer--a retrospective analysis. Acta Med Austriaca. 2001;28:135-140. |
| 16. | Díaz R, Aparicio J, Gironés R, Molina J, Palomar L, Segura A, Montalar J. Analysis of prognostic factors and applicability of Kohne's prognostic groups in patients with metastatic colorectal cancer treated with first-line irinotecan or oxaliplatin-based chemotherapy. Clin Colorectal Cancer. 2005;5:197-202. |
| 17. | Machida N, Yoshino T, Boku N, Hironaka S, Onozawa Y, Fukutomi A, Yamazaki K, Yasui H, Taku K, Asaka M. Impact of baseline sum of longest diameter in target lesions by RECIST on survival of patients with metastatic colorectal cancer. Jpn J Clin Oncol. 2008;38:689-694. |
| 18. | Rocklin MS, Senagore AJ, Talbott TM. Role of carcinoembryonic antigen and liver function tests in the detection of recurrent colorectal carcinoma. Dis Colon Rectum. 1991;34:794-797. |
| 19. | Bakalakos EA, Burak WE Jr, Young DC, Martin EW Jr. Is carcino-embryonic antigen useful in the follow-up management of patients with colorectal liver metastases? Am J Surg. 1999;177:2-6. |
| 20. | Tartter PI, Slater G, Gelernt I, Aufses AH Jr. Screening for liver metastases from colorectal cancer with carcinoembryonic antigen and alkaline phosphatase. Ann Surg. 1981;193:357-360. |
| 21. | Steele L, Cooper EH, Mackay AM, Losowsky MS, Goligher JC. Combination of carcinoembryonic antigen and gamma glutamyl transpeptidase in the study of the evolution of colorectal cancer. Br J Cancer. 1974;30:319-324. |