Published online Aug 28, 2010. doi: 10.3748/wjg.v16.i32.4039
Revised: February 10, 2010
Accepted: February 17, 2010
Published online: August 28, 2010
AIM: To investigate whether curcumin could attenuate nuclear factor (NF)-κB p65 expression and macromolecular leakage in the gastric mucosa of Helicobacter pylori (H. pylori)-infected rats.
METHODS: Twenty-five male Sprague-Dawley rats were equally divided into five groups: control rats (Control), control rats supplemented with 600 mg/kg curcumin, H. pylori-infected rats (Hp), H. pylori-infected rats supplemented with 200 mg/kg curcumin (Hp + curI), and H. pylori-infected rats supplemented with 600 mg/kg curcumin (Hp + curII). In H. pylori-infected groups, rats were inoculated with H. pylori suspension twice a day at an interval of 4 h for 3 d. Two weeks later, 200 or 600 mg/kg curcumin was given once daily to curcumin-supplemented groups for 7 d. On the day of the experiment, macromolecular leakage in gastric mucosa was examined by intravital fluorescence microscopy. The stomach tissue was removed to examine NF-κB p65 expression in gastric epithelial cells by immunohistochemistry.
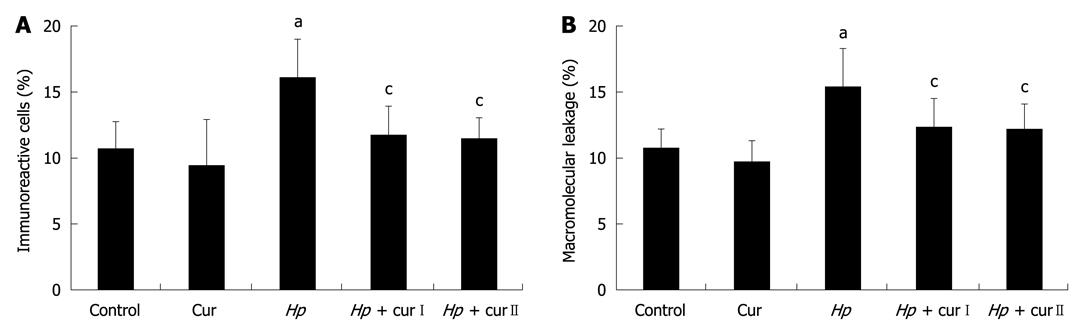
RESULTS: The expression of NF-κB p65 in gastric epithelial cells and the macromolecular leakage from gastric mucosal microcirculation significantly increased in the Hp group compared with the Control group. The percentages of NF-κB p65 immunoreactive cells in Control and Hp groups were 10.72% ± 2.10% vs 16.02% ± 2.98%, P = 0.004, respectively. The percentages of macromolecular leakage in Control and Hp groups were 10.69% ± 1.43% vs 15.41% ± 2.83%, P = 0.001, respectively. Curcumin supplementation in Hp + curI and Hp + curII groups significantly decreased NF-κB p65 immunoreactive cells and macromolecular leakage compared with results in the Hp group. The percentages of NF-κB p65 immunoreactive cells in Hp + curI and Hp + curII groups were 11.79% ± 2.13% (P = 0.017) and 11.42% ± 1.68% (P = 0.010), respectively. The percentages of macromolecular leakage in Hp + curI and Hp + curII groups were 12.32% ± 2.13% (P = 0.025) and 12.14% ± 1.86% (P = 0.018), respectively.
CONCLUSION: H. pylori-induced gastric inflammation in rats is associated with increased NF-κB activation and macromolecular leakage which can be reduced by curcumin supplementation.
-
Citation: Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N, Chatsuwan T. Curcumin suppresses gastric NF-κB activation and macromolecular leakage in
Helicobacter pylori -infected rats. World J Gastroenterol 2010; 16(32): 4039-4046 - URL: https://www.wjgnet.com/1007-9327/full/v16/i32/4039.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i32.4039
Helicobacter pylori (H. pylori) is a spiral-shaped Gram-negative bacterium. The infection causes chronic gastritis and peptic ulcer diseases in patients. H. pylori infection is also related to mucosa-associated lymphoid tissue lymphoma and gastric cancer diseases in patients[1,2]. The pathogenesis of H. pylori infection is associated with the bacterial virulence factors. After H. pylori bacteria adhere to gastric epithelial cells, they inject their virulence factors into the host cells via a type IV secretory system[3]. The virulence factors can induce the activation of nuclear factor (NF)-κB in gastric epithelial cells[4].
NF-κB is an important regulator of many cellular processes including the control of the immune response and inflammation[5,6]. NF-κB is a dimeric complex composed of the five mammalian Rel proteins, p65, c-Rel, p50/NF-κB1, p52/NF-κB2, and RelB, in almost any combination. In resting cells, the inhibitors of NF-κB (IκB) form complexes with NF-κB. Upon stimulation, specific intracellular signalling pathways are activated, leading to the activation of the IκB kinase complex (IKK complex). The activated IKK complex phosphorylates the IκB at specific amino acids for the poly-ubiquitination of these NF-κB inhibitors. The ubiquitination of IκB and its subsequent degradation by a proteasome are required for NF-κB activation. NF-κB is now free to translocate into the nucleus and regulate NF-κB-dependent gene expression[7]. The target of activated NF-κB includes the genes encoding proinflammatory cytokines and chemokines that are the causes of H. pylori-induced gastric inflammation[4,8,9].
In H. pylori-associated gastric inflammation, inflammatory mediators could induce vascular damage. A previous study demonstrated that H. pylori-infected patients showed erythema, edema, and vasodilation as well as neutrophil infiltration in the mucosa[10]. Our previous study suggested that leukocyte adhesion in postcapillary venules was increased in H. pylori-infected rats. Moreover, the degree of leukocyte adhesion was correlated with the level of the proinflammatory cytokine, tumor necrosis factor (TNF)-α[11]. In addition, previous studies have demonstrated that water-soluble extracts of H. pylori induced leakage of macromolecules from rat gastric mucosal microcirculation[12-14].
Curcumin (diferuloylmethane) is an active ingredient of Curcuma longa (turmeric) and is pharmacologically safe for human and animals. Curcumin has many biological activities, including anti-inflammatory properties[15]. Most of the anti-inflammatory effects can be explained by the efficient inhibition of NF-κB mediated by this substance[16-18]. Recently, a previous study showed that curcumin can inhibit NF-κB activation in H. pylori-infected gastric epithelial cells[19]. Curcumin is also a potent antibacterial agent against H. pylori as shown in in vitro study[20]. In contrast, curcumin did not eradicate H. pylori in H. pylori-infected patients[21].
However, it is not clear whether curcumin has any in vivo effects in H. pylori-induced gastric inflammation. Therefore, we examined the anti-inflammatory effect of curcumin, which may reduce mucosal macromolecular leakage through the suppression of gastric epithelial NF-κB p65 expression induced by H. pylori infection in rats.
Male Sprague-Dawley rats (National Laboratory Animal Center, Mahidol University, Nakorn pathom, Thailand) weighing 200-250 g were used. All experiments and procedures carried out on the animals were approved by the Ethics Committee of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Rats were housed in a controlled temperature room at 25 ± 1°C under standard conditions (12-h day-night rhythm). Twenty-five rats were divided into five groups (five rats each) as follows.
Control rats (Control): rats were fed normal saline (1 mL/rat) orally via intragastric tube twice a day at an interval of 4 h for 3 consecutive days. Two weeks later, 0.1% DMSO (1 mL/rat) was given once daily to the rats by intragastric tube for 7 d.
Control rats supplemented with 600 mg/kg curcumin (Cur): rats were fed normal saline (1 mL/rat) orally via intragastric tube twice a day at an interval of 4 h for 3 consecutive days. Two weeks later, 600 mg/kg curcumin (95% purified curcumin, Cayman Chemical, Ann Arbor, MI, USA) dissolved in 0.1% DMSO (1 mL/rat) was given once daily to the rats by intragastric tube for 7 d.
H. pylori-infected rats (Hp): rats were inoculated with H. pylori suspension according to Thong-Ngam et al[22]. Briefly, H. pylori suspension (1010 CFU/mL; 1 mL/rat) was given to the rats by intragastric tube twice a day at an interval of 4 h for 3 consecutive days. Two weeks later, 0.1% DMSO (1 mL/rat) was given once daily to the rats by intragastric tube for 7 d.
H. pylori-infected rats supplemented with 200 mg/kg curcumin (Hp + curI): 2 wk after H. pylori inoculation, 200 mg/kg curcumin dissolved in 0.1% DMSO (1 mL/rat) was given once daily to the rats by intragastric tube for 7 d.
H. pylori-infected rats supplemented with 600 mg/kg curcumin (Hp + curII): 2 wk after H. pylori inoculation, 600 mg/kg curcumin dissolved in 0.1% DMSO (1 mL/rat) was given once daily to the rats by intragastric tube for 7 d.
H. pylori strains used for all experiments were originally obtained from peptic ulcer patients who visited the King Chulalongkorn Memorial Hospital. The bacteria were grown in Brucella broth (pH 7.0) supplemented with 10% goat serum for 24 h at 37°C in an automatic CO2-O2 incubator (85% N2, 10% CO2, and 5% O2).
The method of preparing animals for in vivo fluorescent microscopy was adapted from a previous study[14]. The animal was anesthetized with thiopental (General Drug House, Thailand; 60 mg/kg, intraperitoneal). A constant level of anesthesia was maintained throughout the experiment by a supplement dose (20% of original dose) every 30-45 min[11]. A tracheotomy was performed. The arterial blood pressure was recorded in the common carotid artery using a pressure transducer (Nihon Kohden, Tokyo, Japan). The abdominal cavity was opened via a midline laparotomy. A 1.0 cm incision was made using an electrical microcautery device (Hyfrecator plus®, Conmed, Utica, NY, USA) at the posterior wall, being parallel to the “limiting ridge” of the exteriorized stomach[12]. Next, the stomach was gently extended and placed on a designed board. The incision in the anterior wall was opened using microclamps and covered with Saran wrap to allow visualization of the posterior mucosal surface[12]. The animals were terminated after studying intravital fluorescent videomicroscopy.
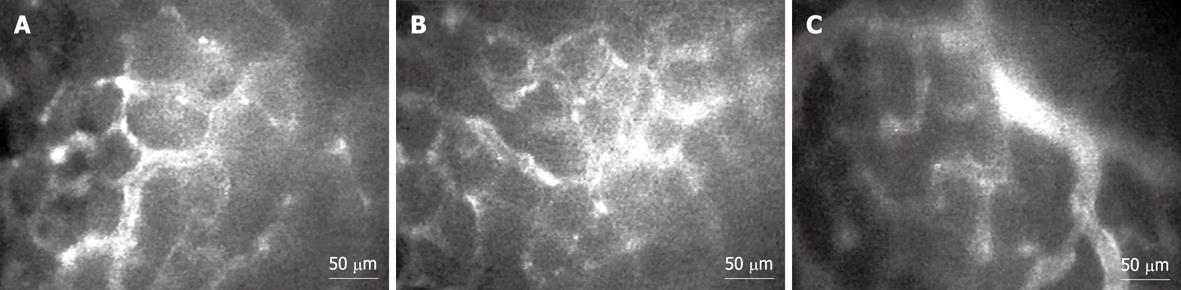
Observations were made from the glandular portion of the posterior mucosa. Fluorescence tracer [0.3 mL of 0.5% fluorescein isothiocyanate (FITC)-labeled dextran (FITC-dx, MW = 250 000, Sigma-Aldrich, USA)] was injected into the jugular vein[23]. The posterior mucosal microcirculation was visualized under an intravital fluorescence videomicroscope (Nikon Optiphot-2, Nikon, Tokyo, Japan), and examined under × 20 objective lens (Nikon). The selected area included the characteristic honeycomb-like network of mucosal capillaries and at least one postcapillary venule (PCV; diameter 15-30 μm)[24]. Five minutes after FITC-dx administration, a recording was performed as a baseline using a video-recorder (Sony SVT-124p, Sony, Tokyo, Japan). Thirty minutes later, recording was performed again.
Based on the recorded video images, we measured macromolecular leakage from the PCV in the selected area. Computerized image analysis (GLOBAL LAB® image II program, USA) was used to measure fluorescence intensity in the interstitial space and in the PCV at both time points during the experiment. The fluorescence intensities between outside and inside vessels (Iout/Iin) at baseline and 30-min time points were measured[25]. The molecular leakage in percentage was calculated using the equation: Macromolecular leakage (%) = [(Iout/Iin) at 30 min - (Iout/Iin) at baseline]/[(Iout/Iin) at baseline] × 100.
The presence of H. pylori infection in individual rats was determined by urease test and histological examination by a pathologist. After studying intravital fluorescent microscopy, the rat was terminated by injection of an overdose of thiopental. Then the stomach was removed and longitudinally dissected along the greater curvature. A 2 mm3 segment of gastric mucosa from the antrum was immediately cut and placed in the urease test tube.
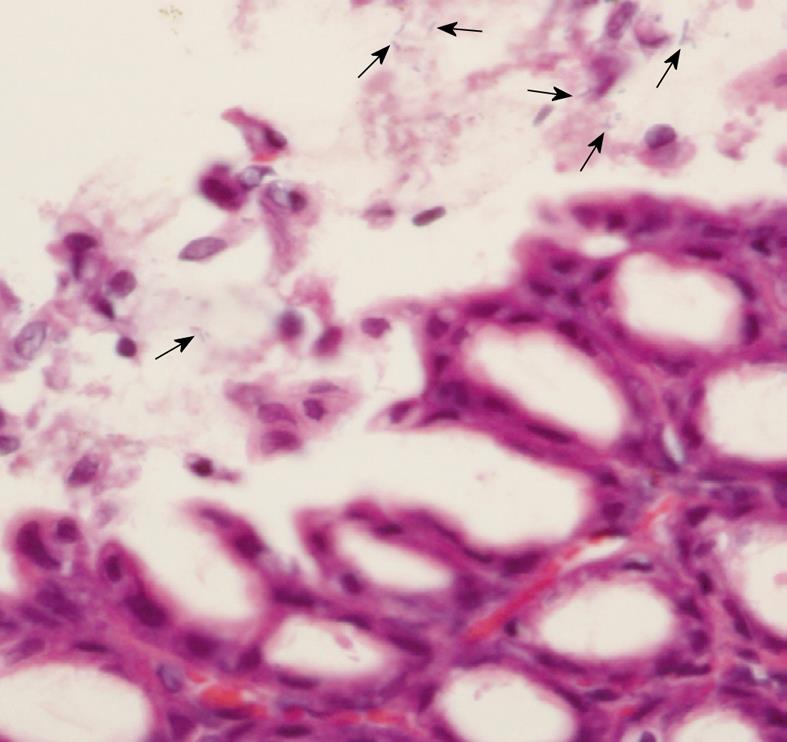
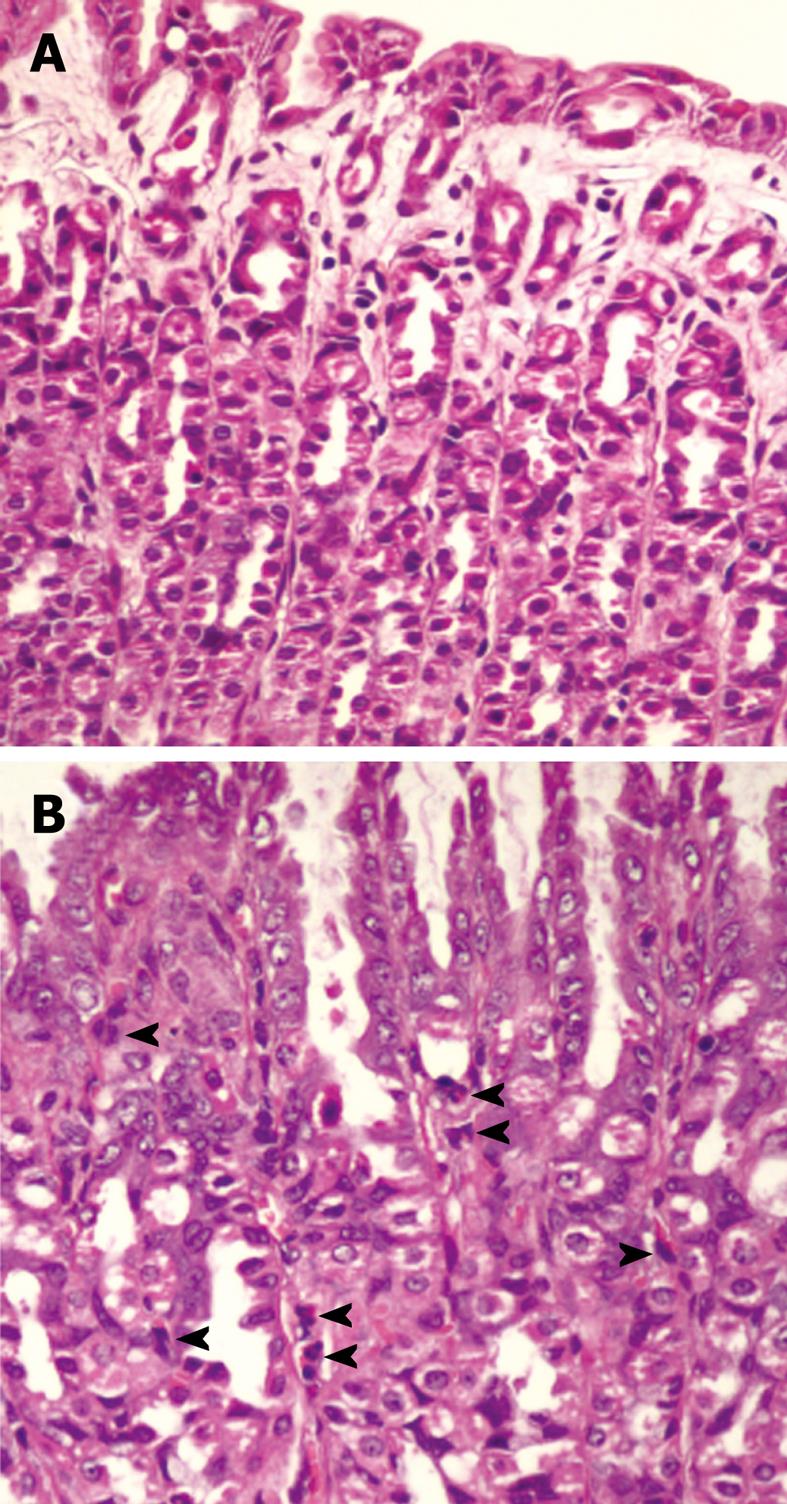
Regarding histological examination, the stomach was fixed with 4% paraformaldehyde in phosphate-buffered saline, pH 7.4 at room temperature for 24 h. The tissue was processed, embedded in paraffin, and cut at 5 μm thickness. The sections were stained with hematoxylin and eosin, and microscopically examined for the presence of H. pylori. The presence of H. pylori was also detected by Warthin-Starry staining in unclear cases. The level of bacterial colonization was recorded by using a grading system as follows, score 0: no bacteria detected; score 1: mild colonization in some gastric crypts; score 2: mild colonization in most gastric crypts; score 3: moderate colonization in all gastric crypts; and score 4: dense colonization in some gastric crypts. The results were presented as the bacterial colonization scores for each group. Moreover, gastric inflammation was classified by using the Sydney system[26]. The infiltrations of mononuclear and polymorphonuclear leukocytes in the gastric mucosa defining the inflammatory scores were recorded. Score 0 to 3 represented normal, mild, moderate, and marked histopathology changes, respectively.
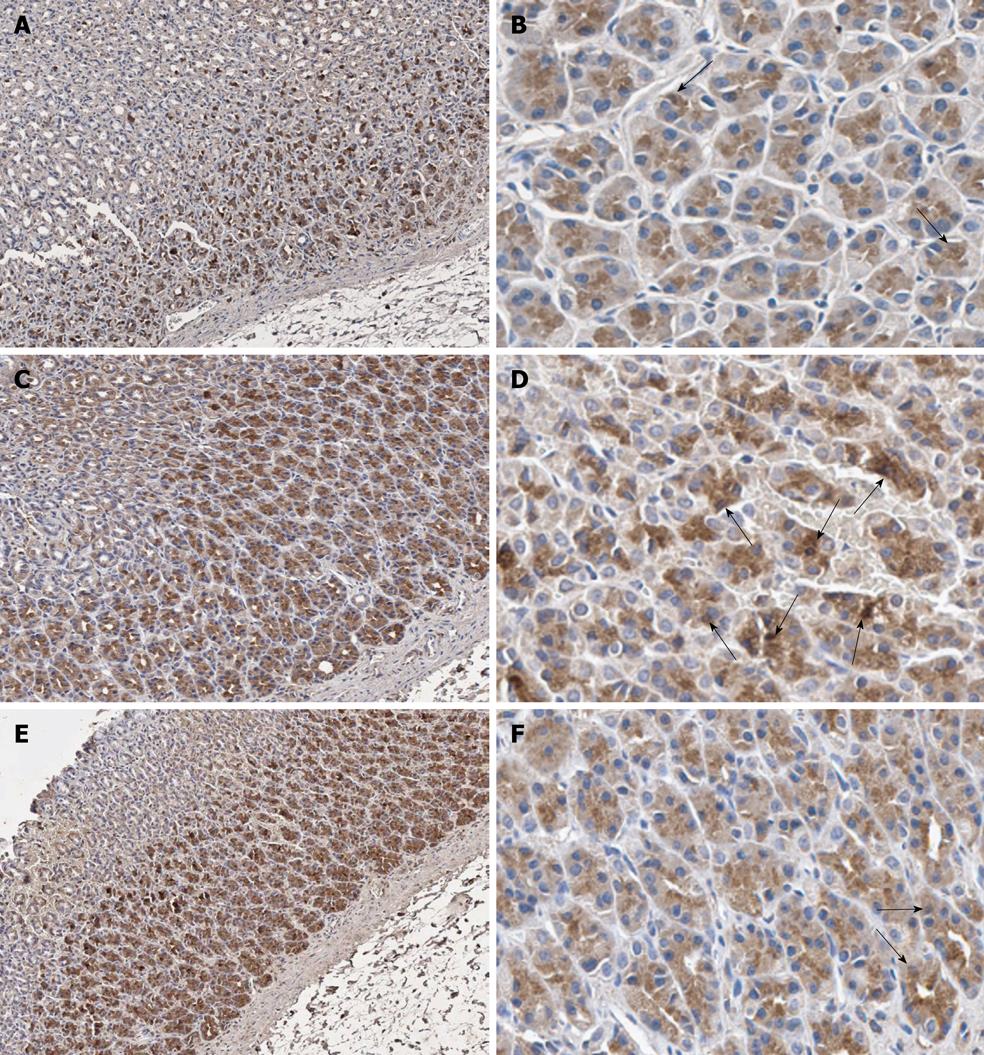
The stomach sections were deparaffinized with xylene and gradually dehydrated in ethyl alcohol. Next, antigen retrieval was performed by immersing the sections in citric acid buffer (pH 6.0) in a microwave oven for 13 min. Endogenous peroxidase activity and nonspecific binding were blocked with 3% hydrogen peroxide (Merck, Hohenbrunn, Germany) for 5 min and 3% normal horse serum (Gibco, Carlsbad, CA, USA) for 20 min, respectively. After that, the sections were incubated with polyclonal antibody against the p65 subunit of NF-κB (sc109; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:100 in a humidified chamber for 1 h at room temperature. Then the sections were incubated with biotinylated anti-rabbit immunoglobulin (DAKO, Glostrup, Denmark) in the humidified chamber for 30 min. The reaction was visualized using the substrate diaminobenzidine (DAKO). The sections were then counterstained with hematoxylin.
Under light microscope, the expression of NF-κB p65 was cytoplasmic with scattered positive nuclear staining[27]. Thus, NF-κB p65 immunoreactive cells were defined as those with dark brown stain in their nuclei. To quantify, one thousand gastric epithelial cells were counted for each rat under × 40 objective lens. All counting was manually performed by an investigator who was blinded to the treatment groups. The data were shown as percentage of immunoreactive cells calculated from this equation: Percentage of immunoreactive cells (%) = (number of immunoreactive cells × 100)/1000.
All data were presented as mean ± SD. The means were compared by one-way analysis of variance (one-way ANOVA) followed by LSD post hoc test. Correlation between the percentages of NF-κB p65 immunoreactive cells and macromolecular leakage was analyzed using Pearson’s correlation. All the statistical tests were performed using the computer program SPSS, version 13.0, for Windows (SPSS Inc., Chicago, IL, USA). Probability value of less than 0.05 was considered to be statistically significant.
H. pylori infection in rats was judged based on a urease test and histological examination. From histological examination, H. pylori colonization was observed in the Hp group (Figure 1) as well as in the Hp + curI and Hp + curII groups, whereas colonization was not observed in the Control and Cur groups. Figure 2 demonstrates histological differences between Control and Hp groups. The data are shown in Table 1.
| Group | No. | Level of H. pylori colonization | Score of gastric inflammation | |||||||
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | ||
| Control | 5 | 5 | - | - | - | - | 5 | - | - | - |
| Cur | 5 | 5 | - | - | - | - | 5 | - | - | - |
| Hp | 5 | - | 5 | - | - | - | - | 3 | 2 | - |
| Hp + curI | 5 | - | 5 | - | - | - | 3 | 2 | - | - |
| Hp + curII | 5 | - | 3 | 2 | - | - | 3 | 2 | - | - |
NF-κB p65 expression in gastric epithelial cells was studied using immunohistochemistry (Figure 3). H. pylori infection increased NF-κB p65 expression in gastric epithelial cells. The percentage of immunoreactive cells significantly increased in the Hp group (16.02% ± 2.98%) compared with the Control group (10.72% ± 2.1%, P = 0.004) (Figure 4A).
The expression of NF-κB p65 in gastric epithelial cells was diminished by curcumin supplementation in both Hp + curI and Hp + curII groups. The percentage of immunoreactive cells significantly decreased in Hp + curI (11.79% ± 2.13%, P = 0.017) and Hp + curII (11.42% ± 1.68%, P = 0.010) compared with the Hp group (Figure 4A). However, there was no significant difference between the number of immunoreactive cells in Hp + curI and Hp + curII. Curcumin administration in the Cur group did not alter the baseline NF-κB p65 expression (Cur group, 9.47% ± 3.46%, P = 0.447) in gastric epithelial cells.
The macromolecular leakage was studied by intravital fluorescent videomicroscopy. The captured images of gastric mucosal microcirculation from Control, Hp, and Hp + curI groups at the 30-min time point are shown in Figure 5. H. pylori infection led to a significant increase of macromolecular leakage in the Hp group (15.41% ± 2.83%) compared with the Control group (10.69% ± 1.43%, P = 0.001) (Figure 4B).
Oral treatment with curcumin over 1 wk attenuated H. pylori infection-induced macromolecular leakage significantly in both Hp + curI (12.32% ± 2.13%, P = 0.025) and Hp + curII (12.14% ± 1.86%, P = 0.018) groups (Figure 4B). However, there was no significant difference between the extent of macromolecular leakage in Hp + curI and Hp + curII. In addition, macromolecular leakage showed no significant differences between the Control group and the Cur group (Cur group, 9.74% ± 1.5%, P = 0.463).
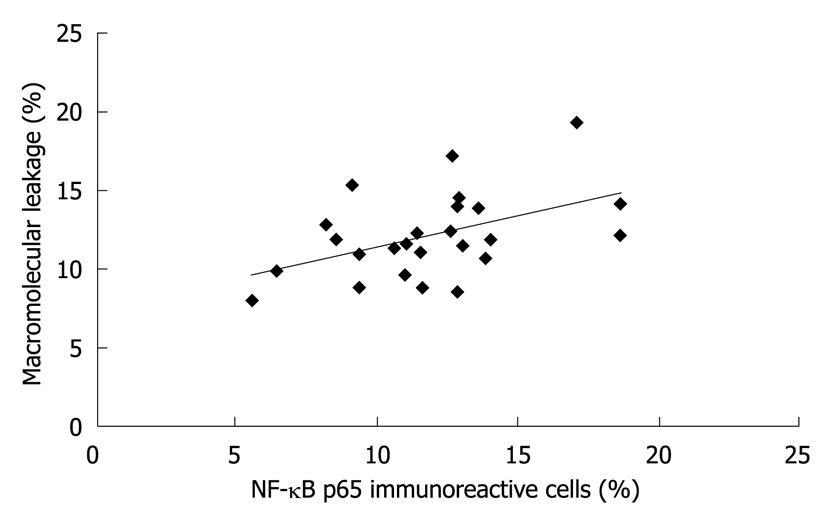
The percentages of NF-κB p65 immunoreactive cells and of macromolecular leakage from the same rat in all groups were plotted against each other (Figure 6). Interestingly, the level of NF-κB p65 expression was moderately correlated with the degree of macromolecular leakage (r2 = 0.2228, P = 0.017).
The present study demonstrated that H. pylori infection activated NF-κB in gastric epithelial cells. This result corresponds to previous observations studied in both in vitro and in vivo models[4,19,28]. NF-κB is an important transcription factor which activates many genes involved in inflammatory and immune responses[5,6]. Activated NF-κB induced by H. pylori infection upregulates cytokine production and is associated with gastric inflammation[9]. Clinically, NF-κB was also seen to be activated in the stomach of patients with H. pylori-induced gastritis[29-31]. Therefore, gastric epithelial NF-κB activation may play an important role in the initiation of H. pylori-induced gastric inflammation.
From the results, this study can also demonstrate an increased macromolecular leakage after H. pylori infection. This alteration is in good agreement with previous reports[12,14]. Several mechanisms may contribute to the increased macromolecular leakage. A previous study demonstrated that the transmigration of activated neutrophils expressing a specific protein could regulate endothelial permeability, allowing macromolecules to leak[32]. Furthermore, many proinflammatory cytokines such as TNF-α and interleukin (IL)-1β could be directly modulating vascular permeability[33-35]. Neutrophil transmigration and proinflammatory cytokine production were also suggested in a NF-κB-dependent manner in gastric mucosa during H. pylori infection[4,8,36-38]. H. pylori infection may directly influence the leakage via the transportation of H. pylori toxin and activation of NF-κB in endothelial cells. Previously, in vitro studies indicated that endothelial cells infected with H. pylori have changes in protein expression and function[39,40]. Our results substantiate these findings by showing that increased NF-κB p65 expression in gastric epithelial cells is accompanied by increased macromolecular leakage during H. pylori infection. Thus, the increased macromolecular leakage may result from inflammatory mediator production and vascular permeability changes through H. pylori-induced NF-κB activation.
Our experiments show that curcumin supplementation can suppress H. pylori-induced gastric inflammation, as indicated by decreased NF-κB p65 expression in gastric epithelial cells and decreased macromolecular leakage in the gastric microcirculation. The activation of NF-κB is essential for transcription of many genes involved in inflammatory and immune responses influencing gastric inflammation induced by H. pylori infection. In the present study, NF-κB p65 expression in the nucleus indicated that curcumin may possibly suppress the translocation of activated NF-κB into transcriptional sites. H. pylori-induced NF-κB activation affects recruitment of neutrophils and vascular permeability that reflect gastric inflammation. Curcumin decreased these parameters, indicating that curcumin could decrease gastric inflammation.
Inhibition of H. pylori growth was unlikely to be a mechanism that contributed to the effect of curcumin observed in this study, since positive results regarding H. pylori infection were still obtained from both urease test and histological examination after curcumin treatment in Hp + curI and Hp + curII animals. Recently, Di Mario et al[21] demonstrated that 7-d treatment with curcumin significantly improved gastric inflammation in H. pylori-positive patients despite H. pylori persistence. However, eradication by curcumin may be dependent on a high dose of curcumin, the safety of which has to be confirmed in animals and humans[41].
A previous study demonstrated that curcumin at the doses of 200 mg/kg and 600 mg/kg had an anti-inflammatory property[42]. In this study, 200 mg/kg curcumin was a sufficient dose for reducing gastric epithelial NF-κB p65 expression and mucosal macromolecular leakage. The possible mechanism cited was that curcumin inhibited H. pylori-induced NF-κB activation. This finding corresponded to an earlier in vitro study showing that H. pylori-induced NF-κB activation and the subsequent release of IL-8 were inhibited by curcumin[19]. In addition, the correlation between NF-κB p65 expression and macromolecular leakage found in our study suggests that H. pylori-induced mucosal macromolecular leakage may be mediated via NF-κB activation in gastric epithelial cells. Thus, the decreased macromolecular leakage may be explained by the reduction of inflammatory mediators due to epithelial NF-κB inhibition by curcumin.
In conclusion, the present study showed that H. pylori infection induced gastric epithelial NF-κB activation and increased mucosal macromolecular leakage. Curcumin supplementation may exert its anti-inflammatory effect by reducing macromolecular leakage through the suppression of NF-κB p65 expression in gastric epithelial cells. Hence, curcumin might be a novel therapeutic strategy against gastric inflammation induced by H. pylori infection.
The pathogenesis of Helicobacter pylori (H. pylori) infection is associated with bacterial virulence factors. The virulence factors can induce the activation of nuclear factor (NF)-κB in gastric epithelial cells, causing gastric inflammation and inducing vascular damage. Curcumin has many biological activities, including anti-inflammatory properties resulting from inhibition of NF-κB.
Curcumin (diferuloylmethane) is an active ingredient of Curcuma longa (turmeric) that has many biological activities mediated by the efficient inhibition of NF-κB. H. pylori infection induces gastric epithelial NF-κB activation and increases mucosal macromolecular leakage. The hotspots of this study indicate that curcumin supplementation may exert its anti-inflammatory effect by reducing macromolecular leakage through the suppression of NF-κB p65 expression in gastric epithelial cells.
A previous study showed that curcumin is a potent antibacterial agent against H. pylori and can inhibit NF-κB activation in H. pylori-infected gastric epithelial cells in vitro. However, it is not clear whether curcumin has any in vivo effects on H. pylori-induced gastric inflammation. Therefore, in this study, the authors examined the anti-inflammatory effect of curcumin, which was shown to reduce mucosal macromolecular leakage through the suppression of gastric epithelial NF-κB p65 expression induced by H. pylori infection in vivo in rats.
Curcumin might be a novel therapeutic strategy against gastric inflammation induced by H. pylori infection.
The merits of the manuscript are in showing the conclusion that curcumin can reduce the gastric inflammation reflected in attenuated levels of NF-κB and leakage of dextran.
Peer reviewer: Ki-Baik Hahm, MD, PhD, Professor, Gachon Graduate School of Medicine, Department of Gastroenterology, Lee Gil Ya Cancer and Diabetes Institute, Lab of Translational Medicine, 7-45 Songdo-dong, Yeonsu-gu, Incheon 406-840, South Korea
S- Editor Wang JL L- Editor Logan S E- Editor Zheng XM
| 1. | Gerhard M, Rad R, Prinz C, Naumann M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2002;7 Suppl 1:17-23. |
| 2. | Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28-37. |
| 3. | Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. |
| 4. | Brandt S, Kwok T, Hartig R, König W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci USA. 2005;102:9300-9305. |
| 6. | Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725-734. |
| 7. | Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621-663. |
| 8. | Kim SG, Kim JS, Kim JM, Chae Jung H, Sung Song I. Inhibition of proinflammatory cytokine expression by NF-kappaB (p65) antisense oligonucleotide in Helicobacter pylori-infected mice. Helicobacter. 2005;10:559-566. |
| 9. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. |
| 10. | Wyatt JI. Histopathology of gastroduodenal inflammation: the impact of Helicobacter pylori. Histopathology. 1995;26:1-15. |
| 11. | Prabjone R, Thong-Ngam D, Videsopas N, Chatsuwan T, Patumraj S. Anti-inflammatory effects of Aloe vera on leukocyte-endothelial interaction in the gastric microcirculation of Helicobacter pylori-infected rats. Clin Hemorheol Microcirc. 2006;35:359-366. |
| 12. | Kalia N, Jacob S, Brown NJ, Reed MW, Morton D, Bardhan KD. Studies on the gastric mucosal microcirculation. 2. Helicobacter pylori water soluble extracts induce platelet aggregation in the gastric mucosal microcirculation in vivo. Gut. 1997;41:748-752. |
| 13. | Kalia N, Bardhan KD, Reed MW, Jacob S, Brown NJ. Mechanisms of Helicobacter pylori-induced rat gastric mucosal microcirculatory disturbances in vivo. Dig Dis Sci. 2000;45:763-772. |
| 14. | Kalia N, Bardhan KD, Atherton JC, Brown NJ. Toxigenic Helicobacter pylori induces changes in the gastric mucosal microcirculation in rats. Gut. 2002;51:641-647. |
| 15. | Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1-7. |
| 16. | Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995;270:24995-25000. |
| 17. | Kumar A, Dhawan S, Hardegen NJ, Aggarwal BB. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem Pharmacol. 1998;55:775-783. |
| 18. | Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474-3483. |
| 19. | Foryst-Ludwig A, Neumann M, Schneider-Brachert W, Naumann M. Curcumin blocks NF-kappaB and the motogenic response in Helicobacter pylori-infected epithelial cells. Biochem Biophys Res Commun. 2004;316:1065-1072. |
| 20. | Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002;22:4179-4181. |
| 21. | Di Mario F, Cavallaro LG, Nouvenne A, Stefani N, Cavestro GM, Iori V, Maino M, Comparato G, Fanigliulo L, Morana E. A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure? Helicobacter. 2007;12:238-243. |
| 22. | Thong-Ngam D, Prabjone R, Videsopas N, Chatsuwan T. A simple rat model of chronic Helicobacter pylori infection for research study. Thai J Gastroenterol. 2005;6:3-7. |
| 23. | Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K. Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol. 1993;143:1055-1062. |
| 24. | Peti-Peterdi J, Kovács G, Hamar P, Rosivall L. Hemodynamics of gastric microcirculation in rats. Am J Physiol. 1998;275:H1404-H1410. |
| 25. | Somboonwong J, Thanamittramanee S, Jariyapongskul A, Patumraj S. Therapeutic effects of Aloe vera on cutaneous microcirculation and wound healing in second degree burn model in rats. J Med Assoc Thai. 2000;83:417-425. |
| 26. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. |
| 27. | Chen FE, Ghosh G. Regulation of DNA binding by Rel/NF-kappaB transcription factors: structural views. Oncogene. 1999;18:6845-6852. |
| 28. | Takahashi S, Fujita T, Yamamoto A. Role of nuclear factor-kappaB in gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1296-G1304. |
| 29. | Zhang X, Ruiz B, Correa P, Miller MJ. Cellular dissociation of NF-kappaB and inducible nitric oxide synthase in Helicobacter pylori infection. Free Radic Biol Med. 2000;29:730-735. |
| 30. | Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, Nishiyama T, Inoue K, Murata I, Kohno S. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am J Gastroenterol. 2000;95:2768-2776. |
| 31. | Yang GF, Deng CS, Xiong YY, Gong LL, Wang BC, Luo J. Expression of nuclear factor-kappa B and target genes in gastric precancerous lesions and adenocarcinoma: association with Helicobactor pylori cagA (+) infection. World J Gastroenterol. 2004;10:491-496. |
| 32. | Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med. 2001;7:1123-1127. |
| 33. | Wang X, Sun Z, Börjesson A, Andersson R. Inhibition of platelet-activating factor, intercellular adhesion molecule 1 and platelet endothelial cell adhesion molecule 1 reduces experimental pancreatitis-associated gut endothelial barrier dysfunction. Br J Surg. 1999;86:411-416. |
| 34. | Byrne MF, Corcoran PA, Atherton JC, Sheehan KM, Murray FE, Fitzgerald DJ, Murphy JF. Stimulation of adhesion molecule expression by Helicobacter pylori and increased neutrophil adhesion to human umbilical vein endothelial cells. FEBS Lett. 2002;532:411-414. |
| 35. | Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645-2650. |
| 36. | Rieder G, Hatz RA, Moran AP, Walz A, Stolte M, Enders G. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect Immun. 1997;65:3622-3630. |
| 37. | Sharma SA, Tummuru MK, Blaser MJ, Kerr LD. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol. 1998;160:2401-2407. |
| 38. | Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744-1752. |
| 39. | Innocenti M, Thoreson AC, Ferrero RL, Strömberg E, Bölin I, Eriksson L, Svennerholm AM, Quiding-Järbrink M. Helicobacter pylori-induced activation of human endothelial cells. Infect Immun. 2002;70:4581-4590. |
| 40. | Tobin NP, Henehan GT, Murphy RP, Atherton JC, Guinan AF, Kerrigan SW, Cox D, Cahill PA, Cummins PM. Helicobacter pylori-induced inhibition of vascular endothelial cell functions: a role for VacA-dependent nitric oxide reduction. Am J Physiol Heart Circ Physiol. 2008;295:H1403-H1413. |
| 41. | Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75:787-809. |
| 42. | Chuang SE, Cheng AL, Lin JK, Kuo ML. Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem Toxicol. 2000;38:991-995. |