Published online Aug 28, 2010. doi: 10.3748/wjg.v16.i32.4031
Revised: May 26, 2010
Accepted: June 2, 2010
Published online: August 28, 2010
AIM: To assess the effects of bile and pancreatic juice on structural and mechanical resistance of extracellular matrices (ECMs) in vitro.
METHODS: Small-intestinal submucosa (SIS), porcine dermal matrix (PDM), porcine pericardial matrix (PPM) and bovine pericardial matrix (BPM) were incubated in human bile and pancreatic juice in vitro. ECMs were examined by macroscopic observation, scanning electron microscopy (SEM) and testing of mechanical resistance.
RESULTS: PDM dissolved within 4 d after exposure to bile or pancreatic juice. SIS, PPM and PDM retained their integrity for > 60 d when incubated in either digestive juice. The effect of bile was found to be far more detrimental to mechanical stability than pancreatic juice in all tested materials. In SIS, the loss of mechanical stability after incubation in either of the digestive secretions was less distinct than in PPM and BPM [mFmax 4.01/14.27 N (SIS) vs 2.08/5.23 N (PPM) vs 1.48/7.89 N (BPM)]. In SIS, the extent of structural damage revealed by SEM was more evident in bile than in pancreatic juice. In PPM and BPM, structural damage was comparable in both media.
CONCLUSION: PDM is less suitable for support of gastrointestinal healing. Besides SIS, PPM and BPM should also be evaluated experimentally for gastrointestinal indications.
-
Citation: Hoeppner J, Marjanovic G, Helwig P, Hopt UT, Keck T. Extracellular matrices for gastrointestinal surgery:
Ex vivo testing and current applications. World J Gastroenterol 2010; 16(32): 4031-4038 - URL: https://www.wjgnet.com/1007-9327/full/v16/i32/4031.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i32.4031
Extracellular matrices (ECMs) have been introduced for clinical therapy of gastrocutaneous, enterocutaneous and anal fistulas and for buttressing of gastrointestinal staple lines. Moreover, they have been evaluated experimentally as bioscaffolds for tissue regeneration in different gastrointestinal hollow organs[1-14] and for reinforcement of gastrointestinal anastomoses[15,16]. In particular, small-intestinal submucosa (SIS) has been tested clinically and experimentally. SIS is a biodegradable, commercially available, acellular, immunologically inert collagen matrix, which is extracted from the submucosal layer of porcine small bowel. Several clinical studies have been performed to evaluate SIS for plug repair of gastrointestinal fistula. Prospective studies have shown high rates of success in the treatment of anal fistulas[17,18], but enterocutaneous[19,20] and gastrocutaneous[21,22] fistulas have also been treated successfully by implantation of SIS plugs. Furthermore, it has been shown that sealing of intestinal anastomoses promotes the healing of intestinal anastomoses[15,16]. For successful application in luminal gastrointestinal organs, temporary maintenance of structure and stability of ECM against gastrointestinal fluids should be guaranteed. To date, SIS has never been examined because of its resistance against gastrointestinal digestive juices. Other biological collagenous acellular scaffolds, which are promising for the support of gastrointestinal healing, are extracted from porcine dermis, porcine pericardium or bovine pericardium.
The aim of our study was to assess the effect of physiological intraluminal intestinal components human pancreatic juice and bile on 3D surface ultrastructure and mechanical resistance of different natural biological scaffolds in vitro.
Single-layer SIS was prepared as previously described[23]. Sections of porcine jejunum were obtained from the local slaughterhouse and immediately placed in 0.9% saline solution. Jejunal sections were cut into 10-cm lengths and luminally cleaned with 0.9% saline solution. The mesenteric tissues were removed from the segment of the small intestine, followed by mechanical removal of the tunica serosa and tunica muscularis from its outer surface by gentle abrasion using a scalpel handle and saline-moistened gauze. The segment was inverted and the tunica mucosa was mechanically removed by similar mechanical abrasion and reverted to its original orientation. The remaining 0.1-0.2-mm thick translucent tube actually consisted of the tunica submucosa. The stratum compactum that originally was in contact with the more superficial luminal mucosa was now the luminal surface of the SIS graft. After sterilization of the SIS graft by 2 h incubation in 0.1% perchloric acid, it was rinsed with sterile normal saline and stored in refrigerated 0.05% gentamicin at 4°C. Storage time for the graft materials ranged from 3 to a maximum of 7 d until the material was used for in vitro testing.
Four-layer SIS was provided as Surgisis® from Cook Surgical (Lafayette, IN, USA). Porcine dermal matrix (PDM) was provided as Xenoderm® from MBP (Neustadt-Gleve, Germany). Cleansed porcine pericardial matrix (PPM) was provided from aap Biomaterials (Dieburg, Germany). Bovine pericardial matrix (BPM) was provided as Lyoplant® from Braun Dexon (Melsungen, Germany). The materials were divided under sterile conditions into pieces of 1 cm × 1 cm for scanning electron microscopy (SEM) and for measurement of degradation time. For assessment of mechanical properties, samples of 1 cm × 3 cm were used.
Human bile had been collected during laparotomy from patients in whom cholecystectomy was performed. Microbiological assessment of bile excluded an infectious biliary syndrome. Pancreatic juice was obtained from patients with pancreatic duct drainage after pancreatic head resection for chronic pancreatitis. Patient consent to use the secretions for the study was given. Both fluids were checked for bacterial contamination in aerobic and anaerobic microbiologic cultures, and only sterile bile and pancreatic juice were used for experiments. ECM specimens of 1 cm × 1 cm and 1 cm × 3 cm were incubated at 37°C for 1, 7, 14 or 60 d in bile and pancreatic juice. For reference, the material was also incubated in sterile phosphate buffered saline (PBS). The pH of human bile used for incubation was 8.37. Pancreatic enzyme concentrations were measured by routine diagnostic methods at the central laboratory of the University Hospital Freiburg. Initial concentration of amylase was on average 86 040 U/L, concentration of lipase was 210 630 U/L in pancreatic juice, and pH was 8.42. After 24 h of incubation, concentration of amylase was on average 68 440 U/L, and concentration of lipase was 28 670 U/L in pancreatic juice. Due to this degradation of active enzymes during incubation, both media were replaced every 24 h under sterile conditions.
All incubated samples were macroscopically inspected daily for signs of degradation for 60 d. After incubation in PBS, human bile and human pancreatic juice, the size of the specimens was measured after 1, 7, 24 and 60 d.
Assessment of mechanical resistance using a sero-hydraulic material testing machine with a 200-N force transducer (UTS 20; UTS Systeme GmbH, Germany) was performed after 24 h and 14 d. Test Expert II Software (Zwick GmbH, Ulm, Germany) was used for analysis of force/distension diagrams. All mechanical experiments were carried out in triplicate. ECM strips of 1 cm × 3 cm were removed from incubation medium, rinsed in PBS and fixed in the testing device of the material testing machine. The sample was distended longitudinally at a speed of 12 mm/min until the sample broke. The required force until failure of the SIS strip was measured and reported as Fmax in Newtons.
After 7 d incubation in PBS, bile or pancreatic juice ECM samples (1 cm × 1 cm) were rinsed in PBS and subsequently fixed with 4% buffered formaldehyde for 48 h at room temperature. The samples were dehydrated in a graded series of acetone, dried in a critical-point dryer, mounted for SEM, and coated with gold in an evaporator unit. The samples were mounted such that one of the sides and the cross-section were visible. Examination was then performed in an LEO 435 VP scanning electron microscope (LEO Electron Microscopy Ltd., Cambridge, UK). A grading system was developed for comparison of ultrastructural alterations after incubation in bile and pancreatic juice. As reference, original materials incubated for 60 min in PBS were used. Changes in porosity, surface fibrillar arrangement and erosion of the ECM surface were measured and scored (Table 1).
| Characteristic | Resistance→Degradation | |||
| Score 0 | Score 1 | Score 2 | Score 3 | |
| Porosity | No change | Light increase | Distinct increase | Strong increase |
| Surface fibrillar arrangement | No change | Light alteration | Distinct alteration | Native arrangement not recognizable |
| Erosion of matrix surface | No change | Light surface erosion | Distinct surface erosion | Native surface structure not recognizable |
SIS one-layer, SIS four-layer, PPM and BPM samples were intact, without macroscopic signs of degradation after 60 d of incubation in bile, pancreatic juice and PBS. Apart from deep green-brown color after incubation in bile, there were no macroscopic differences recognizable after 7, 14 and 60 d of incubation. No shrinkage and no change in size of the patches were macroscopically detectable, regardless of the medium of incubation in SIS, PPM and BPM samples. PDM was dissolved within 40 d in PBS, within 2 d in bile, and within 4 d in pancreatic juice (Table 2).
For SIS, PPM and BPM, testing revealed the most distinctive loss of mechanical strength after incubation in bile at 24 h and 14 d. Incubation in pancreatic juice also caused a significant decrease of breaking strength at 14 d. No marked difference could be detected between saline and pancreatic juice incubation for SIS four-layer, PPM and BPM after 24 h. As a result of early structural degradation, PDM could only be tested after 24 h of incubation. No marked differences in mechanical resistance in the three different media were detected for PDM after 24 h. Numerical mean values of mechanical testing are shown in Table 3.
| PBS | Bile | Pancreatic juice | ||||
| 24 h | 14 d | 24 h | 14 d | 24 h | 14 d | |
| SIS 4-layer | 31.40 ± 4.3 | 26.03 ± 3.2 | 13.70 ± 0.9 | 4.01 ± 0.3 | 30.20 ± 1.0 | 14.27 ± 0.6 |
| SIS 1-layer | 4.56 ± 1.2 | 3.42 ± 0.4 | 3.60 ± 1.6 | 1.96 ± 0.2 | 2.56 ± 0.4 | 2.59 ± 0.4 |
| PDM | 1.05 ± 0.2 | 0.74 ± 0.1 | 0.81 ± 0.1 | Dissolved | 0.97 ± 0.1 | Dissolved |
| PPM | 19.03 ± 2.9 | 13.8 ± 4.9 | 10.45 ± 1.4 | 2.08 ± 0.6 | 21.95 ± 7.2 | 5.23 ± 1.3 |
| BPM | 27.13 ± 7.3 | 31.07 ± 5.2 | 10.34 ± 1.3 | 1.48 ± 0.3 | 30.07 ± 2.2 | 7.89 ± 1.8 |
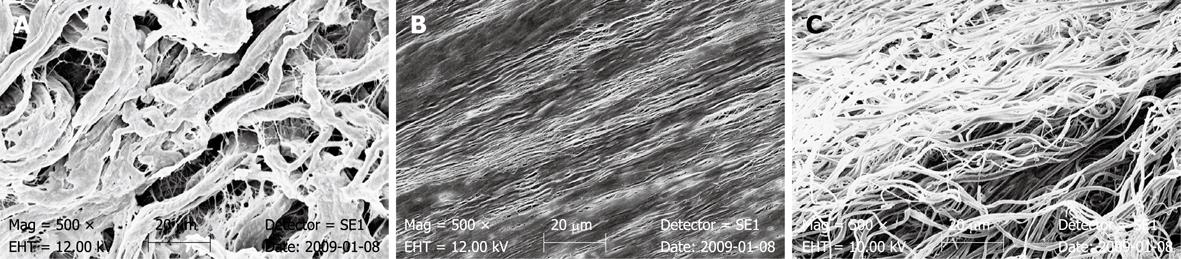
SEM of SIS showed a non-directed fibrous and fine surface structure of abluminal surface of the material. On the opposite side, which represents the stratum compactum of the submucosal layer of the porcine bowel wall, the surface appeared dense without a porous aspect (Figure 1). The single layers of the 4-layer SIS were distinguishable in the cross-sectional view. Arrangement of collagenous fibers in PDM appeared to be more directed. Single fibers looked chubby and showed a scaly surface (Figure 2). The collagenous fibers in PPM appeared fine and were assembled almost straight. Porosity of the material was verified by SEM, although some parts of the heterogeneous surface appeared dense (Figure 2). In BPM, SEM showed a directed but wormed arrangement of the fibers. Single collagenous fibers appeared fine-structured with a slick surface (Figure 2).
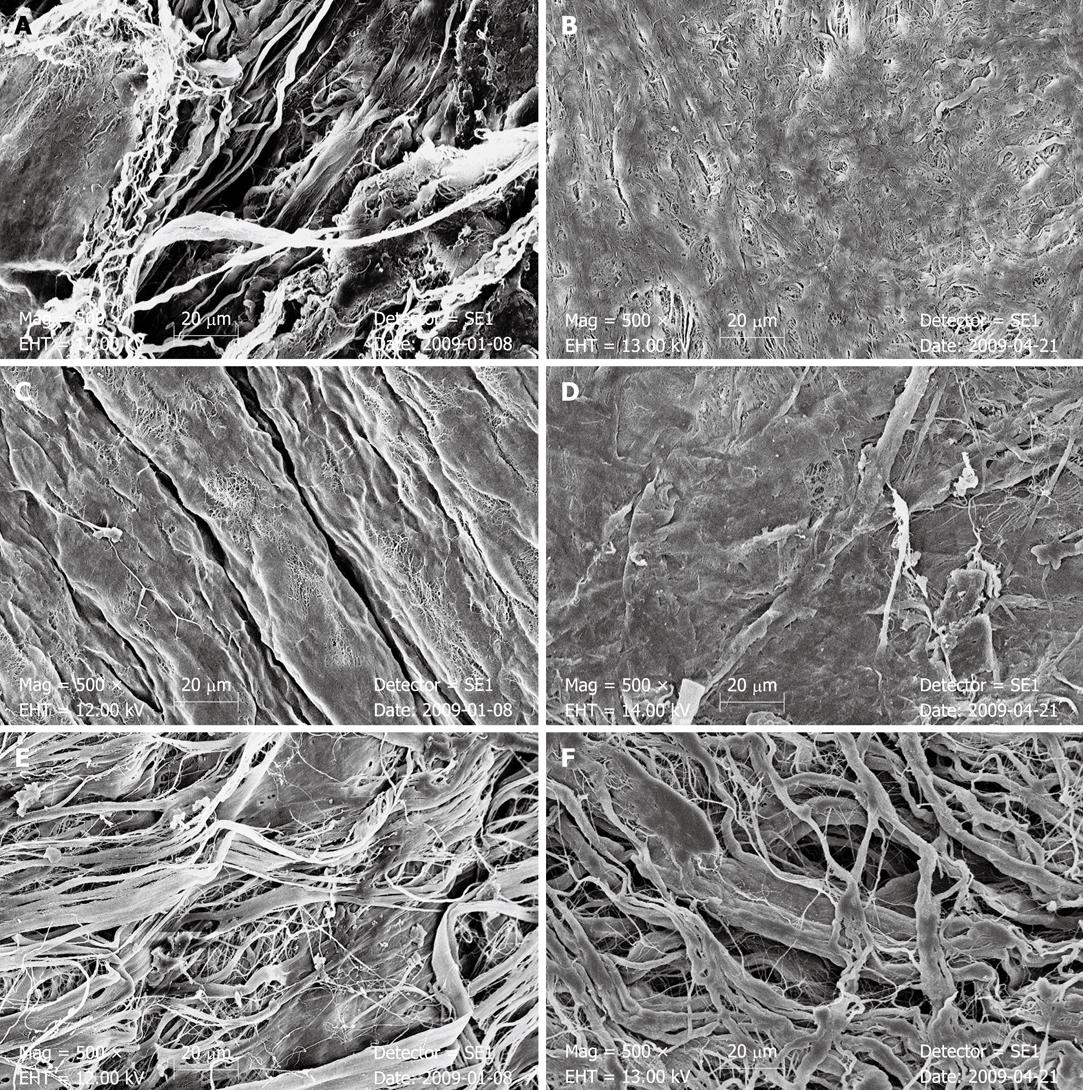
After 7 d of incubation in bile, the stratum compactum surface of SIS appeared clearly damaged. The dense surface was scarified and a fibrous structure of the deeper parts of the material could be recognized. After biliary incubation in PPM, collagenous fibers were grouped together with a gross scaly surface. The dense surface of the single strands appeared eroded and very fine reticular structures were recognized. In BPM, SEM after 7 d exposure to bile also revealed the phenomenon of collagenous fibrous structures that appeared to be grouped together (Figure 3 and Table 4).
| Bile | Pancreatic juice1 | |||||||
| Porosity | Surface fibrillar arrangement | Erosion of matrix surface | Σ | Porosity | Surface fibrillar arrangement | Erosion of matrix surface | Σ | |
| SIS 4-layer | 3 | 2 | 3 | 8 | 2 | 1 | 1 | 4 |
| SIS 1-layer | 3 | 2 | 3 | 8 | 2 | 1 | 1 | 4 |
| PPM | 1 | 3 | 1 | 5 | 1 | 3 | 3 | 7 |
| BPM | 0 | 2 | 2 | 4 | 1 | 2 | 1 | 4 |
After exposure to pancreatic juice, SEM revealed structural changes in SIS. In the stratum compactum, surface porosity of SIS was increased and the fibrillar structure of the deeper parts was recognizable. Surface structure of the single fibrils and the surface texture of the SIS sheet appeared to be altered. A slight decrease in thickness of the SIS was seen after pancreatic juice incubation for both types of SIS samples in the cross-sectional view. Single layers of four-layer SIS could not be separated in the cross-sectional view. In PPM, the straight arrangement of the collagenous fibers was no longer recognizable after pancreatic juice incubation. The PPM surface appeared dense and partially eroded with a fine undirected fibrillar aspect. In BPM, the fibers of the matrix appeared grouped together with a chubby surface (Figure 3 and Table 4).
A prerequisite to the in vivo implantation of a biomaterial as a tissue substitute in a gastrointestinal luminal organ is its resistance against gastrointestinal contents, with maintenance of integrity until gastrointestinal healing has progressed and integrity of the host tissue is restored. Physiological digestive fluids are extremely aggressive substances that are able to destroy most biological tissues. At present, only one experimental study has examined the resistance of ECM against digestive juices[24]. Human placental extracts, human collagen patches, bovine elastin, and bovine collagen matrices were tested for their resistance against bile and pancreatic juice. Only human collagen patches showed sufficient maintenance of integrity, whereas human placental extracts and all bovine materials failed in in vitro incubation in bile and pancreatic juice. Recent bioscaffolds like SIS, which have been successfully evaluated for therapy of gastrointestinal fistula, but also have proven potential for gastrointestinal tissue substitution and regeneration at different locations and organs, have so far not been examined systematically for structural and mechanical resistance against digestive contents. This study was designed to examine the effects of biological digestive fluids on the mechanical and structural resistance of current ECM.
It has been shown that ECM, implanted in vivo in gastrointestinal luminal organs, is able to induce regenerative responses in the host, and that anatomical tissue structure and tissue function are restored[1-13]. Different expressions of morphological and functional regeneration have been reported in the literature (Table 5). In the past, the majority of experimental work on ECM as a bioscaffold for gastrointestinal regeneration has been performed with SIS[1-13]. Other biological ECMs have only been tested in one single study in an in vivo setting in rodents[14]. Nearly complete and anatomical regeneration with SIS as a bioscaffold has been shown for the esophagus and the small bowel. Complete mucosal regeneration and regeneration of muscular layers has been reported within 3-6 mo[2,4,10,11]. After implantation in the rodent stomach, mucosal and muscular restoration, along with regeneration of innervation of the stomach wall were evident within 6 mo[6]. In contrast, limited mucosal regeneration and complete absence of muscular regeneration have been reported in the repair of duodenal and lower colonic defects with SIS[7-9]. These differences in SIS-induced gastrointestinal tissue regeneration could be explained by the effects of varying intraluminal chemical and bacterial environments on the integrity and intactness of the collagenous matrix and the 3D structure of the matrix. Biliary and pancreatic enzyme aggression is likely to play a major role in these processes. Toxicity of bile is explained by the detergent capacity of bile salts and alkaline pH of bile. The destructive effects of pancreatic juice on biological tissues and membranes are best explained by its content and the high concentration of enzymes like proteases, glucosidases, elastases and lipases. It is assumed that these enzymes are able to degrade the ECM components that are present in biological and artificial scaffolds[24]. Physiologically, bile and pancreatic juice are slowly secreted and diluted by other intraluminal gastrointestinal contents such as chyme. In our study, ECM was exposed to concentrated and very aggressive media in vitro, although they almost never encounter such demanding conditions in vivo.
| Autor | Location | Model | Structural regeneration | ||
| Mucosa | Muscularis | Nerve | |||
| Badylak et al[4] | Esophagus | Dog | + | + | NR |
| Lopes et al[11] | Esophagus | Rat | + | + | + |
| de la Fuente et al[3] | Stomach | Rat | (+) | - | - |
| Ueno et al[6] | Stomach | Rat | + | + | + |
| Rosen et al[1] | Bile duct | Dog | + | - | NR |
| De Ugarte et al[9] | Duodenum | Rat | (+) | - | NR |
| Souza Filho et al[8] | Duodenum | Dog | (+) | - | NR |
| Demirbilek et al[12] | Jejunum | Rabbit | + | - | NR |
| Chen et al[2] | Small bowel | Dog | + | + | NR |
| Ansaloni et al[10] | Ileum (isolated loop) | Rat | + | + | + |
| Wang et al[13] | Ileum | Rat | + | (+) | NR |
| Ueno et al[5] | Ceacum | Rat | + | + | + |
| Hoeppner et al[7] | Colon | Pig | (+) | (+) | - |
The most pronounced effects of bile and pancreatic juice in our study were seen in PDM. It was dissolved within a few days in both digestive juices. SIS, PPM and BPM maintained their integrity for at least 60 d of incubation in pure bile or pancreatic juice. In ECM of porcine origin, SIS and PPM, the loss of mechanical stability after incubation in the two digestive media was less distinct than in BPM. Our examination revealed bile to be much more efficacious in degrading ECM than pancreatic juice in all tested materials. In SEM, ECM incubated in bile was more eroded than after incubation in pancreatic juice. Moreover, SEM showed obvious changes in the 3D surface structure and arrangement of the collagenous fibers in SIS, PPM and BPM. It is assumed that the 3D structure of the fibrillar collagens and adhesive glycoproteins in the naturally occurring biopolymer SIS are involved in tissue regeneration induced by SIS[25], therefore, it has to be assumed that those changes in 3D structure impair gastrointestinal tissue regeneration. Moreover, destruction of regulatory proteins that are present in SIS, such as fibronectin, heparin sulfate proteoglycan, fibroblast growth factor-2, transforming growth factor-β and vascular endothelial growth factor, by aggressive contents of bile and pancreatic juice could also impair tissue regeneration[25-27]. This is a possible explanation for limited mucosal and missing muscular regeneration in duodenal patch repair by SIS[8,9]. Although nearly complete regeneration of biliary epithelium was reported, no formation of a muscular layer was seen when SIS was used for defect repair of the common bile duct in a canine model[1]. Based on these in vivo reports, it can be assumed that in the presence of higher intraluminal concentrations of bile and pancreatic enzymes, in particular, muscular regeneration is impaired if SIS is used as a bioscaffold.
Different clinical studies have reported successful application of SIS as a plug system for the therapy of enterocutaneous fistulas. For this indication, SIS is commercially available as Surgisis AFP Anal Fistula Plug® (Cook Biotech Inc.). For plug repair of anorectal fistulas, Champagne et al[17] have reported an overall success rate of 83% in a prospective study in 46 patients with a follow-up of 24 mo. In another prospective examination in 60 patients, Schwandner et al[18] have reported effective closure in anorectal fistula systems in 62% of cases, with a follow-up of 12 mo. In both studies, no serious adverse effects like impairment of continence function were reported.
Besides the prospective trials for therapy of anorectal fistulas, only case reports have been published for other enterocutaneous fistulas. Small-bowel-derived fistulas have been reported to be closed successfully in three patients[19,20]. Recently Toussaint et al[21] have reported a case series of five patients with gastrocutaneous fistulas after gastric sleeve and gastric bypass with a success rate of 80%. Furthermore, effective closure of persistent gastrocutaneous fistulas after removal of a gastric feeding tube[22], as well as successful therapeutic approaches for rectovaginal and ileal pouch-vaginal fistulas have been reported in case series and reports[28].
Although only experimental work has been published, SIS is commercially available for reinforcement of linear gastrointestinal staple lines (Surgisis Biodesign Staple Line Reinforcement®, Cook Biotech Inc.). It has been proven experimentally that SIS-reinforced staple lines in the porcine small bowel have increased mechanical stability if tested for bursting pressure in small bowel in vitro[29] and immediately after application of staple lines in vivo[30]. To date, no information is available concerning the effects of SIS in buttressing circular staple lines and its consequences for the intestinal healing process in staple lines. Most experimental and clinical examinations concerning buttressing of staple lines have been carried out on bovine pericardium. Especially in the field of obesity surgery, bovine pericardium is commercially available and widely used for reinforcement of staple lines. In a prospective randomized trial, Angrisani et al[31] have reported significant effects in prevention of staple line bleeding and a reduction of operating time due to dry operating fields compared to non-buttressed staple lines in laparoscopic gastric bypass. However, not only beneficial effects have been reported for bovine pericardium. Ibele et al[32] have recently published a retrospective analysis of 500 patients in which buttressing of the circular staple lines with bovine pericardium during laparoscopic Roux-en-Y gastric bypass was associated with an increased staple line leak rate.
Evaluation of ECM for anastomotic reinforcement in terms of sealing of colonic anastomoses by SIS has been experimentally performed in animal models. In rodents, sealing of colonic anastomoses by SIS showed microscopically and mechanically improved intestinal healing in the most vulnerable early phase of anastomotic healing[16]. In the porcine model, although no information about the effects of SIS on early anastomotic healing and long-term effects of SIS on circular stapled colonic anastomoses beyond 30 d was gained, the feasibility and safety of anastomotic sealing by SIS were demonstrated[15].
In summary, most clinical and experimental evaluation of ECM application in gastrointestinal surgery has been performed on SIS. Apart from staple line reinforcement, other biologically derived ECMs have only been rarely examined for gastrointestinal tissues. We therefore compared the mechanical and ultrastructural characteristics of SIS with ECM derived from porcine dermis, porcine pericardium and bovine pericardium in the presence of aggressive gastrointestinal fluids. Compared to SIS and PPM, mechanical resistance of BPM after 2 wk of exposure to bile or pancreatic juice is clearly weaker. These differences, however, could not be reproduced for structural degradation. Therefore, neither ECM of porcine nor bovine origin can be judged as more resistant to one or other of the human digestive juices. Early degradation of PDM in the presence of bile and pancreatic juice could be an explanation for the failure of acellular dermal matrix as a bioscaffold for intestinal regeneration placed in gastrointestinal continuity, whereas after prevention of exposure to bile and pancreatic juice by implantation in a defunctionalized blind jejunal limb, acellular dermal matrix remained sufficient and allowed mucosal regeneration[14]. These findings are important for application of ECM to gastrointestinal luminal organs, because our data suggest that deviation of bile and pancreatic juice upstream from the repair, should be applied in further experimental and clinical testing.
SIS, PPM and BPM retain their integrity in the presence of high concentrations of human bile and pancreatic juice. Ultrastructural degradation with changes in porosity, surface fibrillar arrangement and erosion of matrix surface were detectable in all three ECMs. The extent of ultrastructural alterations in SIS was more pronounced after incubation in bile than in pancreatic juice. In PPM and BPM, these differences were less distinct. Therefore, we conclude that PPM and BPM should also be evaluated as bioscaffolds for intestinal regeneration, as sealing materials for anastomotic reinforcement, and for plug repair of gastrointestinal fistulas in in vivo studies. As a result of early dissolution in the presence of digestive juices, PDM is less suitable for application in gastrointestinal luminal organs. To verify the results from in vitro testing, the bioscaffolds used should be tested in vivo by implantation in different gastrointestinal luminal organs, with and without deviation from bile, pancreatic juice and stool at the site of repair in upcoming experimental studies. Finally, PPM and BPM should also be evaluated experimentally for treatment of gastrointestinal fistula and reinforcement of intestinal anastomoses in vivo.
Extracellular matrices (ECMs) have been introduced for clinical therapy of gastrocutaneous, enterocutaneous and anal fistulas and for buttressing of gastrointestinal staple lines. They have been experimentally evaluated as bioscaffolds for tissue regeneration at different gastrointestinal hollow organs and for reinforcement of gastrointestinal anastomoses.
ECMs have been tested for different indications and at different locations in the gastrointestinal tract. It is not known if there are any relevant structural and mechanical changes in ECM caused by exposure to digestive juices. In vivo models have reported varying degrees of morphological intestinal regeneration after implantation of ECM as a bioscaffold at different gastrointestinal locations. This phenomenon could be explained by the effects of digestive juices on the structural and mechanic traits of the ECM. In this study, the authors investigated the effects of bile and pancreatic juice on different ECMs.
Small-intestinal submucosa (SIS) was found to be mechanically the most resistant tested material. It was demonstrated that porcine dermal matrix (PDM) is not suitable for therapeutic purposes in intestinal tissue regeneration due to its early degradation. However, SIS, porcine pericardial matrix (PPM) and PDM retained their integrity for up to 60 d when exposed to bile and pancreatic juices. Ultrastructural alterations were found to be more important in SIS when exposed to the juices.
As a result of their proven ultrastructural and mechanical resistance, PPM and bovine pericardial matrix (BPM) should be evaluated for treatment of gastrointestinal fistulas, as bioscaffolds for intestinal regeneration, and for reinforcement of intestinal anastomoses in vivo.
ECMs are biodegradable, acellular collagen matrices that are derived from biological tissues. SIS is extracted from the submucosal layer of porcine small bowel. PDM is extracted from the porcine dermis. PPM and BPM are extracted from porcine and bovine pericardia.
In general, this is an interesting study with new perspectives on various biomatrices that can be used for intestinal regeneration. These analyses could have an impact on the development of therapeutic approaches in the field of bioengineering.
Peer reviewer: Nathalie Perreault, PhD, Associate Professor, Department of Anatomy and Cell Biology, University of Sherbrooke, 3001, 12e avenue nord, Sherbrooke, j1H5N4, Canada
S- Editor Wang YR L- Editor Kerr C E- Editor Zheng XM
| 1. | Rosen M, Ponsky J, Petras R, Fanning A, Brody F, Duperier F. Small intestinal submucosa as a bioscaffold for biliary tract regeneration. Surgery. 2002;132:480-486. |
| 2. | Chen MK, Badylak SF. Small bowel tissue engineering using small intestinal submucosa as a scaffold. J Surg Res. 2001;99:352-358. |
| 3. | de la Fuente SG, Gottfried MR, Lawson DC, Harris MB, Mantyh CR, Pappas TN. Evaluation of porcine-derived small intestine submucosa as a biodegradable graft for gastrointestinal healing. J Gastrointest Surg. 2003;7:96-101. |
| 4. | Badylak S, Meurling S, Chen M, Spievack A, Simmons-Byrd A. Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg. 2000;35:1097-1103. |
| 5. | Ueno T, Oga A, Takahashi T, Pappas TN. Small intestinal submucosa (SIS) in the repair of a cecal wound in unprepared bowel in rats. J Gastrointest Surg. 2007;11:918-922. |
| 6. | Ueno T, de la Fuente SG, Abdel-Wahab OI, Takahashi T, Gottfried M, Harris MB, Tatewaki M, Uemura K, Lawson DC, Mantyh CR. Functional evaluation of the grafted wall with porcine-derived small intestinal submucosa (SIS) to a stomach defect in rats. Surgery. 2007;142:376-383. |
| 7. | Hoeppner J, Crnogorac V, Marjanovic G, Jüttner E, Karcz W, Weiser HF, Hopt UT. Small intestinal submucosa as a bioscaffold for tissue regeneration in defects of the colonic wall. J Gastrointest Surg. 2009;13:113-119. |
| 8. | Souza Filho ZA, Greca FH, Rocha SL, Ioshii SO, Domanski AC, Kfouri D, Campos PD, Silva RF. [Porcine submucosa graft for the treatment of duodenal injuries in dogs]. Acta Cir Bras. 2005;20:394-398. |
| 9. | De Ugarte DA, Choi E, Weitzbuch H, Wulur I, Caulkins C, Wu B, Fonkalsrud EW, Atkinson JB, Dunn JC. Mucosal regeneration of a duodenal defect using small intestine submucosa. Am Surg. 2004;70:49-51. |
| 10. | Ansaloni L, Bonasoni P, Cambrini P, Catena F, De Cataldis A, Gagliardi S, Gazzotti F, Peruzzi S, Santini D, Taffurelli M. Experimental evaluation of Surgisis as scaffold for neointestine regeneration in a rat model. Transplant Proc. 2006;38:1844-1848. |
| 11. | Lopes MF, Cabrita A, Ilharco J, Pessa P, Patrício J. Grafts of porcine intestinal submucosa for repair of cervical and abdominal esophageal defects in the rat. J Invest Surg. 2006;19:105-111. |
| 12. | Demirbilek S, Kanmaz T, Ozardali I, Edali MN, Yücesan S. Using porcine small intestinal submucosa in intestinal regeneration. Pediatr Surg Int. 2003;19:588-592. |
| 13. | Wang ZQ, Watanabe Y, Noda T, Yoshida A, Oyama T, Toki A. Morphologic evaluation of regenerated small bowel by small intestinal submucosa. J Pediatr Surg. 2005;40:1898-1902. |
| 14. | Pahari MP, Raman A, Bloomenthal A, Costa MA, Bradley SP, Banner B, Rastellini C, Cicalese L. A novel approach for intestinal elongation using acellular dermal matrix: an experimental study in rats. Transplant Proc. 2006;38:1849-1850. |
| 15. | Hoeppner J, Crnogorac V, Marjanovic G, Jüttner E, Keck T, Weiser HF, Hopt UT. Small intestinal submucosa for reinforcement of colonic anastomosis. Int J Colorectal Dis. 2009;24:543-550. |
| 16. | Hoeppner J, Wassmuth B, Marjanovic G, Timme S, Hopt UT, Keck T. Anastomotic sealing by extracellular matrices (ECM) improves healing of colonic anastomoses in the critical early phase. J Gastrointest Surg. 2010;14:977-986. |
| 17. | Champagne BJ, O'Connor LM, Ferguson M, Orangio GR, Schertzer ME, Armstrong DN. Efficacy of anal fistula plug in closure of cryptoglandular fistulas: long-term follow-up. Dis Colon Rectum. 2006;49:1817-1821. |
| 18. | Schwandner T, Roblick MH, Kierer W, Brom A, Padberg W, Hirschburger M. Surgical treatment of complex anal fistulas with the anal fistula plug: a prospective, multicenter study. Dis Colon Rectum. 2009;52:1578-1583. |
| 19. | Schultz DJ, Brasel KJ, Spinelli KS, Rasmussen J, Weigelt JA. Porcine small intestine submucosa as a treatment for enterocutaneous fistulas. J Am Coll Surg. 2002;194:541-543. |
| 20. | Satya R, Satya RJ. Successful treatment of an enterocutaneous fistula with an anal fistula plug after an abdominal stab wound. J Vasc Interv Radiol. 2010;21:414-415. |
| 21. | Toussaint E, Eisendrath P, Kwan V, Dugardeyn S, Devière J, Le Moine O. Endoscopic treatment of postoperative enterocutaneous fistulas after bariatric surgery with the use of a fistula plug: report of five cases. Endoscopy. 2009;41:560-563. |
| 22. | Wood J, Leong S, McCarter M, Pearlman N, Stiegmann G, Gonzalez RJ. Endoscopic-assisted closure of persistent gastrocutaneous fistula with a porcine fistula plug: report of a new technique. Surg Innov. 2010;17:53-56. |
| 23. | Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47:74-80. |
| 24. | Aprahamian M, Damgé C, Kerr-Conte J, Mutter D, Evrard S, Marescaux J. In vitro resistance of artificial connective tissues to human bile and pancreatic juice. Biomaterials. 1992;13:697-703. |
| 25. | Hodde J. Naturally occurring scaffolds for soft tissue repair and regeneration. Tissue Eng. 2002;8:295-308. |
| 26. | Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478-491. |
| 27. | Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8:11-24. |
| 28. | Gonsalves S, Sagar P, Lengyel J, Morrison C, Dunham R. Assessment of the efficacy of the rectovaginal button fistula plug for the treatment of ileal pouch-vaginal and rectovaginal fistulas. Dis Colon Rectum. 2009;52:1877-1881. |
| 29. | Burugapalli K, Chan JC, Kelly JL, Pandit A. Buttressing staples with cholecyst-derived extracellular matrix (CEM) reinforces staple lines in an ex vivo peristaltic inflation model. Obes Surg. 2008;18:1418-1423. |
| 30. | Downey DM, Harre JG, Dolan JP. Increased burst pressure in gastrointestinal staple-lines using reinforcement with a bioprosthetic material. Obes Surg. 2005;15:1379-1383. |
| 31. | Angrisani L, Lorenzo M, Borrelli V, Ciannella M, Bassi UA, Scarano P. The use of bovine pericardial strips on linear stapler to reduce extraluminal bleeding during laparoscopic gastric bypass: prospective randomized clinical trial. Obes Surg. 2004;14:1198-1202. |
| 32. | Ibele A, Garren M, Gould J. Effect of circular staple line buttressing material on gastrojejunostomy failure in laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:64-67. |