INTRODUCTION
Colorectal cancer (CRC) is the third most common type of cancer to develop and to cause death in the United States[1]. Although the pattern of spread of CRC may vary, the initial step involves lymphatic invasion and metastasis to regional lymph nodes[2]. Patients with lymphatic invasion have a less favorable outcome, and lymph node metastasis is one of the most important prognostic factors in CRC[3]. In fact, both the Dukes and TNM staging systems, which have been the most widely used staging systems for CRC, are based on the assessment of lymph node metastasis in addition to the extent of primary tumor and distant metastatic disease[4,5]. Patients with an early stage tumor without evidence of lymph node metastasis (Dukes A, TNM stage I) have an excellent post-operative prognosis and a 5-year survival rate of 80%-90%, while patients with advanced tumors with regional lymph node disease (Dukes C, TNM stage III) have a 5-year rate of 25%-60%. Furthermore, patients with distant metastatic disease (Dukes D, TNM stage IV) have a 5-year rate of less than 10%[6-8]. In addition, the number of nodes with metastatic disease has an important impact on the prognosis of patients with CRC. In fact, the influence of lymph node metastatic disease on prognosis in CRC is so great that there is not only a difference between N1 and N2 (1 to 3 nodes vs 4 or more nodes), but based upon separate analyses by the Surveillance Epidemiology and End Results program, there is also a significant prognostic difference within each of these groups. Accordingly, N1 is subdivided into N1a (1 node involvement) and N1b (2-3 node involvement), and N2 is subdivided into N2a (4 to 6 node involvement) and N2b (7 or more). This new TNM staging system lymph node sub-categorization is based on survival[8], which is in further agreement with the importance of lymph node metastatic disease in CRC patient outcome. Moreover, detailed analysis of lymph node status allows for accurate staging, which is now shown to be associated with better outcomes[8].
While local or regional CRC can be controlled with complete surgical resection, combination therapy is required to treat systemic disease. Among patients with newly diagnosed CRC, 25% will first present with metastatic disease[9]. Even among patients who present with localized, resectable disease, 30% will have a recurrence with metastatic disease[9]. There has been remarkable progress in the treatment of metastatic CRC during the last decade in the fields of surgery, radiation, chemotherapy, and targeted therapy[9-11]. Over the last decade, a better understanding of the processes involved in tumorigenesis and cancer metastasis has led to the development of a new category of systemic drugs called targeted therapies. The term targeted therapy refers to drugs that selectively target specific molecular pathways involved in tumorigenesis and/or tumor metastatic progression[11]. In CRC, two targets have been intensively investigated and are currently under Phase III clinical trials: the vascular endothelial growth factor (VEGF) pathway that controls angiogenesis, the phenomenon where new blood vessels are formed to feed the enlarging tumor and develop access to the blood stream; and the epidermal growth factor pathway that controls cell survival and proliferation. The former is targeted by the anti-VEGF monoclonal antibody, bevacizumab (Avastin™), and the latter is targeted by the anti-EGFR monoclonal antibodies, cetuximab or panitumumab[9,12]. Recently, there has been growing evidence that not only angiogenesis, but also lymphangiogenesis, the formation of new lymphatic vessels, is important in CRC metastatic progression[2,13-23]. This article will review the latest reports on lymphangiogenesis not only in experimental models, but also in clinical studies, and also review its clinical application as a biomarker and as a new targeted therapy.
WHY IS ANGIOGENESIS IMPORTANT FOR CANCER PROGRESSION AND METASTASIS?
In order for cancer to progress and metastasize, the primary tumor must have access to the systemic circulation, either through blood or lymphatic vessels. Angiogenesis is defined as the process whereby new blood vessels are formed from existing vessels, and as such, is a natural physiological process. Under normal physiologic conditions, angiogenesis only occurs in adults during menstruation, gestation and wound healing. At other times, anti-angiogenic factors maintain the endothelial cells that form blood vessels in a quiescent state[24]. The theory that angiogenesis could support tumor metastatic progression and therefore be a target for cancer therapy was proposed by Folkman et al[25,26] in the 1970s. He hypothesized that cancer requires angiogenesis to “feed” the cancer enabling it to grow beyond a certain size, and to allow for systemic spread. After 2 decades of developing this theory, modern molecular and cell biology techniques verified the role of angiogenesis in cancer growth via animal tumor models and clinical trials of bevacizumab, a humanized monoclonal antibody that neutralizes VEGF[27,28].
Tumors secrete multiple angiogenic factors and/or down-regulate angiogenesis inhibitors to induce tumor angiogenesis. VEGF-A is one of the key factors responsible for stimulation and maintenance of the disorganized, leaky, and torturous tumor vasculature. Other factors include members of the platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), insulin-like growth factor, angiopoietin, and hepatocyte growth factor (HGF) families[29]. Conversely, blockade of VEGF function inhibits angiogenesis and suppresses tumor growth in vivo[30]. While the discovery of factors important for angiogenesis has not yet led to a new cure for cancer, understanding that this process is essential for tumor metastasis has revealed several possibilities for targeted therapy. Applying this research approach to lymphangiogenesis can produce new potential targeted therapies.
WHY IS LYMPHANGIOGENESIS IMPORTANT FOR CANCER PROGRESSION AND METASTASIS?
The importance of lymph node metastasis in the progression of CRC has been well established and has a great impact on prognosis[8]. For cancer metastasis to the lymph node to occur, the cancer cells must access the lymphatic vessels to reach the regional lymph nodes. Applying approaches similar to the ones used to understand angiogenesis is expected to identify molecular mechanisms that control the processes related to lymphangiogenesis[31]. Therefore, understanding how lymphatic vessels are formed under physiologic and non-cancerous pathologic conditions can help provide an understanding of lymphangiogenesis in cancer in order to provide new avenues for targeted therapy development.
Although the ancient Greeks had already described aspects of the lymphatic system, the lymphatic vasculature was only properly considered to be a distinct circulatory system in 1622 by Asselli[32]. The lymphatic vasculature forms a vessel network that drains interstitial fluid from tissues and returns it to the blood circulation via the thoracic duct. Lymphatic vessels are also known to be an essential part of the body’s immune defense. Descriptions of the metastatic spread of cancer can be found as far back as the 14th century[33], and the involvement of the lymphatic system in metastatic progression has been described since the 18th century[34]. The traditional theory was that tumor cells metastasized to lymph nodes by utilizing pre-existing lymphatic vessels, and that lymphatic vessel entry occurred by permeation or embolization, not through the creation of new lymphatic vessels in response to cancer. Although the regeneration of lymphatic vessels was observed by Clark and Clark in 1932, cancer metastasis and the concept of lymphangiogenesis were not linked until the last two decades[35]. Despite an acceptance for centuries of the important role of the lymphatic system as the primary pathway for the metastatic spread of tumor cells to regional lymph nodes, and possibly even also to distant organs, the exact mechanism of this process has remained unclear until recently[36].
Over the past few years, understanding of the cellular and molecular aspects of physiologic lymphangiogenesis and tumor-induced lymphangiogenesis has advanced after the discovery of VEGF-C and its function to promote the growth of lymphatic vessels[37]. Initially, the study of lymphangiogenesis largely focused on the primary site of tumor growth and adjacent tissues, which is known as “tumoral lymphangiogenesis”[38,39]. However, lymphangiogenesis was also observed around regional lymph nodes, in particular the sentinel nodes where tumor cells first metastasize, a phenomenon now known as “lymph node lymphangiogenesis”[6,40]. Lymph node lymphangiogenesis and increased lymph flow through tumor-draining lymph nodes are speculated to actively promote metastasis via the lymphatics[41]. Recent evidence indicates that tumor cells can also induce lymph node lymphangiogenesis - even before they metastasize - and that metastatic tumor cells continue to induce lymphatic vessel growth within sentinel lymph nodes, theoretically promoting their further metastatic dissemination[42,43].
As expected, the majority of studies point to a positive correlation between tumor-induced lymphangiogenesis and lymphatic metastasis[6,13-17,44,45]. Because the physiologic role of the lymphatic system is to collect interstitial fluid from peripheral tissues and return it to the systemic blood circulation, it is hypothesized that tumor-induced lymphangiogenesis occurs in order to drain interstitial fluid away from the tumor. Therefore, targeting this process provides a potential avenue for cancer therapy[46]. In fact, experimental inhibition of this process in animal models suggested that lymphangiogenic growth factors facilitate the metastatic spread of tumor cells via the lymphatics[14,18-20]. The results highlight the key role that lymphangiogenic growth factors and new lymphatic vessels play in tumor metastatic progression. These early studies indicate that targeting lymphangiogenic growth factors in tumors could be a strategy for restricting the metastatic spread of cancer[31].
IN VIVO AND IN VITRO MODELS OF LYMPHANGIOGENESIS
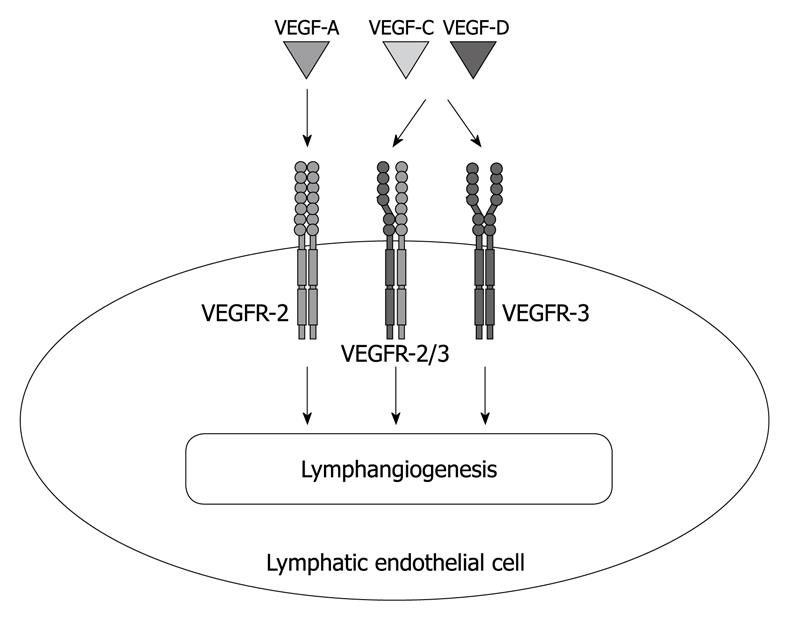
The recent discovery of the key lymphangiogenic factors VEGF-C and VEGF-D, other proteins related to these factors, and their receptor VEGF receptor (VEGFR)-3, have provided novel insights into how the lymphatic vessels and blood vessels coordinately grow and affect human disease[47]. In fact, these factors are associated with a number of human tumor types[31]. These secreted glycoproteins largely signal via the cell surface tyrosine kinase receptor VEGFR-3/Flt4 present on the surface of lymphatic endothelial cells (LECs), and VEGFR-3 activation promotes LEC proliferation, migration, and survival, which result in lymphatic vessel proliferation in vitro and in vivo[6]. Furthermore, recent studies indicate that the VEGF-A/VEGFR-2 signaling pathway plays a major role not only in angiogenesis, but also in lymphangiogenesis[48,49] (Figure 1).
Figure 1 Lymphangiogenic growth factors and their receptors expressed by lymphatic endothelium.
Vascular endothelial growth factor (VEGF) receptor (VEGFR)-3 is a member of the fms-like tyrosine kinase family and specifically binds VEGF-C and VEGF-D, but not VEGF-A. Recent studies also indicate an important role for the VEGF-A/VEGFR-2 signaling pathway in lymphangiogenesis.
In vitro techniques to study lymphangiogenesis have evolved with the development of methods to isolate and culture LECs. LECs have been isolated from lymphatic vessels or skin followed by enzymatic digestion and flow cytometric cell sorting using markers specific to LECs. Several LEC markers have been recently identified, including: lymphatic vessel endothelial hyaluronan receptor-1[50]; glomerular podocyte membrane mucoprotein, podoplanin (D2-40)[51]; the homeobox gene product, Prox-1[52,53]; and VEGFR-3[54,55]. Although these markers have aided in the purification of LECs, the limited quantity of cells obtained and the reduced growth potential of these cells have posed a challenge. To address the challenge of only having a limited quantity of cells after the purification of LECs, immortalization with SV40 large T antigen[56] or transformation with human telomerase reverse transcriptase[57] have been utilized to extend the life span of LECs, and transgenic mice have been developed to harvest immortalized LECs[58]. Protocols for the isolation of LECs from microlymphatic vessels in different tissues in rats have recently been established[59,60]. In most experimental assays, LECs are seeded as monolayers on culture plates or onto the surface of matrix-coated plates. While 2-D cultures cannot undergo all of the steps of lymphatic vessel formation, these culture systems allow the analysis of each step individually, using various assays of cell activity (e.g. gene expression profiling), cell proliferation, apoptosis, adhesion, migration (wound scratch assay, Boyden chamber assay), and morphogenesis (tubulogenesis)[61].
Numerous in vivo models to study the growth of lymphatic vessels have utilized the same techniques as those used for blood vessel growth. The growth of vessels into the avascular cornea in response to specific factors or inflammation has been historically utilized as a model to study lymphangiogenesis[62-64]. Another extensively used model is the development of lymphedema and lymphangiomas. Lymphedema is swelling due to the failure of fluid drainage by the lymphatics which occurs as a result of obstruction or secondary changes impairing lymph flow. Several mouse models carrying mutations or chromosomal aberrations recapitulate this phenotype[65,66], and surgical ablation of lymphatic vessels can induce lymphedema and subsequently lymphangiogenesis[67,68]. Lymphangiomas, characterized as benign malformations of the lymphatic system, have been induced by injection of incomplete Freund’s adjuvant, either into the mouse ear[69] or intraperitoneally[70], causing lymphatic vessel hyperplasia leading to inflammation and lymphangiogenesis[71].
Breast, gastric or CRC cells over-expressing VEGF-C implanted into transgenic mice induced tumor-associated lymphangiogenesis in orthotopic mouse models[13,72,73]. Skin carcinogenesis models in transgenic mice over-expressing VEGF-A or C showed that tumors in these mice were significantly more likely to metastasize[40,74]. As compared to VEGF-A, VEGF-C did not increase the size of the primary tumors, but induced the expansion of metastatic networks within the lymph nodes and promoted metastasis to distant sites such as distant lymph nodes and the lungs[74]. In addition to VEGF, PDGF-BB, FGF-2, HGF and angiopoietin enhance lymphangiogenesis[75-79]. Sphingosine-1-phosphate (S1P) also stimulates lymphangiogenesis in both in vitro and in vivo models[80,81]. S1P is generated by the action of two sphingosine kinases, sphingosine kinase 1 and 2[82,83]. Tumor cells, which are characterized by high levels of sphingosine kinase 1 expression, can release S1P into the extracellular space[84], which in turn can lead to paracrine-induced angiogenesis and lymphangiogenesis[81]. Interestingly, LEC-specific deletion of sphingosine kinase 1 in the sphingosine kinase 2 knockout mouse inhibits lymphatic vessel maturation[85].
In vivo models demonstrate that lymphangiogenesis promotes CRC metastasis, suggesting new avenues for the development of targeted therapy and prognostic markers. VEGF-C and VEGF-D, which are up-regulated in CRC, appear to drive tumor lymphangiogenesis through the VEGF-C/VEGF-D/VEGFR-3 pathway, while other growth factors, such as VEGF-A, have modulatory effects on this process[48,49]. VEGF-A expression levels significantly correlate with metastasis to the lymph nodes in CRC[48,49]. Orthotopic implantation of VEGF-C over-expressing DLDA colon cancer cells demonstrated that VEGF-C induced lymphangiogenesis-mediated tumor spread and the formation of metastatic disease in the lymph nodes[13], while the inhibition of VEGF-C expression reduced lymphangiogenesis, the extent of lymph node metastatic disease, and enhanced survival in mice[14]. The inhibition of VEGF-C expression also dramatically suppresses tumor lymphangiogenesis, tumor growth, and regional lymph node metastasis in mice[18]. Inhibition of VEGFR-3 using small interfering RNA also significantly inhibited tumor growth[19,20]. These in vitro and in vivo mouse data demonstrate the possible clinical application of lymphangiogenesis as a biomarker and/or as a new target for therapy in CRC in humans.
LYMPHANGIOGENESIS IN HUMAN SAMPLES
The role of intra- and peri-tumoral lymphatics in tumor biology and the initial steps of lymphatic metastatic progression, i.e. the invasion of tumor cells into the lymphatic vessels, are just beginning to be elucidated in human samples[44]. Animal studies have demonstrated that intra-tumoral lymphatic vessels are poorly functional due to high intra-tumoral pressure and may not be required for lymphatic metastatic progression. Conversely, lymphatic vessels in the tumor periphery are functional and can drain colloids from the tumor. In several common human tumors, such as cutaneous melanoma[86,87], head and neck squamous cell carcinoma[38,88], transitional cell carcinoma of the bladder[89,90] and non-small cell lung cancer[91], tumoral lymphangiogenesis detected by lymphatic vessel density (LVD) can be readily appreciated and has been shown to be of prognostic significance. In contrast, in breast[92,93], cervical and prostate carcinoma[94] tumors that metastasize to the lymph nodes, there is little evidence of significant tumoral lymphangiogenesis detected by LVD, with most proliferating vessels lying within the peritumoral tissues[6]. Another important factor to consider is the location of the tumor in relationship to the amount of pre-existing lymphatic vessels, such as in biliary cancer, which is very prone to metastasize via the lymphatic system[95,96]. Furthermore, mouse models of cecal cancer metastasis to the liver have demonstrated that both VEGF-C and VEGF-D produced less metastatic disease in the liver compared to primary cecal tumors, suggesting the importance of the tumor microenvironment for the production of these lymphangiogenic factors[97]. Taken together, the pattern of tumoral lymphangiogenesis and metastasis to the lymph node varies between tumor histological type and anatomic location of the tumor, involving both the lymphatic system and the microenvironment. Clearly, further studies are awaited to understand this complex process.
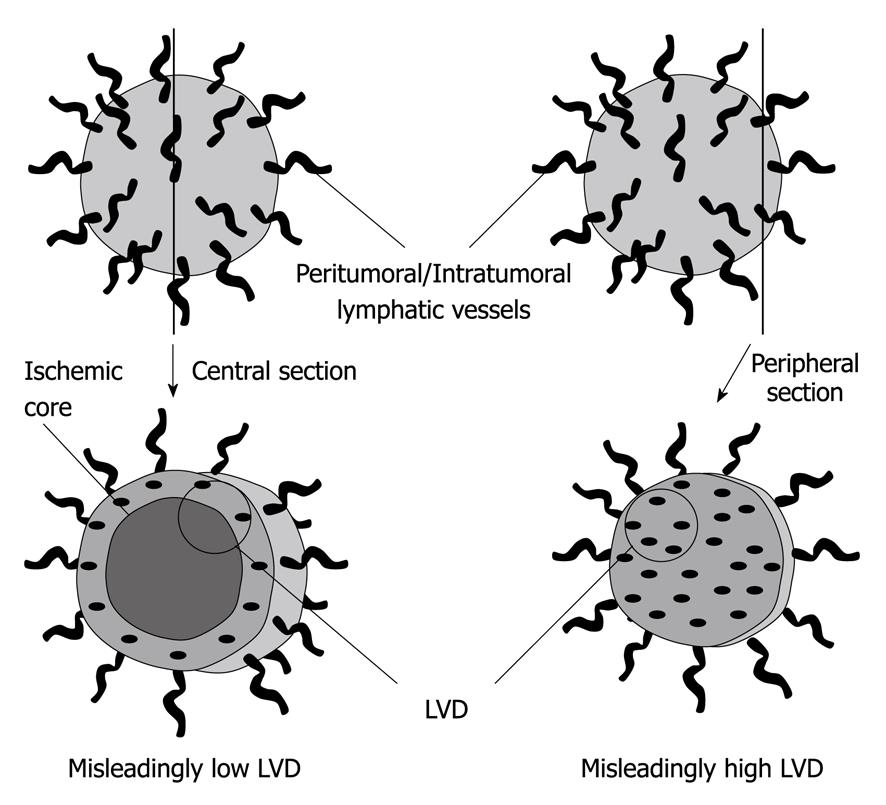
Although several studies have reported the discrepancy between LVD measurements and clinical outcome, it should be noted that there is a great deal of variability in their methodologies and consequently also in their results. In addition to tumor characteristics, the discrepancies in terms of the correlation of LVD with metastasis to the lymph nodes and prognosis in these studies can be attributed to the limitations of the methodologies used. The limitations include the different types of tissue preservation, variable immunostaining techniques, different LVD quantification methods employed, and the lack of standardization in the estimation of lymphangiogenesis[98] (Figure 2). To address the limitations of this qualitative analysis methodology, some studies have attempted to quantify the expression of VEGF-C and VEGF-D mRNA or protein in excised primary tumor tissue of patients with various cancers. They frequently report that the levels of these molecules exhibit a strong correlation with parameters associated with poor patient outcome, such as the invasion of lymphatic vessels by tumor cells, the extent of lymph node metastatic disease, and disease-free as well as overall survival[31]. However, even these attempts to quantifiably measure lymphangiogenesis have not been entirely successful. In fact, recent epigenetic studies demonstrated that the analysis of mRNA or protein expression may not reflect actual lymphangiogenesis due to posttranscriptional modifications of proteins. In order to adequately assess the degree of lymphangiogenesis, a better method to accurately quantify the amount of lymphangiogenesis is needed.
Figure 2 Limitations of lymphatic vessel density estimations.
The central section of a tumor with a necrotic central core may estimate a misleadingly low lymphatic vessel density (LVD) (left), while the peripheral section of the same tumor may estimate a misleadingly high LVD (right).
LYMPHANGIOGENESIS AS A BIOMARKER FOR CRC PROGRESSION
Although animal models show a strong relationship between lymphangiogenesis and lymph node metastasis and survival, the clinical significance of lymphangiogenesis in CRC remains uncertain, as is the case for other tumors[6]. Parr et al[21] showed that the expression of VEGFR-3 receptor, prox-1, 5’-nucleotidase expression, and podoplanin expression in cancer tissue were significantly higher than in the normal background tissue. Jia et al[16] showed that the extent of lymph node metastatic disease in VEGF-C-positive patients (81.1%) was significantly higher than that in the negative group (42.1%). Lu et al[17] showed that quantitative analysis of podoplanin in CRC specimens correlates with metastasis to regional lymph nodes. Yan et al[22] showed that the co-accounting of LVD and microvessel density (MVD) was an independent prognostic factor in CRC. Moehler et al[15] showed that the expression of VEGF-D is significantly associated with lymphatic involvement in CRC patients and that cetuximab can block such expression effectively. In addition, the quantification of VEGF-C and VEGF-D in blood samples has the potential to serve as a biomarker to predict the extent of lymph node metastatic disease[23]. Interestingly, Sundlisaeter et al[99] showed that LVD was significantly increased in tumor tissue compared with the normal mucosa, but there were no changes in LVD between stage II and III CRC. This indicates that lymphangiogenesis occurs in CRC, and indeed suggests that it is triggered at an early stage of tumor development. Taken together, these studies suggest that these lymphangiogenesis-related markers indicate an increase in lymphangiogenesis in CRC, and might therefore have prognostic value for CRC patients.
However, other reports failed to find an association between higher LVD, the aggressiveness of tumor behavior and poorer clinicopathological variables. Kazama et al[100] revealed that the expression of VEGF-C was significantly correlated with lymphatic involvement, lymph node metastatic disease and tumor size, but not with venous involvement, metastasis to the liver in invasive carcinomas, or overall survival. Miyazaki et al[101] showed that an elevated level of plasma VEGF-C correlated with deeper invasion, and more severe venous and lymphatic invasion of the primary tumor, although there was no significant difference in the plasma level between patients with CRC and the healthy controls. Gao et al[98] showed that MVD and LVD were higher in the tumor compared with the corresponding normal mucosa, but they were not related to clinicopathological variables and overall survival. However, it should be noted that these studies rely on qualitative analysis methodologies, which are not objectively quantifiable assays. Duff et al[102] showed that the balance between the expression of VEGF-C and VEGF-D at the invading tumor edge may enhance lymphatic metastasis by promoting tumor lymphangiogenesis or by activating pre-existing lymphatic vessels. However, no relationship was identified between LVD and clinicopathological variables. Again, it should be noted that these reports rely on qualitative analysis methodologies, which are not objectively quantifiable assays. Taken together, lymphangiogenesis occurs in CRC development, but it has not been clearly linked to CRC patient prognosis. The conflicting reports in the literature regarding the possible correlation of LVD with clinical factors can be attributed to the use of qualitative analysis methodologies. Therefore, the development of a new quantifiable assay that uses standardized metrics is necessary.
In the future, the intra-tumoral expression of specific molecules, e.g. deleted in CRC[103,104] or 18q loss of heterozygosity[104], DNA microsatellite instability[103-105], KRAS mutation[103-105], or thymidylate synthase[103,104] could become biomarkers to predict prognosis or the response to therapy, independently of TNM stage group or histologic grade. It is now clear that there is an interaction between the T and N designations that is likely to rely on the expression of specific molecules within the cancer. In the latest edition of their cancer staging manual, the American Joint Committee on Cancer has stated that they will add molecular profiling information to the TNM classification to enhance the prediction of prognosis and/or even response to therapy[8]. Because lymphatic invasion and metastasis to the lymph nodes have a great impact on patient prognosis, lymphangiogenesis-related molecules are good candidates for the biomarkers that will be included in future editions of the TNM staging system.
LYMPHANGIOGENESIS AS A NEW THERAPEUTIC TARGET FOR CRC
Based upon the importance of angiogenesis and lymphangiogenesis in cancer progression, specific antibodies against angiogenic factors have been developed. The humanized VEGF antibody, known as bevacizumab (Avastin™), has been approved by the United States Food and Drug Administration (FDA) for treating metastatic carcinoma of the colon or rectum, and recurrent or metastatic nonsquamous non-small cell lung cancer. Recently, bevacizumab also received accelerated FDA approval for the treatment of metastatic HER2-negative breast cancer[31]. However, the addition of bevacizumab to chemotherapy as adjuvant therapy in CRC did not improve disease-free survival[106]. Bevacizumab is being tested in other clinical settings such as adjuvant therapy, maintenance therapy, and in combination with both cytotoxic chemotherapy and other targeted agents, such as the epidermal growth factor receptor kinase inhibitor, erlotinib[106]. In addition to bevacizumab, other antibody-based therapies targeting the VEGF pathway are being tested. Ramucirumab and IMC-18F1 are monoclonal antibodies that target the VEGF receptors VEGFR-2 and VEGFR-1, respectively.
In addition to anti-angiogenesis therapies, many clinical trials in cancer patients are underway or have been completed with inhibitors that have the potential to suppress tumor-induced lymphangiogenesis. However, analysis of the effects of these treatments on tumor lymphatics is not always explicitly mentioned in the trial descriptions listed by the U.S. National Institutes of Health. There is only one study that has mentioned the role of VEGF-C in tumor progression, with VEGFR-3 being considered a target. This study is a Phase II trial of sunitinib for patients with chemo-refractory metastatic gastric cancer[34]. It is hoped that more clinical trials will consistently address the possible effects of novel cancer therapeutics on tumor-induced lymphangiogenesis so that correlative data regarding the possible effects of interfering with tumor lymphatics on patient survival can be generated[34]. It is also important to note that treatment with the VEGFR tyrosine-kinase inhibitors sunitinib and sorafenib is associated with a significant increase in the risk of bleeding[107]. Further assessments need to be performed for treatment with these inhibitors.
Inhibition of metastatic spread may be achieved by restriction of lymphatic vessel growth by using targeted therapeutic strategies against molecules involved in lymphangiogenic signaling, in addition to the inhibition of angiogenesis. Because VEGF-A has been shown to promote tumor lymphangiogenesis, and because VEGF-C and VEGF-D are also able to activate VEGFR-2, the combined inhibition of VEGFR-2 and VEGFR-3, or of VEGF-A and VEGF-C/D, may result in an even more potent blockade of tumor-induced lymphatic vessel growth. Indeed, a combination of both anti-VEGFR-2 and anti-VEGFR-3 blocking antibodies has been shown to be more efficient in reducing experimental lymph node and distant breast cancer metastatic disease than each antibody alone, and it will be of interest to see whether a recently developed biospecific antibody against VEGFR-2 and VEGFR-3 will also show enhanced activity in vivo[42]. On the other hand, several recent trials have shown that the addition of anti-EGFR monoclonal antibodies to bevacizumab and chemotherapy resulted in worse outcomes. This was surprising, given that preclinical and early clinical studies had suggested a benefit in combining anti-VEGF and anti-EGFR antibodies. Taken together, further clinical trials are required to reveal the efficacy of the combination of targeted therapies against lymphangiogenesis with other targeted therapies, and/or other anti-cancer therapies.
Which patients will benefit from anti-angiogenesis and anti-lymphangiogenic therapies? Considering that tumors appear to undergo angiogenesis and lymphangiogenesis at an early stage[99], the anti-lymphangiogenic effect may have an even greater impact on the micrometastatic and/or the in-transit metastatic disease of concern after the resection of early stage malignancies. Therefore, these anti-angiogenesis and anti-lymphangiogenesis therapies may be more effective in patients with early stage CRC. However, the role of adjuvant therapy in stage II CRC is still controversial[108]. Although there is a cohort of stage II CRC patients who will have recurrent disease even after complete resection, there are no markers to identify this cohort. This subgroup appears to be a good candidate for anti-angiogenesis and anti-lymphangiogenesis therapies, if they could be identified with the appropriate biomarkers. Lymphangiogenesis factors have the potential to be used as biomarkers to predict which patients would benefit from adjuvant therapy with anti-lymphangiogenesis therapies to both prevent recurrence and improve overall survival. In the future, tailored treatments consisting of combinations of chemotherapy, other targeted therapies, and anti-angiogenesis and anti-lymphangiogenesis agents will hopefully result in better patient outcomes.
In addition to the development of the ideal combination therapies, the prevention of CRC is also essential to improve patient outcomes. Cancer chemoprevention is a strategy that uses treatments with natural or synthetic agents to inhibit, delay, or reverse the carcinogenesis process even before the development of invasive cancer[109,110]. The rationale for chemopreventive approaches to prevent CRC comes from epidemiologic and observational studies indicating that the long term ingestion of aspirin may reduce mortality in CRC[111]. Recent clinical trial studies demonstrated that celecoxib, a selective COX-2 inhibitor, is equally effective in reducing colorectal adenomas in animal models and patients with familial adenomatous polyposis (FAP), and it is approved by FDA for the chemoprevention of CRC in patients with FAP[112]. Prostaglandin E2 (PGE2) induced by COX-2 exerts several biological properties that may be advantageous for carcinogenesis, including promoting angiogenesis with increased VEGF, bFGF, and PDGF production[112]. Celecoxib enhances tumor cell apoptosis, thereby inhibiting the growth and angiogenesis of tumors by inhibiting COX-2, PGE2 synthesis, and VEGF expression in tumors in a mouse model of human CRC[113]. Interestingly, VEGF-C and COX-2 are coexpressed and are significantly associated with metastasis to the lymph nodes as well as prognosis in human CRC[114]. Moreover, celecoxib inhibits not only angiogenesis, but also lymphangiogenesis by blocking the VEGF pathway in mouse lung cancer models[115,116]. Taken together, lymphangiogenesis appears to play an important part in carcinogenesis in connection with the COX-2 pathway, and to be one of the important targets in chemoprevention, although the role of lymphangiogenesis in CRC within the adenoma-carcinoma sequence is still unknown. Further investigation will be required in this field.
CONCLUSION
Understanding the molecular pathways that regulate lymphangiogenesis is mandatory to pave the way for the development of new targeted therapies for cancer patients. A new quantifiable assay using standardized metrics is required to measure lymphangiogenesis and evaluate its impact on clinical outcome. In the future, tailored treatments consisting of combinations of chemotherapy, other targeted therapies, and anti-lymphangiogenesis agents will hopefully improve patient outcomes. This progression to the clinic may be guided by new avenues of research such as the identification of biomarkers that predict response to treatment.
Peer reviewer: Alessandro Fichera, MD, FACS, FASCRS, Assistant Professor, Department of Surgery, University of Chicago, 5841 S. Maryland Ave, MC 5031, Chicago, IL 60637, United States
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM