Published online Aug 21, 2010. doi: 10.3748/wjg.v16.i31.3911
Revised: April 20, 2010
Accepted: April 27, 2010
Published online: August 21, 2010
AIM: To investigate the effect of tectorigenin on proliferation and apoptosis of hepatic stellate cells (HSC)-T6 cells.
METHODS: HSC-T6 cells were incubated with tectorigenin at different concentrations, and their proliferation was assessed by bromodeoxyuridine incorporation assay. Apoptosis was detected by flow cytometry assay with Hoechst 33342 staining. Also, generation of reactive oxygen species (ROS), intracellular [Ca2+]i, potential of mitochondrial membrane, activities of cytochrome c and caspase-9 and -3 were investigated to explore a conceivable apoptotic pathway.
RESULTS: Tectorigenin suppressed the proliferation of HSC-T6 cells and induced apoptosis of HSC-T6 cells in a time- and dose-dependent manner. Tectorigenin at the concentration of 100 μg/mL greatly inhibited the viability of HSC-T6 cells and induced the condensation of chromatin and fragmentation of nuclei. When treated for 48 h, the percentage of cell growth and apoptosis reached 46.3% ± 2.37% (P = 0.004) and 50.67% ± 3.24% (P = 0.003), respectively. Furthermore, tectorigenin-induced apoptosis of HSC-T6 cells was associated with the generation of ROS, increased intracellular [Ca2+]i, loss of mitochondrial membrane potential, translocation of cytochrome c, and activation of caspase-9 and -3.
CONCLUSION: Tectorigenin inhibits proliferation of HSC-T6 cells and induces apoptosis of HSC-T6 cells.
- Citation: Wu JH, Wang YR, Huang WY, Tan RX. Anti-proliferative and pro-apoptotic effects of tectorigenin on hepatic stellate cells. World J Gastroenterol 2010; 16(31): 3911-3918
- URL: https://www.wjgnet.com/1007-9327/full/v16/i31/3911.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i31.3911
Hepatic fibrosis, a common wound-healing response to diseases such as chronic hepatitis and alcoholic liver damage, is a result of destruction in architecture of the liver parenchyma and an imbalance between fibrogenesis and fibrolysis forming scars or fibrous tissues. Hepatic stellate cells (HSC), the major cells in hepatic fibrosis, are responsible for the development of fibrosis[1,2]. Activation and proliferation of HSC are the key to fibrogenesis while apoptosis of HSC is associated with the resolution of fibrosis. So, HSC have attracted increasing attention due to their essential role in liver fibrosis. With a better understanding of their biological properties, inhibiting the activation and proliferation of HSC and inducing apoptosis of activated HSC have been proposed as potential anti-fibrosis strategies.
In traditional Chinese medicine, Iris tectorum (I. tectorum) has been used in treatment of liver injury for a long time. It has been shown that tectorigenin, an important bioactive compound isolated from I. tectorum, has antioxidant, anti-inflammatory, and anticancer activities[3-5]. Experiments on animal models have also demonstrated that tectorigenin exhibits a hepatoprotective effect on CCl4-induced or t-BHP-induced hepatic injury in rats[6,7]. Since hepatic fibrosis is a common wound-healing response to liver injury, we studied whether tectorigenin has anti-fibrosis potentials and exhibits its hepatoprotective effect by playing a role in liver fibrosis.
The present study was therefore performed to investigate the effects of tectorigenin on proliferation and apoptosis-related events of activated HSC-T6 cells and disclose its possible mechanism underlying apoptosis of HSC-T6 cells.
Tectorigenin was isolated from I. tectorum with a purity of over 98% as confirmed by high-performance liquid chromatography (HPLC) analysis.
HSC-T6 cells, an immortalized rat hepatic stellate cell line, exhibit an activated HSC phenotype[8]. The cells, purchased from Cancer Institute and Hospital, Chinese Academy of Medical Sciences (Beijing, China), were cultured in Dulbecco’s-modified Eagle’s medium (DMEM; Gibco, NY, USA) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 12% new bovine serum (Hangzhou Sijiqing Co., Ltd., Hangzhou, China), in a humidified atmosphere containing 5% (v/v) CO2 at 37°C. L02 cells (a human hepatocyte cell line), purchased from Xiangya Central Experiment Laboratory, Central South University, China, were cultured in DMEM medium (Gibco, NY, USA) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum (Hangzhou Sijiqing Co., Ltd., Hangzhou, China), in a humidified atmosphere containing 5% (v/v) CO2 at 37°C.
Cells were plated in 96-well plates at a density of 5 × 105 cells/well and grown for 24 h. Tectorigenin at different concentrations was added to the cells while only DMSO (solvent) was added as a negative control. After growing for 12, 24, and 48 h, cell viability was evaluated by the reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT; Amresco, OH, USA)[9].
HSC-T6 cells were incubated for 48 h with 20, 40, 60 and 100 μg/mL tectorigenin, respectively. Two hours before the cells were harvested, bromodeoxyuridine (BrdU; GenMed Scienfics Inc., USA) was added. The cells were fixed in 4% paraformaldehyde and stained with Hoechst 33342 following the manufacturer’s protocol[10].
HSC-T6 cells were incubated for 48 h with tectorigenin at 20, 40, 60 and 100 μg/mL tectorigenin, respectively. Nuclear morphological change was assessed using Hoechst 33342 staining[11]. In brief, cells were fixed in 4% paraformaldehyde for 10 min, washed three times with pre-chilled PBS and exposed to 5 μg/mL of Hoechst 33342 at 37°C in dark for 15 min. Samples were observed under a fluorescent microscope (Nikon UFX-II, Japan). Cells showing cytoplasmic and nuclear shrinkage, chromatin condensation or fragmentation, were defined as apoptotic cells.
To quantify apoptotic cells, HSC-T6 cells were harvested after exposed to tectorigenin for 24 and 48 h, respectively, washed twice with cold PBS, resuspended in PBS containing fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide (PI) for 10 min, and measured using a FACScan flow cytometer[12,13] (Becton Dickinson, Franklin Lakes, NJ, USA).
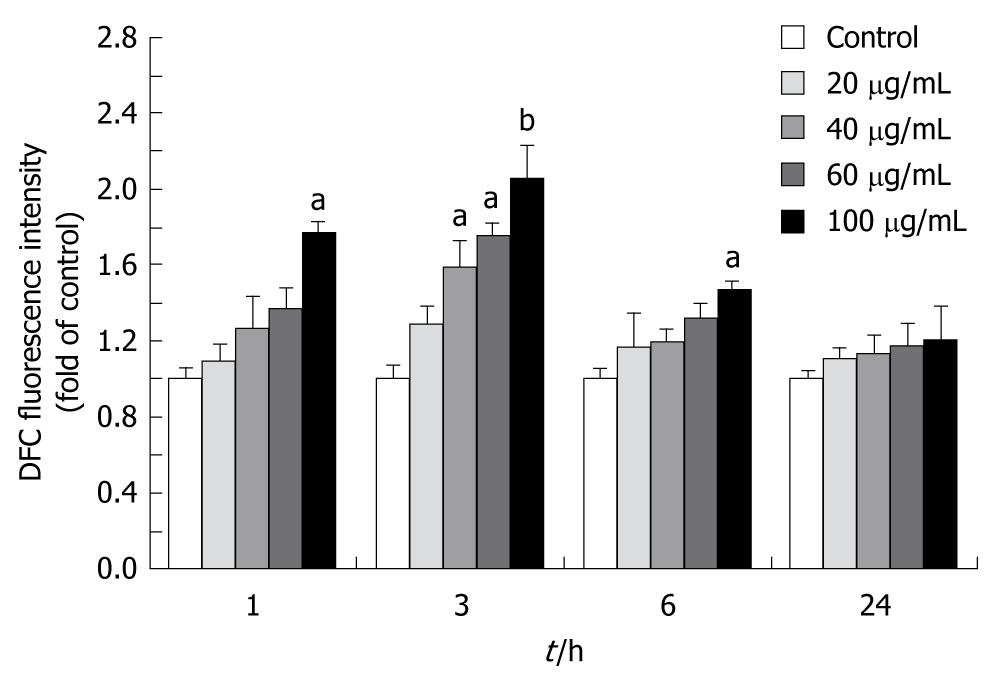
Intracellular ROS was quantified with a fluorescence plate reader using 2, 7-dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma)[14]. The cells on black 96-well plates were treated with tectorigenin at with tectorigenin at 20, 40, 60 and 100 μg/mL for 1, 3, 6 and 24 h, respectively, and incubated with DCFH-DA at 37°C for 30 min. After DCFH-DA was removed, the cells were washed with phosphate buffered saline (PBS). DCFH-DA-loaded cells were read on a Safire fluorescence plate reader (Tecan, Crailsheim, Germany).
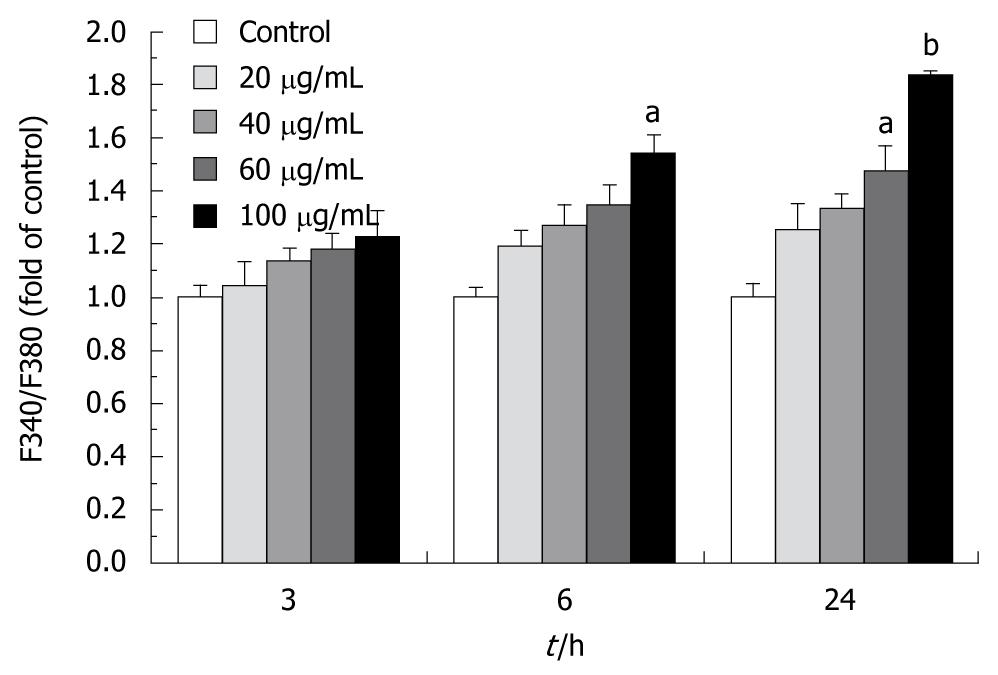
[Ca2+]i was monitored using fluorescent Ca2+-sensitive dye, a Fura 2-acetoxymethyl ester (Fura 2-AM)[15]. Cells were cultured and treated with tectorigenin for 1, 3, 6 and 24 h, respectively, and preloaded with 1 μmol/L Fura2-AM for 30 min in dark at 37°C in a humidified incubator. After loading with Fura2-AM, cells were collected, gently rinsed three times with D-Hanks’ solution, and resuspended in D-Hanks’ solution containing 0.2% BSA at 106cells/mL. Intracellular [Ca2+]i was measured at an emission wavelength of 510 nm and an excitation wavelength of 340 and 380 nm on a Safire fluorescence plate reader (Tecan, Crailsheim, Germany). The ratio of fluorescence intensity at 340 to 380 nm (F340/F380) was used to estimate intracellular free calcium.
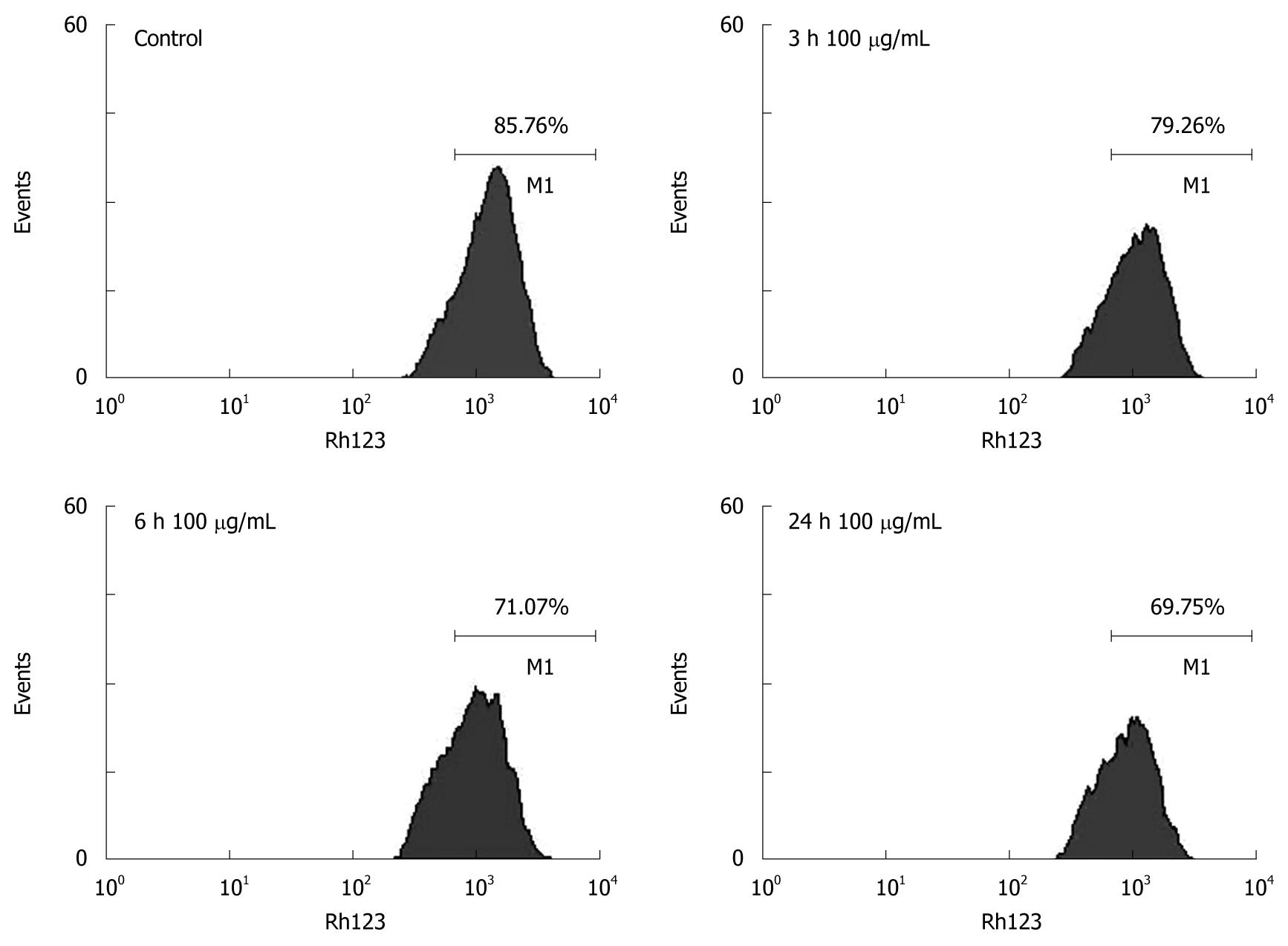
Change in mitochondrial membrane potential (MMP) was monitored using Rhodamine 123 (Rh-123)[16]. In brief, Rh-123 was added to cells to attain a final concentration of 3 μg/mL. After incubated at 37°C for 30 min, cells were collected, washed twice with PBS, and analyzed with a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
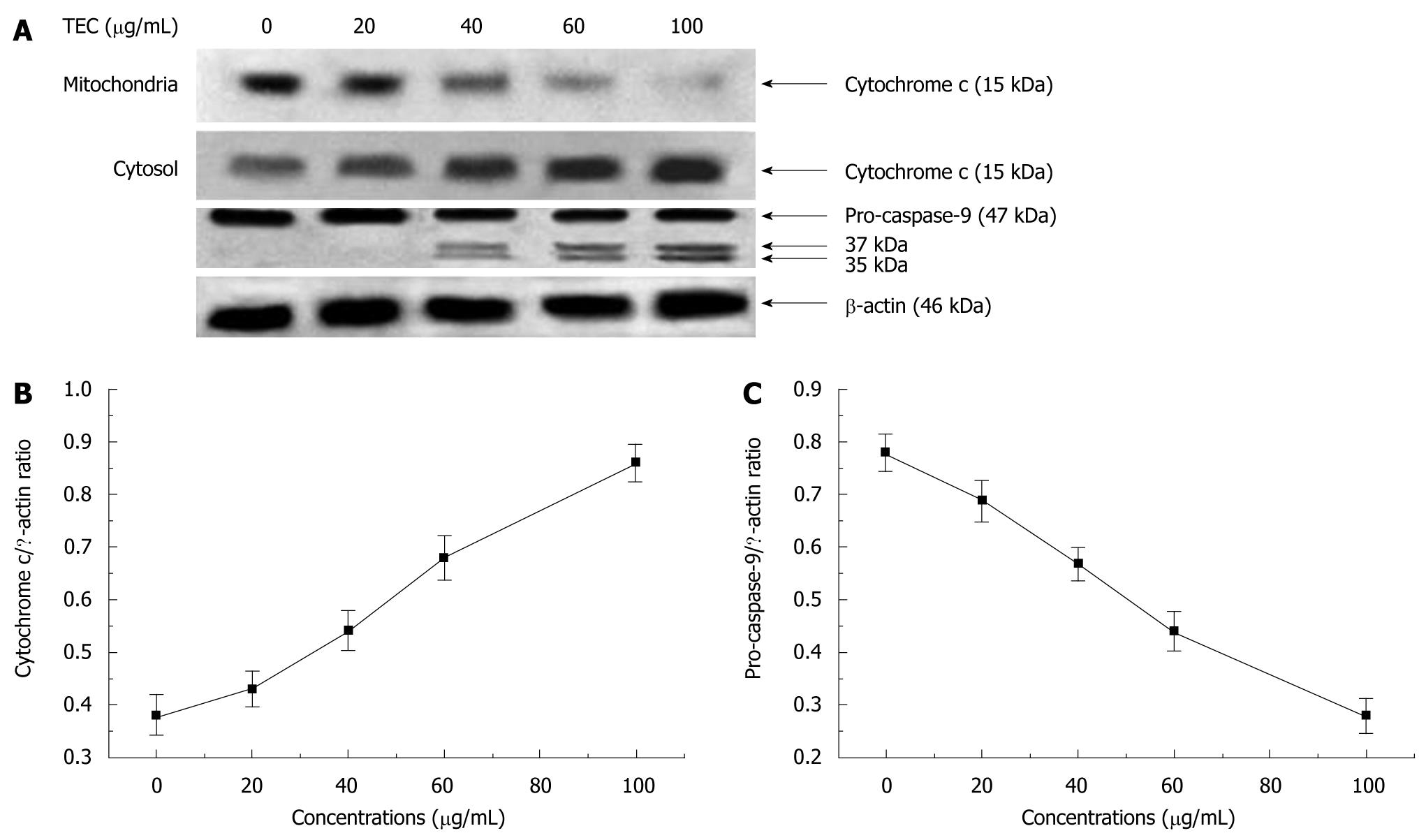
HSC-T6 cells were seeded into 60-mm dishes (1 × 106 cells/dish). After treated on the next day for 48 h with tectorigenin at 0, 20, 40, 60 and 100 μg/mL, respectively, HSC-T6 cells were harvested, resuspended in an ice-cold lysis buffer consisting of 50 mmol/L Tris-HCl, pH 8.0, 50 mmol/L KCl, 5 mmol/L DTT, 1 mmol/L EDTA, 0.1% SDS, 0.5% Triton X-100, and protease inhibitor cocktail tablets (Roche, IN), incubated for 10 min on ice, disrupted in a micro ultrasonic cell disrupter for 10 s and centrifuged at 750 g for 15 min at 4°C. The supernatant (cytosolic fraction) was removed and maintained at -80°C. The pellet containing mitochondria was resolved in a lysis buffer. Protein level was measured using a standard colorimetric assay kit (BCA kit). Proteins were separated by polyacrylamide/SDS gel electrophoresis and transferred onto polyvinylidene fluoride (PVDF) membranes (Roche, IN). The membranes were probed with antibodies (cytochrome c and caspase-9 diluted at 1:1000, Cell Signaling Technology, MA, USA) overnight at 4°C, and incubated with a HRP coupled secondary antibody (HRP; 1:5000, Cell Signaling Technology, MA, USA). Detection was performed using a LumiGLO chemiluminescent subtract system (KPL, Guildford, UK). β-actin (1:200, Boster, Wuhan, China) as a loading control. Results were quantified with a scanning densitometer (Bio-Rad, USA).
Activity of caspase-3, the main execution caspase, was detected with a caspase-3 colorimetric assay kit (KenGen Biotech, Nanjing, China) according to its manufacturer’s instructions. Cultured HSC-T6 cells were washed twice with cold PBS, resuspended in a lysis buffer and left on ice for 20 min. Lysate was centrifuged at 10 000 r/min for 3 min at 4°C. Supernatants were collected and protein concentrations were measured with a BCA kit. Proteins (100 μg) were incubated for 4 h at 37°C with a reaction buffer in a total volume of 105 μL containing 5 μL caspase-3 substrate, and detected with a fluorescence microplate reader (Tecan, Crailsheim, Germany) at λ 405 nm.
All data were expressed as mean ± SD. Origin Pro 7.0 statistical package was used to determine statistical significance. Difference between two groups was analyzed by two-tailed Student’s t-test, and difference among three or more groups was analyzed by one-way ANOVA multiple comparisons. P < 0.05 or P < 0.01 was considered statistically significant.
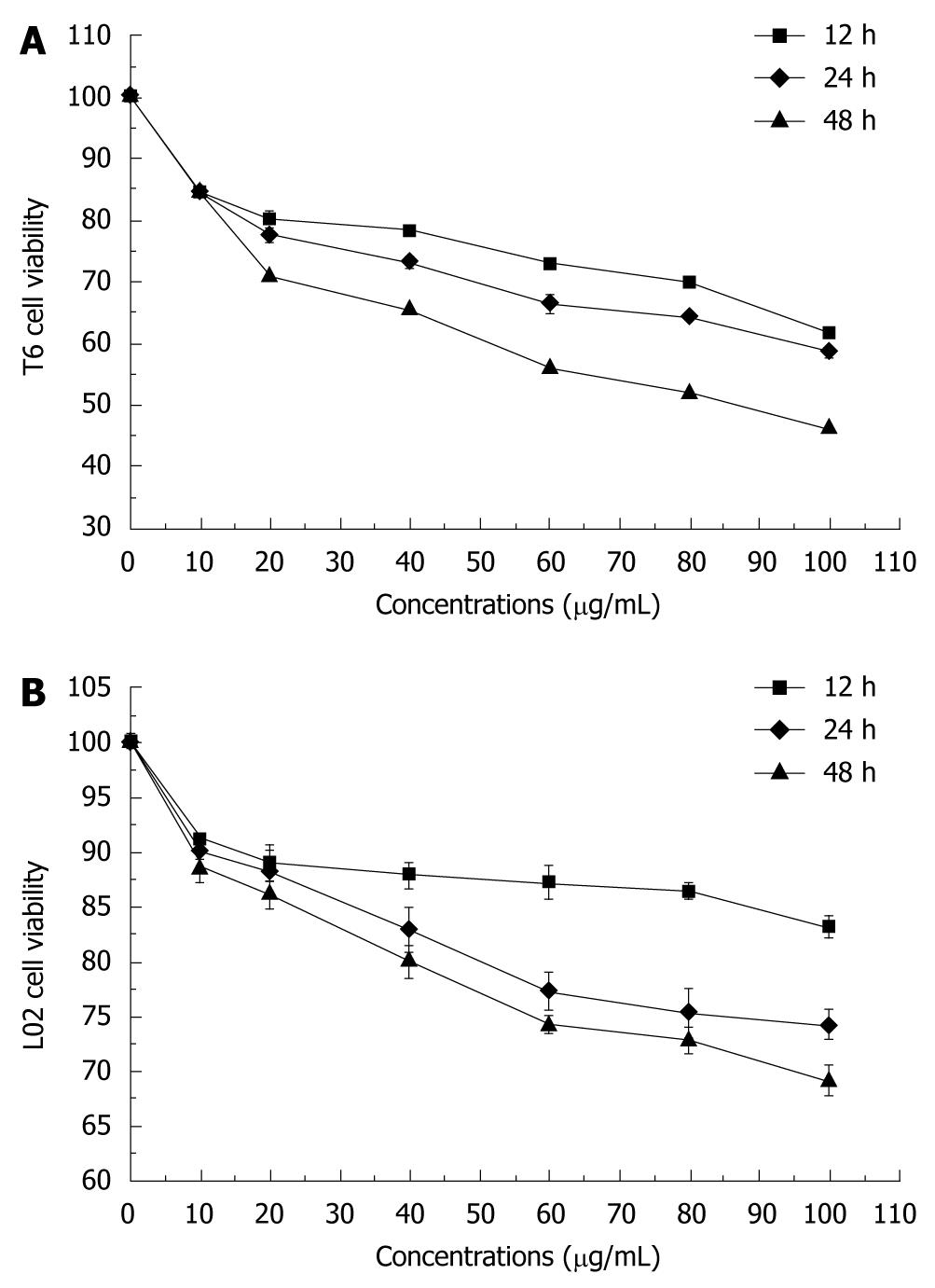
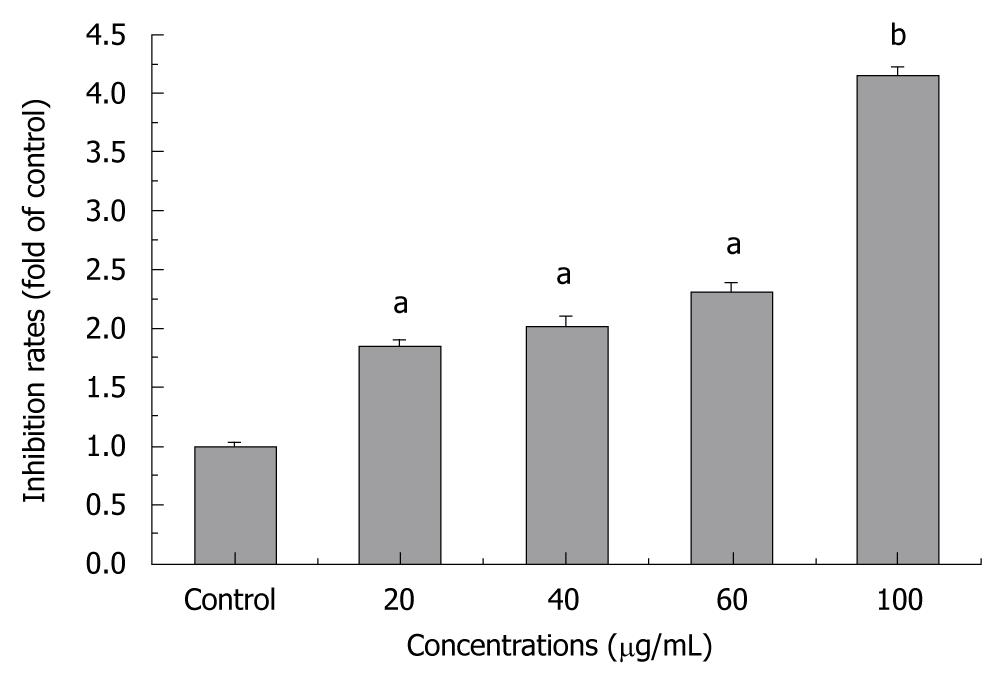
The percentage of cell growth was significantly different between tectorigenin-treated and untreated groups. Tectorigenin inhibited the growth of HSC-T6 cells in a dose-dependent manner (Figure 1A). Increased incubation time of HSC-T6 cells decreased the percentage of cell growth, indicating that tectorigenin also inhibits the growth of HSC-T6 cells in a time-dependent manner. Treatment with tectorigenin at 100 μg/mL resulted in a moderate cytotoxicity to L02 cells after incubated for 48 h (Figure 1B). To further investigate the inhibitory effect of tectorigenin on proliferation of HSC-T6 cells, BrdU incorporation, another indicator of cell proliferation, was detected. Tectorigenin at 20-100 μg/mL could significantly inhibit the proliferation of HSC-T6 cells (Figure 2).
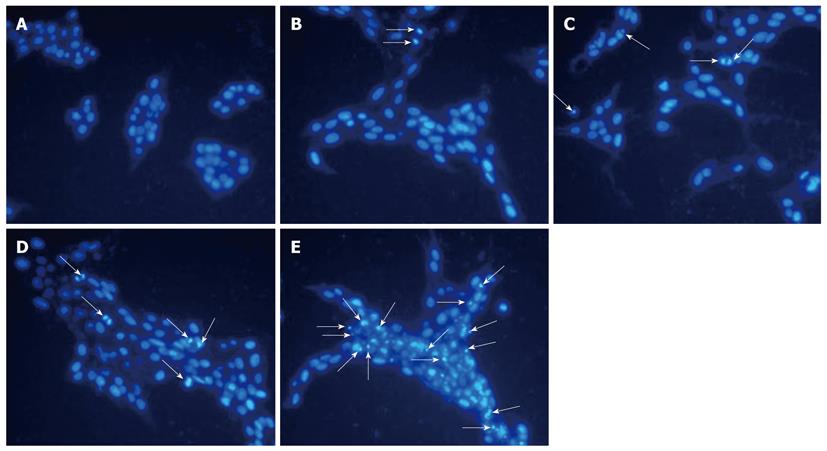
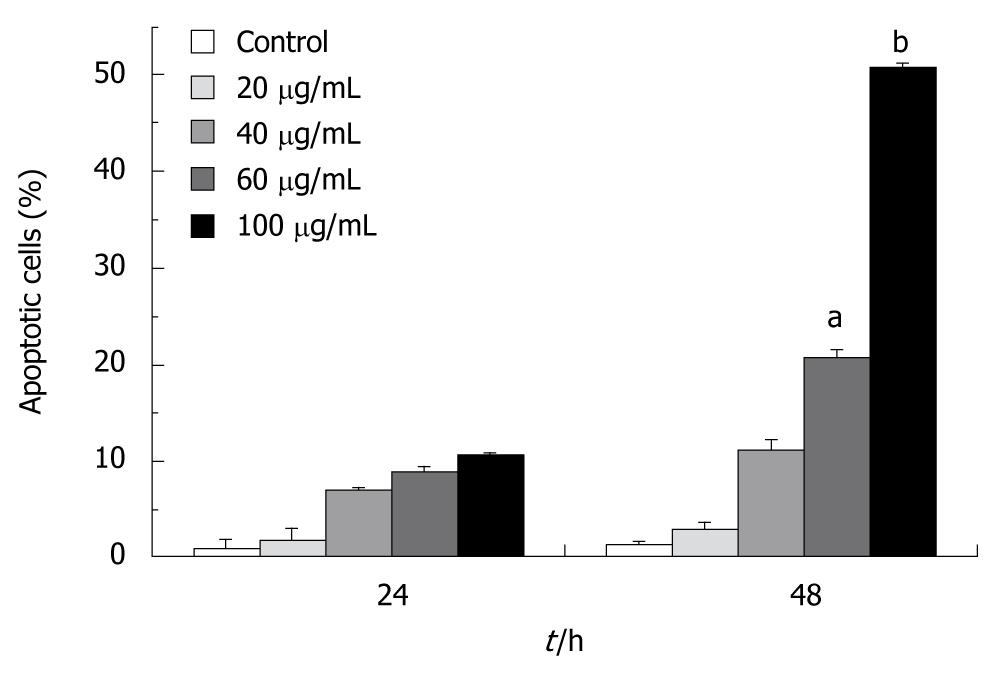
We studied if tectorigenin can induce apoptosis of HSC-T6 cells. In brief, apoptotic cells were visualized using DNA-binding Hoechst 33342. Regular and round-shaped nuclei were observed in control HSC-T6 cells (Figure 3). After treated for 48 h with tectorigenin at 40 and 60 μg/mL, condensed nuclei were found in HSC-T6 cells, which became smaller in size, and eventually fragmented into apoptotic bodies. Moreover, after treated with 100 μg/mL tectorigenin, the nuclei of HSC-T6 cells were further condensed with the number of apoptotic bodies sharply increased. These data suggest that tectorigenin greatly induces the condensation of chromatin and fragmentation of nuclei. The apoptosis rate of HSC-T6 cells was determined by flow cytometry analysis with annexin V-FITC and PI staining. In the control group, most cells were viable. When HSC-T6 cells were exposed for 48 h to tectorigenin at 60 and 100 μg/mL, the percentage of apoptotic cells increased to 20.69% ± 2.57% and 50.67% ± 3.24% (P = 0.003), respectively (Figure 4), which was significantly higher than that of those not exposed to tectorigenin. In addition, tectorigenin induced apoptosis of HSC-T6 cells in a dose- and time-dependent manner.
To determine whether tectorigenin is able to induce ROS generation in HSC-T6 cells, the level of ROS, measured using the fluorescence probe DCFH-DA, was significantly higher in HSC-T6 cells than in control cells after treated with 100 μg/mL tectorigenin for 1, 3 (peaked) and 6 h (Figure 5).
To determine whether tectorigenin influences the level of intracellular Ca2+, the level of intracellular Ca2+ was measured with Fura 2-AM staining. After treated with 100 μg/mL tectorigenin for 3 h, the fluorescence ratio (F340/F380) increased to 123.1% ± 7.18% compared to that not treated with tectorigenin (Figure 6). In addition, tectorigenin increased cytoplasmic Ca2+ in a time-dependent manner. When the incubation time was prolonged to 24 h, the F340/F380 value increased from 123.1% ± 7.18% to 183.3% ± 8.64% (P = 0.002).
To determine whether tectorigenin decreases mitochondrial membrane potential in HSC-T6 cells, flow cytometry analysis was carried out using Rhodamine 123. Compared to control cells not treated with tectorigenin, HSC-T6 cells treated with 100 μg/mL tectorigenin for 24 h decreased the mitochondrial membrane potential (MMP) from 85.76% ± 6.39% to 69.75% ± 5.28% (P = 0.03) (Figure 7).
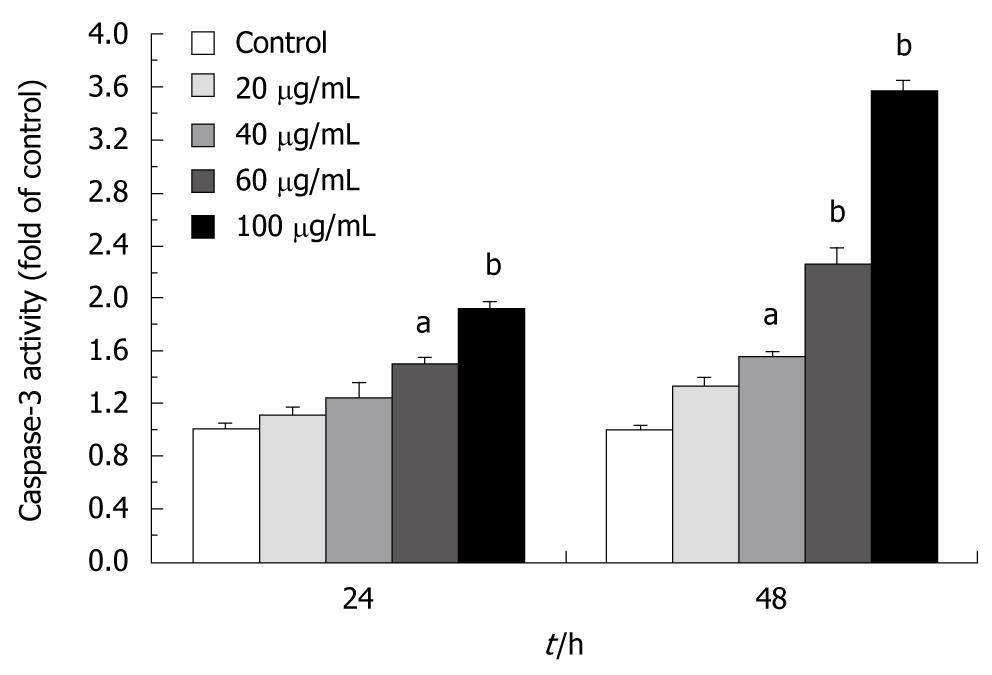
To assess whether tectorigenin-treated cells accompany increased cytosolic translocation of cytochrome c, the activated caspases-9 and -3 were detected, and the cytosolic and mitochondrial levels in cytochrome c and intracellular caspases-9 and -3 were measured. The results reveal that tectorigenin releases mitochondrial cytochrome c into cytosol in a dose-dependent manner (Figure 8B). Western blotting also showed that tectorigenin induced proteolytic cleavage of pro-caspase-9 into the active form of HSC-T6 cells and tectorigenin activated the caspase-9 in a dose-dependent manner (Figure 8C). Tectorigenin (100 μg/mL) significantly increased the activity of caspase-3 in HSC-T6 cells (Figure 9).
In the present study, tectorigenin, a bioactive compound isolated from I. tectorum used traditionally for severe liver disorder, suppressed the proliferation of HSC and induced apoptosis of HSC in a time- and dose-dependent manner.
As an immortalized rat liver stellate cell line, HSC-T6 cell line exhibits an activated phenotype of HSC and a fibroblast-like morphology, which presents as a useful tool in exploring hepatic fibrosis[17]. MTT and BrdU incorporation assay demonstrated that tectorigenin could inhibit the proliferation of HSC-T6 cells, with a lower cytotoxicity to human hepatocytes (L02). Furthermore, flow cytometry analysis indicated that tectorigenin could induce apoptosis of HSC-T6 cells in a dose- and time-dependent manner.
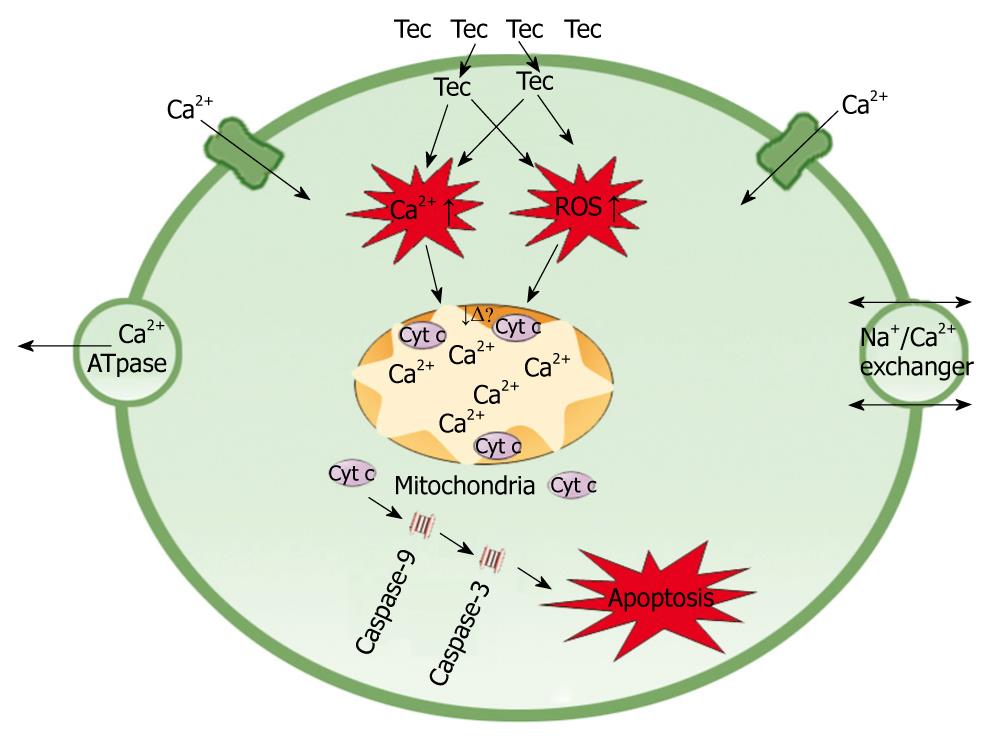
Clarification of the molecular mechanism of tectorigenin underlying the discerned apoptosis is of great importance. It has been shown that ROS generation increases membrane permeability of mitochondria, triggers abrupt mitochondrial depolarization and release of cytochrome c from inner mitochondrial membrane[18]. Calcium homeostasis is an essential mechanism underlying physiological process. Since increased cytosolic calcium may induce mitochondria to take up intracellular overloaded Ca2+, a much larger amount of Ca2+ will accumulate in mitochondria, which can potentially damage the electron transfer chain, leading to failure in maintaining the mitochondrial membrane potential[19-21]. Damages to mitochondrion result in loss of its function. For example, release of cytochrome c from mitochondria leads to cell apoptosis[22]. In this study, tectorigenin increased the ROS production and Ca2+ concentration in cytoplasm of HSC-T6 cells, the intracellular accumulation of ROS and Ca2+ further induced the loss of MMP. The disruption of MMP caused release of cytochrome c from mitochondria to cytosol. Cytosolic cytochrome c activated the pro-caspase-9 and subsequently, caspase-9 activated the downstream effector caspases-3, eventually triggered apoptosis of HSC-T6 cells (Figure 10).
In conclusion, tectorigenin suppresses the proliferation of HSC-T6 cells in a dose- and time-dependent manner, and produces a slight cytotoxicity to L02 cells. More importantly, tectorigenin induces apoptosis of HSC-T6 cells and may have anti-fibrosis potentials.
Liver fibrosis represents a significant health problem worldwide without any currently available and effective therapeutic approach. Advanced liver fibrosis results in cirrhosis, liver failure, and portal hypertension, and often requires liver transplantation. The most characteristic feature of liver fibrosis is excess deposition of type I collagen. A great number of researches have been performed to understand the molecular mechanism underlying liver fibrosis. Activated hepatic stellate cells (HSC) are the primary cell type responsible for the excess production of collagen.
HSC are the major cells responsible for the development of liver fibrosis and cirrhosis. Activated HSC are proliferative and fibrogenic, with accumulation of extra cellular matrix, including α-smooth muscle actin (α-SMA) and type I collagen. Suppression of activation and proliferation, and induction of apoptosis in HSC have been reported as the therapeutic strategies against liver fibrosis. Tectorigenin, a bioactive compound of Iris tectorum (I. tectorum), shows antioxidant, anti-inflammatory, anticancer, and hepatoprotective activities, and has been used in therapy for liver injury. However, how tectorigenin works in live fibrosis has not been unequivocally addressed. In this study, tectorigenin suppressed proliferation of HSC and induced apoptotis of HSC.
Currently used drugs such as corticosteroids and colchicine usually show various side effects such as immunosuppression or cytotoxicity. Tectorigenin, an antioxidant, anti-inflammatory, and hepatoprotective compound isolated from I. tectorum, shows its inhibitory effect on proliferation of HSC and produces a low toxicity. Furthermore, in vitro studies suggest that tectorigenin produces its pro-apoptotic effect on HSC is probably via the mitochondrial pathway to some extent.
By showing how tectorigenin works in HSC, this study may represent a new and future strategy for managing hepatic fibrosis.
This study showed that tectorigenin inhibited the growth and viability of rat hepatic HSC-T6 cells in a dose-dependent manner. The authors conclude that the anti-proliferative and pro-apoptotic effects of this component on HSC might explain its anti-fibrotic properties observed in vivo. This is an interesting study. However, the mechanism of tectorigenin underlying the growth and viability of HSC-T6 cells needs to be further studied.
Peer reviewers: Richard Hu, MD, MSc, Division of Gastroenterology, Department of Medicine, Olive view-UCLA Medical Center, 14445 Olive View Drive, Los Angeles, CA 91342, United States; Dr. Katja Breitkopf, Department of Medicine II, University Hospital Mannheim, University of Heidelberg, Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, Germany; Maurizio Parola, Professor, Department Medicina e Oncologia Sperimentale, University of Torino Corso Raffaello 30, 10125 Torino, Italy
S- Editor Wang JL L- Editor Wang XL E- Editor Ma WH
| 1. | de Villiers WJ, Song Z, Nasser MS, Deaciuc IV, McClain CJ. 4-Hydroxynonenal-induced apoptosis in rat hepatic stellate cells: mechanistic approach. J Gastroenterol Hepatol. 2007;22:414-422. |
| 2. | Chong LW, Hsu YC, Chiu YT, Yang KC, Huang YT. Anti-fibrotic effects of thalidomide on hepatic stellate cells and dimethylnitrosamine-intoxicated rats. J Biomed Sci. 2006;13:403-418. |
| 3. | Kang KA, Lee KH, Chae S, Zhang R, Jung MS, Kim SY, Kim HS, Kim DH, Hyun JW. Cytoprotective effect of tectorigenin, a metabolite formed by transformation of tectoridin by intestinal microflora, on oxidative stress induced by hydrogen peroxide. Eur J Pharmacol. 2005;519:16-23. |
| 4. | Lee HU, Bae EA, Kim DH. Hepatoprotective effect of tectoridin and tectorigenin on tert-butyl hyperoxide-induced liver injury. J Pharmacol Sci. 2005;97:541-544. |
| 5. | Fang R, Houghton PJ, Hylands PJ. Cytotoxic effects of compounds from Iris tectorum on human cancer cell lines. J Ethnopharmacol. 2008;118:257-263. |
| 6. | Jung SH, Lee YS, Lim SS, Lee S, Shin KH, Kim YS. Antioxidant activities of isoflavones from the rhizomes of Belamcanda chinensis on carbon tetrachloride-induced hepatic injury in rats. Arch Pharm Res. 2004;27:184-188. |
| 7. | Lee HU, Bae EA, Kim DH. Hepatoprotective effect of tectoridin and tectorigenin on tert-butyl hyperoxide-induced liver injury. J Pharmacol Sci. 2005;97:541-544. |
| 8. | Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437-451. |
| 9. | Lee TF, Lin YL, Huang YT. Studies on antiproliferative effects of phthalides from Ligusticum chuanxiong in hepatic stellate cells. Planta Med. 2007;73:527-534. |
| 10. | Lin YL, Lee TF, Huang YJ, Huang YT. Inhibitory effects of Ligusticum chuanxiong on the proliferation of rat hepatic stellate cells. J Gastroenterol Hepatol. 2006;21:1257-1265. |
| 11. | Ermak N, Lacour B, Drüeke TB, Vicca S. Role of reactive oxygen species and Bax in oxidized low density lipoprotein-induced apoptosis of human monocytes. Atherosclerosis. 2008;200:247-256. |
| 12. | Shi YQ, Qu XJ, Liao YX, Xie CF, Cheng YN, Li S, Lou HX. Reversal effect of a macrocyclic bisbibenzyl plagiochin E on multidrug resistance in adriamycin-resistant K562/A02 cells. Eur J Pharmacol. 2008;584:66-71. |
| 13. | Yu N, Xu W, Jiang Z, Cao Q, Chu Y, Xiong S. Inhibition of tumor growth in vitro and in vivo by a monoclonal antibody against human chorionic gonadotropin beta. Immunol Lett. 2007;114:94-102. |
| 14. | Shi DH, Wu JH, Ge HM, Tan RX. Protective effect of hopeahainol A, a novel acetylcholinesterase inhibitor, on hydrogen peroxide-induced injury in PC12 cells. Environ Toxicol Phar. 2009;28: 30-36. |
| 15. | Hong H, Liu GQ. Protection against hydrogen peroxide-induced cytotoxicity in PC12 cells by scutellarin. Life Sci. 2004;74:2959-2973. |
| 16. | Gong Y, Han XD. Nonylphenol-induced oxidative stress and cytotoxicity in testicular Sertoli cells. Reprod Toxicol. 2006;22:623-630. |
| 17. | Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, Blaner WS. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882-893. |
| 18. | Kakkar P, Singh BK. Mitochondria: a hub of redox activities and cellular distress control. Mol Cell Biochem. 2007;305:235-253. |
| 19. | Chakraborti T, Das S, Mondal M, Roychoudhury S, Chakraborti S. Oxidant, mitochondria and calcium: an overview. Cell Signal. 1999;11:77-85. |
| 20. | Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552-565. |
| 22. | Xiao Y, He J, Gilbert RD, Zhang L. Cocaine induces apoptosis in fetal myocardial cells through a mitochondria-dependent pathway. J Pharmacol Exp Ther. 2000;292:8-14. |