Published online Jan 21, 2010. doi: 10.3748/wjg.v16.i3.398
Revised: November 2, 2009
Accepted: November 9, 2009
Published online: January 21, 2010
Cerebral lipiodol embolism (CLE) is an extremely rare complication of transcatheter arterial chemoembolization for hepatocellular carcinoma (HCC). The authors present a case of CLE that occurred after the second hepatic arterial chemoembolization for HCC, and attempt to introduce several plausible mechanisms of CLE, after reporting the clinical and radiological findings and reviewing the medical literature.
- Citation: Wu L, Yang YF, Liang J, Shen SQ, Ge NJ, Wu MC. Cerebral lipiodol embolism following transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2010; 16(3): 398-402
- URL: https://www.wjgnet.com/1007-9327/full/v16/i3/398.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i3.398
Transcatheter arterial chemoembolization (TACE) is utilized worldwide in the treatment of patients with unresectable hepatocellular carcinoma (HCC). The documented complications of TACE include post-embolization syndrome, septicemia, acute hepatic failure, liver infarction or abscess, intrahepatic biloma, embolization of extrahepatic organs, pseudoaneurysm formation, cholecystitis, tumor rupture, splenic infarction, gastritis, duodenitis, gastroduodenal ulceration, variceal bleeding, and iatrogenic dissection[1-5]. Cerebral lipiodol embolism (CLE) is a rare complication of TACE. The authors report a case of CLE that occurred after the second TACE, and present its clinical and imaging findings, as well as a review of the literature.
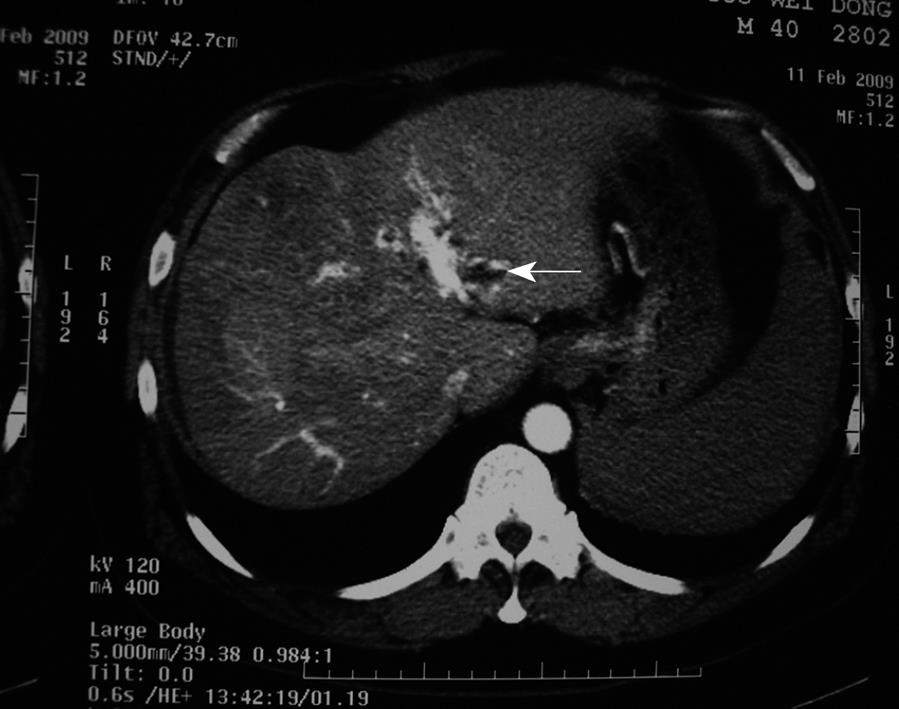
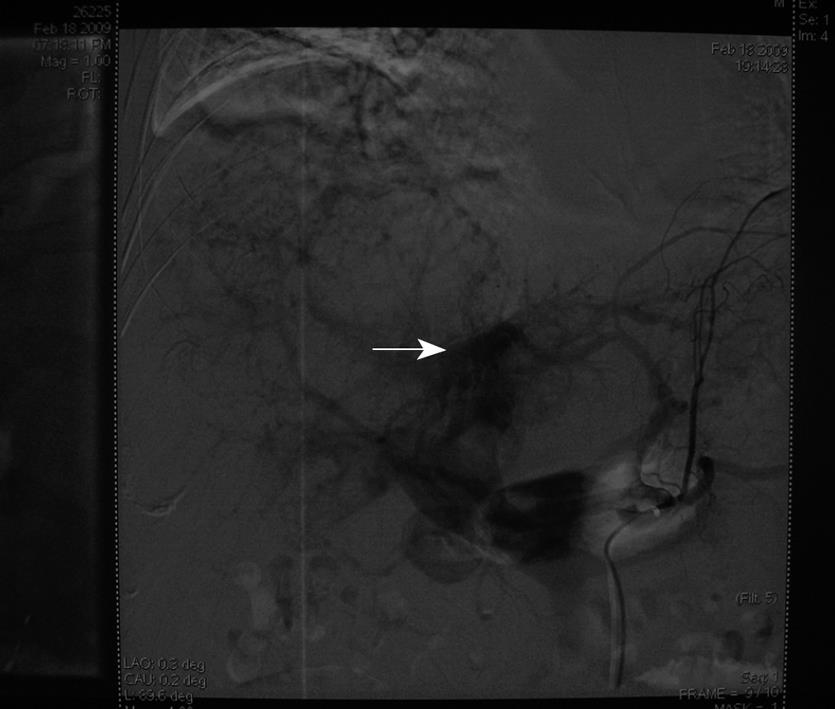
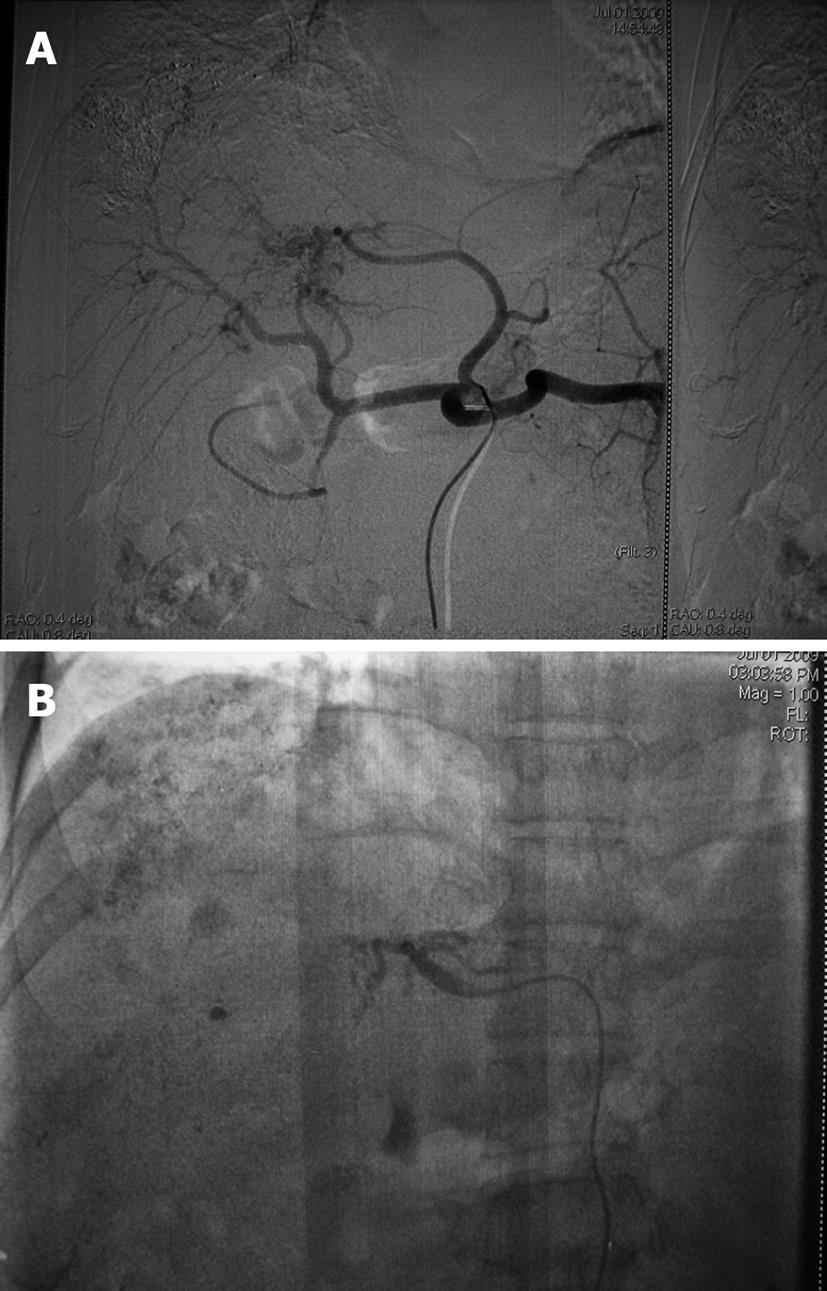
A 41-year-old man with multiple HCC accompanied by portal vein invasion (Figures 1 and 2) underwent a second course of TACE via the proper hepatic artery by using a mixture of 30 mL lipiodol, 10 mg hydroxyl camptothecin and 40 mg pirarubicin, as well as gelatin sponge particles (1400-2000 μm) and coils (5 mm × 8 mm) (Figure 3). The tumor marker test showed 136.9 μg/L alpha fetoprotein (AFP), 0.7 μg/L carcinoembryonic antigen (CEA), and of 28.2 μg/mL carbohydrate antigen 19-9 (CA19-9). The liver function was ranked A in Child-Pugh classification. During the procedure, the patient experienced cough, visual loss, headache and motor weakness of the left upper limb and left lower limb. Upon physical examination, blood pressure was 158/93 mmHg, and pulse rate, respiratory rate and body temperature were normal. The muscle strength of the left upper limb was III+, while the left lower limb was IV+. No neurological pathological reflex was observed. The peripheral oxygen saturation was 94%, and with a nasal catheter, the arterial oxygen saturation was elevated and maintained higher than 97%. The patient was given neurotrophins, cerebrovascular dilators and supportive therapy immediately. Functional exercise was added in the following days.
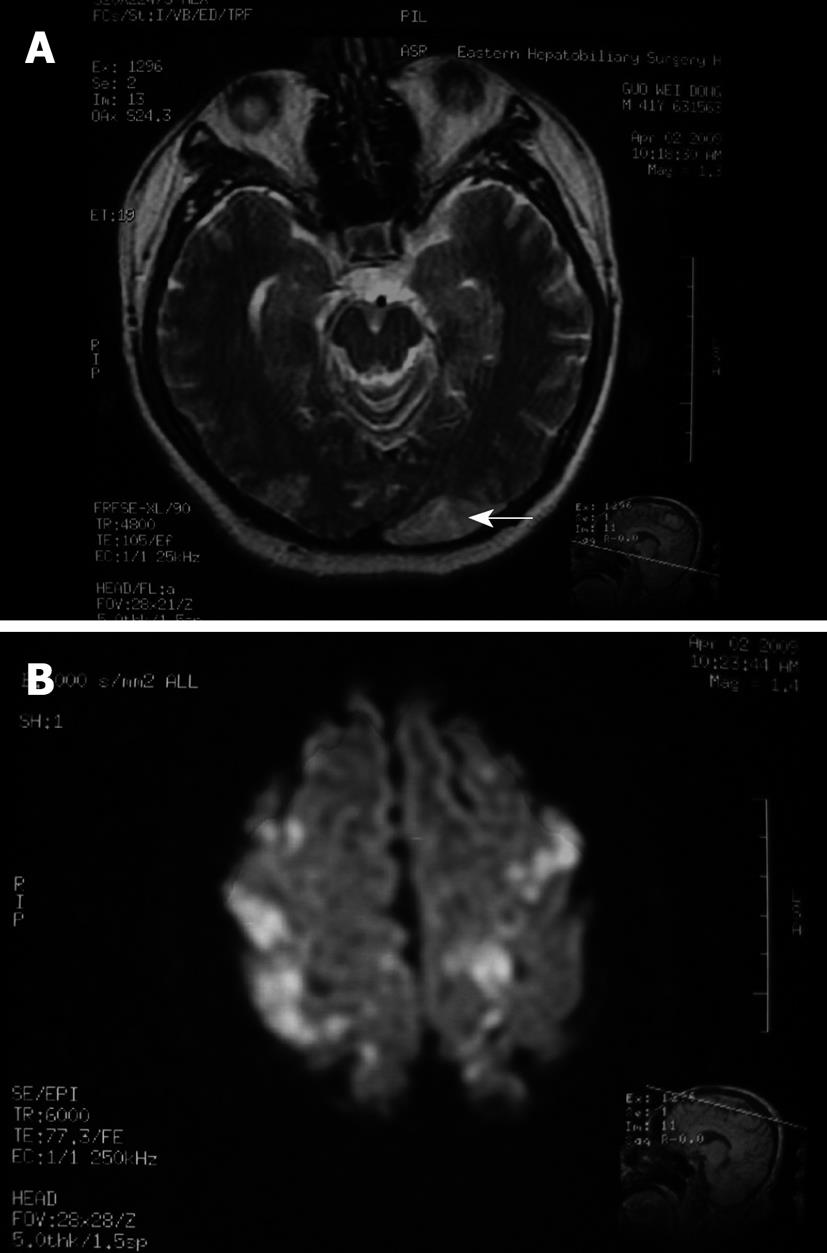
The laboratory data after TACE revealed a white blood cell count of 13.51 × 109/L (normal: 4 × 109 to 10 × 109/L), platelet count of 83 × 109/L (normal: 100 × 109 to 300 × 109/L), total bilirubin of 28.6 μmol/L, direct bilirubin of 11.0 μmol/L, alanine aminotransferase of 187.4 U/L, aspartate aminotransferase of 429.7 U/L, and normal renal function. Consultation with a neurologist suggested a diagnosis of cerebral embolism induced by the introduction of iodized oil. Magnetic resonance imaging (MRI) was performed 19 h after the TACE procedure, which revealed multiple abnormal signals that indicated ischemic foci of the centrum semiovale, and both parietal and occipital lobes (Figure 4). On the 10th day, with the appearance of melena, erythrocyte suspension and plasma were transfused along with hemostatic agents. Melena disappeared 2 d later. Vision and muscle strength of the affected extremities also improved gradually. Six weeks after CLE, the patient completely recovered without any neurological sequelae.
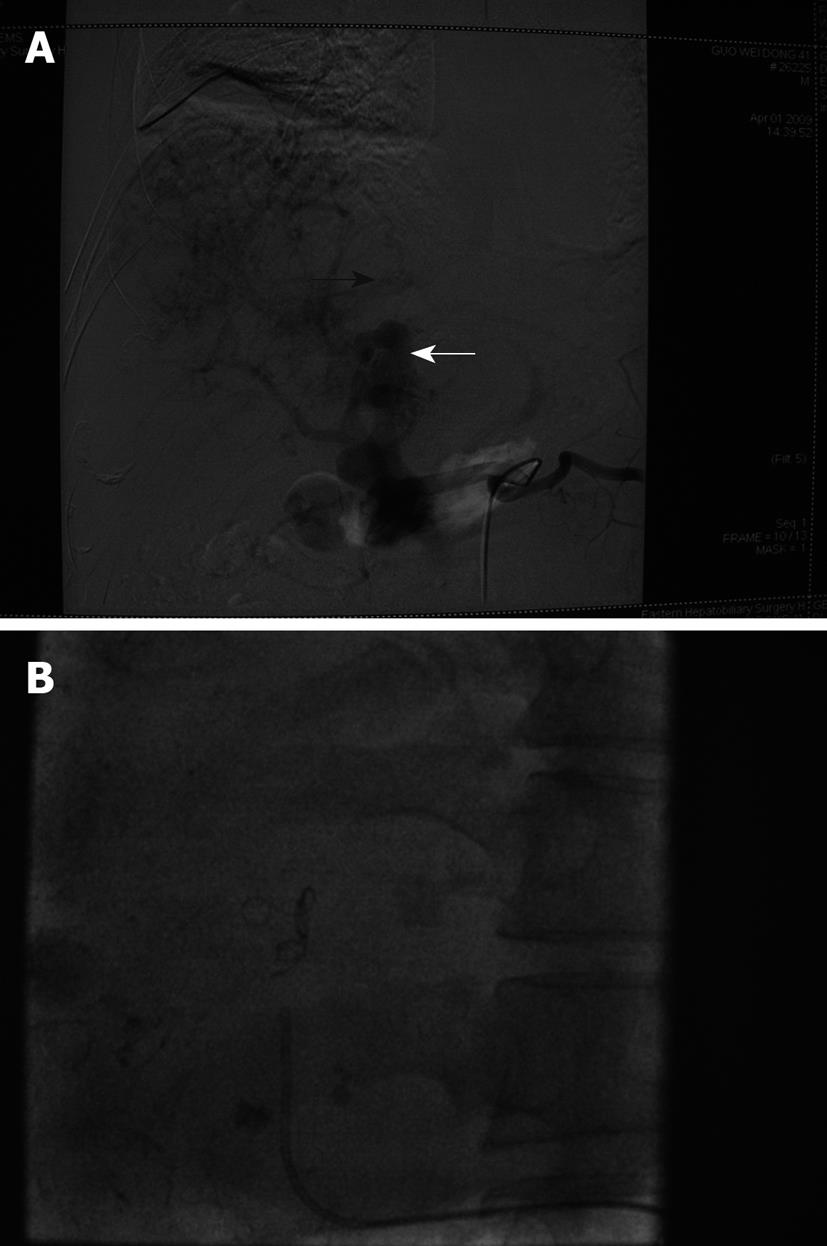
Two months after the second TACE procedure, the patient came back for follow-up. The tumor markers were tested: AFP 11.0 μg/L, CEA 0.8 μg/L, CA-19-9 12.5 U/mL, and digital subtraction angiography (DSA) revealed that an arterial-portal fistula was still present but improved, meanwhile, hepatic arteriovenous fistula was not seen. Therefore, we performed a third course of TACE. A mixture of 8 mL lipiodol, 10 mg hydroxyl camptothecin and 40 mg pirarubicin, as well as gelatin sponge particles (760-1000 μm) was injected through the right hepatic artery, left hepatic artery and left gastric artery (Figure 5). The patient did not have any respiratory or neurological complaints and was discharged 2 d later, and was confirmed to be in good condition until the present follow-up.
CLE is an extremely rare complication of the invasive procedure TACE[1-3,5]. Only 11 papers have reported 16 cases of lipiodol-associated embolic brain damage following TACE. In China, the first case report of CLE was by Li et al[6] in 2001, while the first overseas was by Yoo et al[1] in 2004.
The symptoms of CLE are nonspecific, including visual loss, headache, motor weakness, and change of mental status. These symptoms vary in severity according to the site of iodized oil deposition. In addition, if the lipiodol enters the lungs as well, the patient complains of chest pain and dyspnea[7]. Actually, among the twelve prior cases presented, 10 were confirmed with pulmonary embolism after initial diagnosis of respiratory manifestations. Most CLE occurs during or immediately after TACE and is identified initially by one or more symptoms mentioned above, however, a delayed type of CLE also has been reported[8]. Although some of the patients who suffered from CLE after TACE die, despite positive interventions, most of them recover completely without any neurological sequelae[5] (Table 1). In our case, the patient developed CLE during the TACE procedure, and after 6 wk of supportive therapy, he recovered, leaving no neurological symptoms.
| n | |
| Sex | |
| Male | 8 |
| Female | 4 |
| Age (yr) | |
| ≥ 50 | 10 |
| < 50 | 2 |
| Course of TACE | |
| First | 3 |
| Second | 6 |
| Third | 2 |
| More than third | 1 |
| Doses of lipiodol (mL) | |
| ≥ 20 | 6 |
| < 20 | 3 |
| ND | 3 |
| Gelatin sponge particles | |
| Yes | 5 |
| No | 7 |
| Embolization through extra-hepatic collateral artery | |
| Yes | 6 |
| No | 4 |
| ND | 2 |
| Tumor site including right lobe | |
| Yes | 8 |
| No | 0 |
| ND | 4 |
| Size of tumor/invasion of diaphragm | |
| Large/multiple | 9 |
| Minor | 1 |
| ND | 2 |
| Vascular invasion | |
| Yes | 3 |
| No | 2 |
| ND | 7 |
| Arteriovenous fistula | |
| Yes | 2 |
| No | 5 |
| ND | 5 |
| Right-to-left shunt | |
| Yes | 0 |
| No | 6 |
| ND | 6 |
| Pulmonary embolism | |
| Yes | 10 |
| No | 1 |
| ND | 1 |
| Time of neurologic symptoms | |
| During or shortly after TACE | 11 |
| 69 h after TACE | 1 |
| Recovery time (All ≤ 6 wk) | |
| ≥ 4 wk | 3 |
| < 4 wk | 5 |
| ND | 1 |
| Death | 3 |
The radiological findings of CLE on computed tomography (CT) and MRI in the previously reported cases are similar. The site of lipiodol deposition includes the basal ganglia, thalamus, gray-white matter conjunction, and both parietal and frontal cortices. Cranial CT/MRI taken after the neurological symptoms disappeared is usually clear, which infers that the lipiodol in the brain could have been cleared entirely[5,8]. In our case, the sites of iodized oil deposition were mainly on the centrum semiovale, and both parietal and occipital lobes.
Ten papers covering 12 cases (the present case included) have described in detail the clinical and radiological data of CLE following TACE, and have been selected to make an analysis (Table 1) (in fact, 12 papers in total were searched, and because Zhao et al[9] and Li et al[10] provided no detailed information, their two cases were excluded). In six TACE procedures that were complicated by CLE, lipiodol was infused through extra-hepatic collateral arteries, and mostly the inferior phrenic arteries. In all 12 cases, only eight of them described the tumor sites, all of which involved the right liver lobes. This implies that invasion of the diaphragm is probably common in patients with CLE after TACE, although it was confirmed only in three cases [2,5,11].
Six authors have analyzed the mechanisms of CLE following TACE treatment of HCC. Wu et al[3] have shown that the most probable cause of lipiodol-induced brain embolism after TACE is a combination of a right-to-left shunt and the dose-dependent effect of the drug[3,12]. The communication between the systemic and pulmonary vessels might develop via adhesive pleurae or tumor invasion of the diaphragm, thus a right-left shunt is formed. When injecting iodized oil via the inferior phrenic artery, some oil droplets might enter the brain, thus bypassing the right-left shunt. Combined with an increased dose of lipiodol during the second course of TACE, the patient has a greater risk of CLE[3]. Wu et al[11] have added that an intra-pulmonary arteriovenous shunt might appear during the pulmonary lipiodol embolism because of increasing pulmonary artery pressure or hypoxia[1,11]. Cui et al[13] have observed that the contrast medium injected into the hepatic artery enters the pulmonary veins directly during angiography in patients with CLE after TACE. This phenomenon supports the mechanism of intra-pulmonary arteriovenous shunting suggested by Wu et al [3]. Choi et al[7] have performed echocardiography and DSA to exclude intra-cardiac and intra-tumoral shunts, to support the mechanism that the communication between the inferior phrenic artery and pulmonary vessels occurs via adherent pleura, and tumor recurrence causes the lipiodol-induced brain embolism after TACE. Li et al[6] and Matsumoto et al[5] have made similar speculation.
Yoo et al[1] have presented three cases of CLE following TACE; all of which had evidence of pulmonary involvement but without a demonstrable intra-cardiac shunt. Yoo et al[1] have speculated that since it has been verified that fat globules < 7 μm in diameter can pass directly through the pulmonary arteriolar network (i.e. trans-pulmonary shunt) and cause cerebral injury[14], the presence of an intra-cardiac right-to-left shunt might not be necessary[1].
In another case reported by Wu et al[8], pulmonary and cerebral embolism occurred 34 and 69 h, respectively, after TACE treatment of HCC. Wu et al[8] have concluded that the rapid-flow, tumor-feeding artery washes out the iodized oil, which leads to embolic damage of the lungs. Then, the lipiodol that is deposited in the lungs is washed out again and enters the systemic circulation, thus causing embolism of the brain.
In the present case, the most probable mechanism of pulmonary and cerebral embolism is attributed to a hepatic venous-arterial shunt and an intra-pulmonary or intra-cardiac shunt. Lipiodol entered the pulmonary circulation through a hepatic arteriovenous fistula (Figure 3), which caused pulmonary embolism that increased pulmonary artery pressure, and produced a temporary pulmonary arteriovenous fistula. Lipiodol entered the brain through this temporary fistula. In addition, the use of a large dose of lipiodol also promotes CLE.
It is well known that CLE after TACE might be associated with intra-cardiac shunt, intra-pulmonary shunt and infusion of large doses of lipiodol[7]. Intra-cardiac right-to-left shunt can occur in some congenital heart diseases, such as patent foramen ovale. Research has revealed that among 1100 non-selected autopsies, 386 cases showed patent foramen ovale and 83% of them were < 0.2 cm in diameter[15]. The small foramen ovale, being often undetectable on routine echocardiography, might allow transient right-to-left shunting when the right heart pressure increases following oil trapping in the lungs[3]. Pulmonary arteriovenous shunts usually are associated with congenital pulmonary vascular malformations, acute or chronic pulmonary diseases, pulmonary tumors and advanced liver diseases[3,16]. The communication that can develop between the inferior phrenic artery and the pulmonary vessels also suggests a possible route for right-to-left shunting[4] (Table 1). Large dose of lipiodol infusion might increase the risk of extra-hepatic embolism. Some authors have suggested that a lipiodol dose less than 15 or 20 mL can prevent ectopic embolism[17,18]. However, in most prior cases of CLE, including the one described in this study, the HCCs were single large or multiple tumors (Table 1). As a result, limiting the dose of lipiodol to < 20 or 15 mL routinely might not be reasonable, especially when there is insufficient evidence to confirm that a large dose of lipiodol is a determinant factor for CLE following TACE. Three previous cases have revealed that CLE also occurs when the lipiodol dose is < 15 mL[1,3,5]. Therefore, all procedures must be individualized.
It is not necessary to search routinely for shunts prior to TACE, especially when right-to-left shunting is often undetectable in routine examination, as in chest or abdominal CT, DSA, and echocardiography[7]. In the present case, the patient was subjected to echocardiography and no positive evidence of right-to-left shunting was revealed. The patient had a history of bleeding gastroesophageal varices, therefore, transesophageal echocardiography was not performed. Direct evidence of a shunt has not yet been reported in previous studies (Table 1). Kim et al[19] have presented one case of CLE following TACE during which the presence of a right-to-left shunt (was demonstrated by the presence of microbubbles in the left middle cerebral artery and left atrium, while trans-cranial Doppler and transesophageal echocardiography were performed during the intravenous injection of agitated saline.
The mechanism of CLE following TACE has not yet been elucidated. Intracardiac or intrapulmonary right-to-left shunts and infusion of large doses of lipiodol might contribute to the increased risk of CLE following TACE. An individualized plan of therapy, including lipiodol dose determination, shunting detection, as well as selecting vessels for the lipiodol infusion prior to TACE is of great importance to achieve an efficient overall result[11].
Peer reviewer: De-Liang Fu, MD, PhD, Professor, Department of Surgery, Pancreatic Disease Institute, Fudan University, 12 Wulumqi Road (M), Shanghai 200040, China
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
| 1. | Yoo KM, Yoo BG, Kim KS, Lee SU, Han BH. Cerebral lipiodol embolism during transcatheter arterial chemoembolization. Neurology. 2004;63:181-183. |
| 2. | Takao H, Makita K, Doi I, Watanabe T. Cerebral lipiodol embolism after transcatheter arterial chemoembolization of hepatocellular carcinoma. J Comput Assist Tomogr. 2005;29:680-682. |
| 3. | Wu RH, Tzeng WS, Chang CM. Iodized oil embolization to brain following transcatheter arterial embolization of liver. J Gastroenterol Hepatol. 2005;20:1465-1467. |
| 4. | Sakamoto I, Aso N, Nagaoki K, Matsuoka Y, Uetani M, Ashizawa K, Iwanaga S, Mori M, Morikawa M, Fukuda T. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18:605-619. |
| 5. | Matsumoto K, Nojiri J, Takase Y, Egashira Y, Azama S, Kato A, Kitahara K, Miyazaki K, Kudo S. Cerebral lipiodol embolism: a complication of transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2007;30:512-514. |
| 6. | Li AM, Wang RP, Zhou S, Wang XJ. Cerebral lipiodol embolism caused by hepatic chemoemboliztion: one case report. Shanghai Yixue Yingxiang. 2001;10:3. |
| 7. | Choi CS, Kim KH, Seo GS, Cho EY, Oh HJ, Choi SC, Kim TH, Kim HC, Roh BS. Cerebral and pulmonary embolisms after transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2008;14:4834-4837. |
| 8. | Wu JJ, Chao M, Zhang GQ, Li B. Delayed cerebral lipiodol embolism after transcatheter arterial chemoembolization of hepatocellular carcinoma. Chin Med J (Engl). 2009;122:878-880. |
| 9. | Zhao H, Wang HQ, Fan QQ, Chen XX, Lou JY. Rare pulmonary and cerebral complications after transarterial chemoembolisation for hepatocellular carcinoma: a case report. World J Gastroenterol. 2008;14:6425-6427. |
| 10. | Li XB, Xu GB, Wang F, Zhou S, Wang XJ. Cerebral infarction caused by transcatheter arterial chemoembolization through infraphrenic artery for hepatocellular carcinoma: A case report. Zhonghua Fangshexue Zazhi. 2004;38:8. |
| 11. | Wu JJ, Chao M, Zhang GQ, Li B, Dong F. Pulmonary and cerebral lipiodol embolism after transcatheter arterial chemoembolization [corrected] in hepatocellular carcinoma. World J Gastroenterol. 2009;15:633-635. |
| 12. | Kishi K, Sonomura T, Satoh M, Nishida N, Terada M, Shioyama Y, Yamada R. Acute toxicity of lipiodol infusion into the hepatic arteries of dogs. Invest Radiol. 1994;29:882-889. |
| 13. | Cui YF, Zu MH, Xu H, Gu YR, Li GJ, Zhang QQ, Wei N. Severe complications after transcatheter arterial chemoembolization for hepatocellular carcinoma. Zhongguo Jieru Yingxiang Yu Zhiliaoxue. 2005;2:31-33. |
| 14. | Sevitt S. The significance and pathology of fat embolism. Ann Clin Res. 1977;9:173-180. |
| 16. | Krowka MJ, Cortese DA. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest. 1994;105:1528-1537. |
| 17. | Sato M, Kishi K, Shioyama Y, Tsuda M, Terada M, Tanaka H, Nakatani K, Sonomura T, Maeda M, Daimon M. [Effects of experimental hepatic artery embolization with lipiodol and gelatin sponge on liver tissue]. Nippon Igaku Hoshasen Gakkai Zasshi. 1990;50:107-113. |
| 18. | Chung JW, Park JH, Im JG, Han JK, Han MC. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1993;187:689-693. |
| 19. | Kim JT, Heo SH, Choi SM, Lee SH, Park MS, Kim BC, Kim Y, Kim MK, Cho KH. Cerebral embolism of iodized oil (lipiodol) after transcatheter arterial chemoembolization for hepatocellular carcinoma. J Neuroimaging. 2009;19:394-397. |