Published online Jan 21, 2010. doi: 10.3748/wjg.v16.i3.339
Revised: November 17, 2009
Accepted: November 24, 2009
Published online: January 21, 2010
AIM: To compare two types of classification of intestinal metaplasia (IM) of the stomach and to explore their relationship to gastric carcinoma.
METHODS: Forty-seven cases of gastric IM were classified into type I, type II or type III according to mucin histochemical staining and compared with a novel classification in which the specimens were classified into simple IM (SIM) or atypical IM according to polymorphism in terms of atypical changes of the metaplastic epithelium. Forty-seven IM and thirty-seven gastric carcinoma samples were stained for p53, c-erbB-2 and Ki67 proteins by Envision immunohistochemical technique.
RESULTS: There were no significant differences in the expression of p53 and c-erbB-2 among type I, type II, type III IM and gastric carcinomas. The positive expression rate of Ki67 was significantly higher in gastric carcinomas than in type I IM while no significant Ki67 expression differences were observed among type II, type III IM and gastric carcinomas. The expression of p53, c-erbB-2 and Ki67 proteins in 20 SIM, 27 Atypical IM and 37 gastric carcinomas showed significant differences between SIM and gastric carcinomas while no significant differences were observed between Atypical IM and gastric carcinomas.
CONCLUSION: Atypical IM may better reveal the precancerous nature of IM and could be a helpful indicator in the clinical follow up of patients.
- Citation: Zheng Y, Wang L, Zhang JP, Yang JY, Zhao ZM, Zhang XY. Expression of p53, c-erbB-2 and Ki67 in intestinal metaplasia and gastric carcinoma. World J Gastroenterol 2010; 16(3): 339-344
- URL: https://www.wjgnet.com/1007-9327/full/v16/i3/339.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i3.339
The relationship between intestinal metaplasia (IM) and gastric carcinoma has always been controversial. Correa postulated that gastric cancer develops through a complex sequence of events from normal mucosa to superficial gastritis, chronic atrophic gastritis, IM, dysplasia, and finally to intestinal type gastric carcinoma[1]. Many investigators have given credence to the preneoplastic nature of IM[2]. Even with common occurrence and presence in gastric biopsies, not all cases of IM may develop into gastric carcinoma. A subtype of IM which has malignant potential should be classified for clinical follow up. Generally, IM is divided into subtypes on the basis of histochemical characteristics, and IM showing sulphomucin secretion has been considered to be a lesion with precancerous nature[3-5], but some others considered this type of IM had no special link to gastric carcinoma[2], and Rothery[6] also found half of the cases with this IM showing sulphomucin secretion exist in benign lesions.
In our study, IM was divided into two subtypes according to the atypical changes of the metaplastic epithelium: simple IM (SIM) and atypical IM (AIM). We detected three tumor-associated proteins, p53, c-erbB-2 and Ki67, in different subtypes of IM in order to find which one is more associated with gastric carcinoma.
Forty-seven formalin-fixed, paraffin-embedded samples for IM were obtained from endoscopic biopsy and thirty-seven gastric carcinoma specimens from gastrectomy at Qilu Hospital of Shandong University. Mucin histochemical staining for IM subtyping was performed.
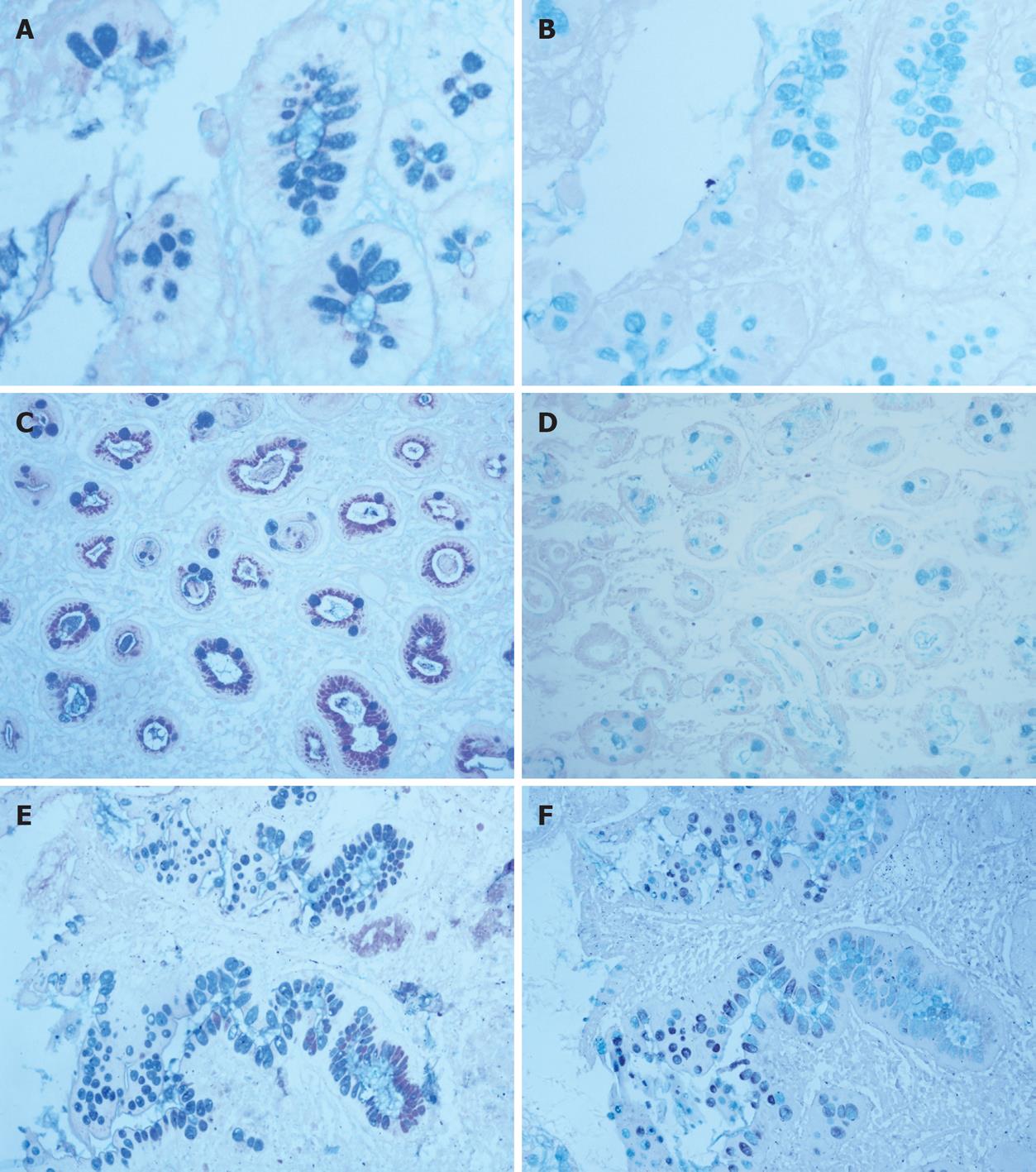
Serial sections were cut, stained with hematoxylin-eosin and the following techniques for mucins: Alcian blue pH 2.5-periodic acid-Schiff and high iron-diamine plus Alcian blue pH 2.5 to identify neutral, sialo- and sulphomucins.
Forty-seven cases of IM were classified in accordance with the system used by Jass and Filipe[7,8]: Type I (complete): mature absorptive and goblet cells, the latter secreting sialomucins (Figure 1); Type II (incomplete): IM cells with few or absent absorptive cells; presence of columnar “intermediate” cells in various stages of differentiation, secreting neutral and acid sialomucins; goblet cells secreting sialomucins and/or occasionally sulphomucins (Figure 1); Type III (incomplete): the cell dedifferentiation is more marked than in type II; “intermediate” cells secrete predominantly sulphomucins. A variable degree of disorganized glandular architecture is often present (Figure 1).
Forty-seven IM samples were classified into SIM and AIM according to atypical changes of the metaplastic epithelium.
Sections 4 μm thick were cut, deparaffinized in xylene, and then dehydrated in descending dilutions of ethanol. For the antigen retrieval regimen, all slides were microwaved in 10 mmol/L sodium citrate buffer (pH 6.0) at 10 min intervals for a total of 20 min. The endogenous peroxidase activity was blocked by 10 min of incubation with 3% hydrogen peroxidase (reagent A) at room temperature. After washing in PBS, the sections were incubated with monoclonal mouse anti-human antibodies p53 (MAB-0364), c-erbB-2 (CB11) and Ki67 (SP6) overnight at 4°C. The sections were washed with PBS and incubated with polymerase auxiliaries (reagent B) for 20 min. After washing in PBS, the sections were incubated with biotinylated secondary antibody (reagent C) for 30 min at room temperature and finally DAB was visualized. Tissues were counterstained with hematoxylin. A negative control was designed by using PBS instead of primary antibody.
Sections were scored by light microscopy. The percentage of positively stained cells was calculated after 100 cells were counted in more than 5 high-power (× 40) fields. The following definitions were made: p53 and Ki67: more than 10% positive staining in nuclei was defined as positive staining; c-erbB-2: more than 10% positive staining in cytoplasm was defined as positive staining in IM, and more than 10% positive staining in cell membrane was defined as positive staining in gastric carcinoma[9].
The significance of associations was determined by the χ2 test or the Fisher’s exact test, P value < 0.05 was considered statistically significant.
In 47 IM samples, type I, type II and type III IM accounted for 17, 18 and 12 samples, respectively. In 20 SIM, type I, type II and type III IM accounted for 11, 7 and 2 samples, respectively. In 27 AIM, type I, type II and type III IM accounted for 6, 11 and 10 samples, respectively (Table 1).
| Type I | Type II | Type III | Total | |
| SIM | 11 | 7 | 2 | 20 |
| AIM | 6 | 11 | 10 | 27 |
| Total | 17 | 18 | 12 | 47 |
The positive expression rates of p53 in type I, type II, type III IM and gastric carcinomas were 41.18%, 27.78%, 25.00% and 54.05%, respectively. The expression of p53 in gastric carcinomas was not significantly higher than in types I, II and III (P > 0.05).
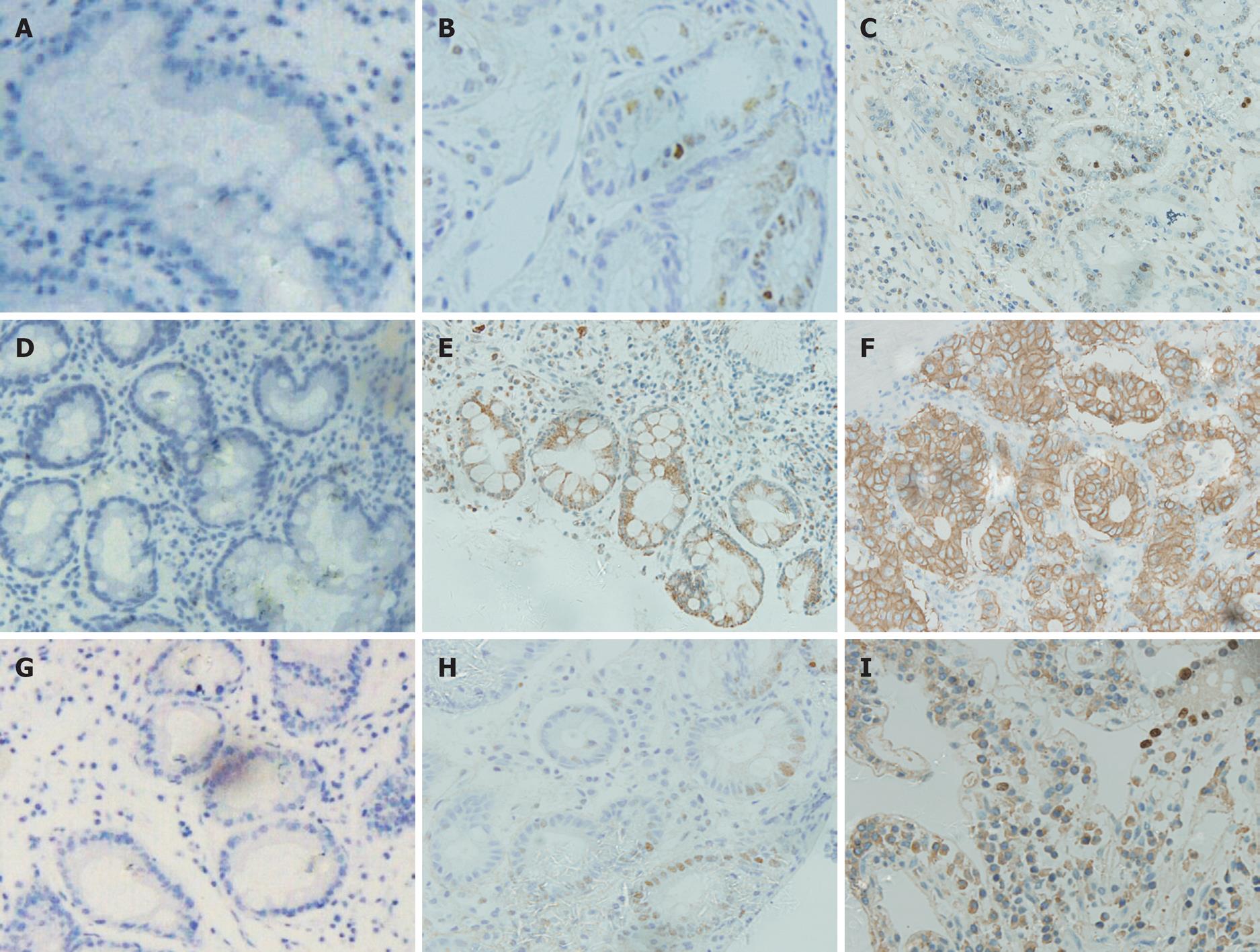
The positive expression rates of p53 in SIM and AIM were 20.00% and 40.74%, respectively. The expression of p53 in gastric carcinomas was significantly higher than in SIM (P < 0.05). There was no significant difference in p53 expression between AIM and gastric carcinomas (P > 0.05) (Table 2, Figure 2).
The positive expression rates of c-erbB-2 in type I, type II, type III IM and gastric carcinomas were 52.94%, 44.44%, 33.33% and 59.46%, respectively. The expression of c-erbB-2 in gastric carcinomas was not significantly higher than in types I, II and III (P > 0.05).
The positive expression rates of c-erbB-2 in SIM and AIM were 30.00% and 656.67%. The expression of c-erbB-2 in gastric carcinomas was significantly higher than SIM (P < 0.05). There was no significant difference between expression in AIM and gastric carcinomas (P > 0.05) (Table 2, Figure 2).
The positive expression rates of Ki67 in type I, type II, type III IM and gastric carcinomas were 29.41%, 50.00%, 41.67% and 75.68%, respectively. The expression of Ki67 in gastric carcinomas was significantly higher than in type I IM (P < 0.05). There was no significant difference in Ki67 expression between gastric carcinomas and type II or type III.
The positive expression rates of Ki67 in SIM and AIM were 25.00% and 51.85%, respectively. The expression of Ki67 in gastric carcinomas was significantly higher than in SIM (P < 0.05) while there was no significant difference in Ki67 expression between AIM and gastric carcinomas (P > 0.05) (Table 2, Figure 2).
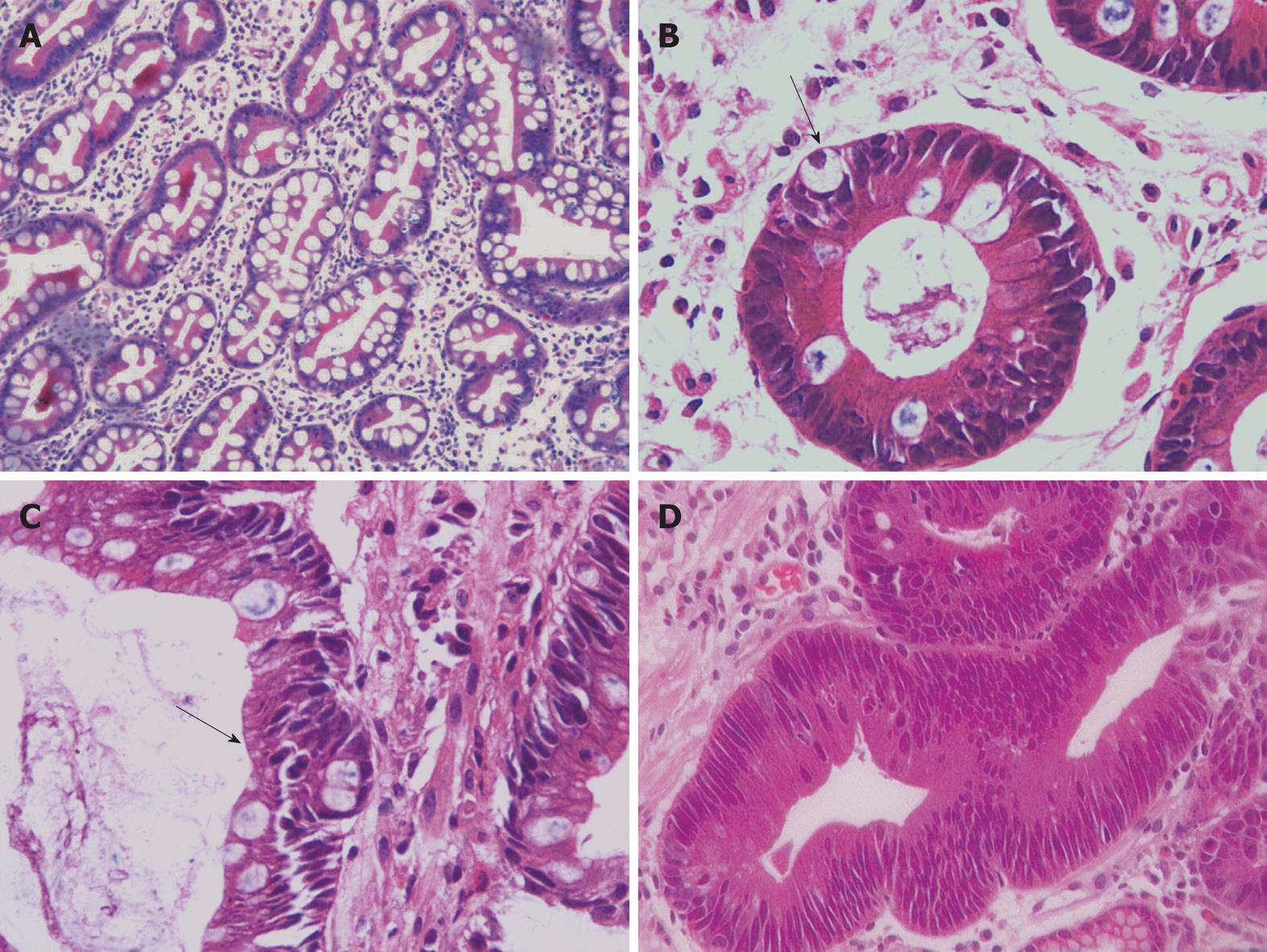
Gastric carcinoma is the fourth most common malignancy worldwide[10], accounting for 10% of newly diagnosed cancers[11]. It is one of the most common causes of cancer mortality in China[12]. Approximately 84% of patients with gastric cancer will have advanced disease and their median survival time is only 3-4 mo if they are not treated with chemotherapy[13]. Therefore, it is necessary to diagnose at an early stage in order to improve the survival rate. Gastric carcinoma is divided into intestinal and diffuse types according to Lauren[14] and the intestinal-type of gastric carcinoma is said to have a strong association with IM[15]. Since Morson[16] pointed out in 1955 that some gastric carcinomas might arise from an area of IM, IM has been considered to be a possible precancerous state. A large number of IM patients have been found in clinical studies, and a research group reported that the detection rate of IM was 37%[4]. Which type of IM is closely related to gastric carcinoma remains an unanswered question. Traditionally, IM is divided according to histochemical characteristics, but research concludes that sulphomucin-positive IM is of no value in surveillance for gastric cancer[17]. In view of the inconsistency of this classification, we assigned IM into the groupings of SIM (Figure 3) and IM with atypical changes as AIM (Figure 3). The predominant difference in the two types of IM is the atypical changes of the metaplastic epithelium: SIM glands are arranged neatly and the goblet cells are in normal forms, the mucosa in the foveolae is flat; while AIM glands are crowded and the goblet cells often are associated with immature differentiation, also the mucosa in the foveolae usually become deeper than in SIM. One question should be clarified regarding the difference between AIM and gastric intraepithelial neoplasia (GIN) (Figure 3): the main distinction lies in the goblet cells. There were more goblet cells (over 10% of total cells) in AIM while there were less or no goblet cells in GIN.
In our study, three tumor-associated proteins, p53, c-erbB-2 and Ki67, were selected for immunohistochemical detection. The p53 gene is localized to chromosome arm 17p13[18]. Evidence from in vitro models suggests that p53 acts as a tumor suppressor gene[19]. The evidence accumulated so far suggests that mutant p53 may be the commonest genetic abnormality in human cancer[20]. p53 gene mutation is known to play a considerable role in the carcinogenesis of colonic carcinoma and gastric carcinoma. Shiao et al[21] reported that 67% of gastric carcinomas, 58% of dysplasias and 38% of metaplastic lesions stained positively for p53. C-erbB-2 (also called NEU and HER2) is a proto-oncogene that codes for a protein product which shows considerable homology with EGFR[22]. Several studies have reported amplification of the c-erbB-2 gene in human neoplasms, particularly in adenocarcinomas[23]. In recent years, researchers have revealed that c-erbB-2 plays an important role in the occurrence and development of gastric carcinoma. Observations suggested that c-erbB-2 gene rearrangement non-randomly associated with carcinomas with glandular origin derived from the gastrointestinal tract[24]. It is reported that over expression of the c-erbB-2 gene is at a frequency of 8.2%-45% in gastric carcinoma[25-27]. Ki67 is a nuclear proliferation-associated antigen expressed in the growth and synthesis phases of the cell cycle but not in the resting phase[28]. This antigen provides information about the proportion of active cells in the cell cycle. Its expression varies greatly during the cell cycle and is increased in many tumors[29]. Studies have revealed that the Ki67 proliferating index increases in the transformation from IM to gastric carcinoma[30]. For the reasons above, p53, c-erbB-2 and Ki67 proteins can be regarded as indicators of the precancerous nature of IM in the gastric mucosa. Our results demonstrated that the expressions of p53 and c-erbB-2 in gastric carcinoma were not significantly higher than in types I, II and III IM. The expression of Ki67 in gastric carcinoma was significantly higher than in type I, but not significantly higher than in type II or type III.
It is difficult to determine which subtype of IM has a definite relationship with gastric carcinomas. In SIM and AIM classification, the expressions of all three proteins in gastric carcinomas were significantly higher than in SIM and no significant differences were observed between gastric carcinomas and AIM. Obviously, AIM may have a much more close relationship with gastric carcinoma.
We deduce that SIM may be merely a response to stimuli caused by the changing environment, while AIM may have malignant transformation and could be regarded as preneoplastic lesions. Clinical follow up of AIM patients may be helpful for the diagnosis of early gastric carcinomas. However, since details of the role of AIM in the multiple steps of carcinogenesis of gastric mucosa are still unknown, further study is necessary with regard to AIM, perhaps using more advanced methods. Furthermore, an adequate long term follow up is indispensable to assess the definite value of AIM in the screening for gastric carcinoma.
Gastric carcinoma remains a significant problem globally. The relationship between intestinal metaplasia (IM) and gastric carcinoma has always been controversial. Generally IM is divided into subtypes on the basis of histochemical characteristics; however, this classification is confusing. A new classification of IM is needed in order to follow up patients selectively.
By detecting three tumor-associated proteins, p53, c-erbB-2 and Ki67, in IM and gastric carcinoma, this study compared two types of classification in IM of the stomach and explored their relationship to gastric carcinoma.
In the past, IM was classified according to histochemical characteristics. In this study, IM was first divided into simple IM (SIM) and atypical IM (AIM) was reported to better reveal the precancerous nature of IM and could be a helpful indicator in the surveillance of patients clinically.
The new classification of IM could be helpful in the surveillance of patients clinically and useful for the diagnosis of early gastric carcinomas.
IM is defined as the appearance of intestinal epithelium in the stomach. Type I, II and III IM are subtypes of IM classified according to the histochemical characteristics of the mucin-secreting cells. SIM and AIM classification is dependent mainly on the atypical changes of the metaplastic epithelium of the stomach. p53, c-erbB-2 and Ki67 are all tumor-associated proteins that are expressed mainly in metaplastic and tumor tissues.
The study provides important new data about the potential risk of gastric cancer in patients with IM. However, it would be important in the future to investigate the expression of p53 and/or Her2Neu in a prospective study in patients with IM, to confirm that only patients with p53/Her2Neu expression in the IM have actually a higher risk for gastric carcinomas.
Peer reviewer: Stefan Kubicka, Professor, Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Carl Neubergstrasse 1, 30625 Hannover, Germany
S- Editor Wang YR L- Editor Logan S E- Editor Zheng XM
| 1. | Cahill RJ, Kilgallen C, Beattie S, Hamilton H, O'Morain C. Gastric epithelial cell kinetics in the progression from normal mucosa to gastric carcinoma. Gut. 1996;38:177-181. |
| 2. | Matsukuma A, Mori M, Enjoji M. Sulphomucin-secreting intestinal metaplasia in the human gastric mucosa. An association with intestinal-type gastric carcinoma. Cancer. 1990;66:689-694. |
| 3. | Heilmann KL, Höpker WW. Loss of differentiation in intestinal metaplasia in cancerous stomachs. A comparative morphologic study. Pathol Res Pract. 1979;164:249-258. |
| 4. | Teglbjaerg PS, Nielsen HO. "Small intestinal type" and "colonic type" intestinal metaplasia of the human stomach, and their relationship to the histogenetic types of gastric adenocarcinoma. Acta Pathol Microbiol Scand A. 1978;86A:351-355. |
| 5. | Sipponen P, Seppälä K, Varis K, Hjelt L, Ihamäki T, Kekki M, Siurala M. Intestinal metaplasia with colonic-type sulphomucins in the gastric mucosa; its association with gastric carcinoma. Acta Pathol Microbiol Scand A. 1980;88:217-224. |
| 6. | Rothery GA, Day DW. Intestinal metaplasia in endoscopic biopsy specimens of gastric mucosa. J Clin Pathol. 1985;38:613-621. |
| 7. | Jass JR, Filipe MI. The mucin profiles of normal gastric mucosa, intestinal metaplasia and its variants and gastric carcinoma. Histochem J. 1981;13:931-939. |
| 8. | Jass JR, Filipe MI. A variant of intestinal metaplasia associated with gastric carcinoma: a histochemical study. Histopathology. 1979;3:191-199. |
| 9. | Wittekind C, Klimpfinger M, Hermanek P, Tannapfel A. Multiple simultaneous gastric carcinomas. Br J Cancer. 1997;76:1604-1609. |
| 10. | Burkitt MD, Varro A, Pritchard DM. Importance of gastrin in the pathogenesis and treatment of gastric tumors. World J Gastroenterol. 2009;15:1-16. |
| 11. | Roder DM. The epidemiology of gastric cancer. Gastric Cancer. 2002;5 Suppl 1:5-11. |
| 12. | Chen XM, Chen GY, Wang ZR, Zhu FS, Wang XL, Zhang X. Detection of micrometastasis of gastric carcinoma in peripheral blood circulation. World J Gastroenterol. 2004;10:804-808. |
| 13. | Rivera F, Vega-Villegas ME, López-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev. 2007;33:315-324. |
| 14. | Wang CH, Yu YZ. Research progress on intestinal metaplasia of the gastric mucosa. Zhongguo Shiyong Neike Zazhi. 2004;9:562-564. |
| 15. | Whitehead R, Skinner JM, Heenan PJ. Incidence of carcinoma of stomach and tumour type. Br J Cancer. 1974;30:370-372. |
| 16. | Morson BC. Carcinoma arising from areas of intestinal metaplasia in the gastric mucosa. Br J Cancer. 1955;9:377-385. |
| 17. | Ectors N, Dixon MF. The prognostic value of sulphomucin positive intestinal metaplasia in the development of gastric cancer. Histopathology. 1986;10:1271-1277. |
| 18. | McBride OW, Merry D, Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13). Proc Natl Acad Sci USA. 1986;83:130-134. |
| 19. | Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989;57:1083-1093. |
| 20. | Harris AL. Mutant p53--the commonest genetic abnormality in human cancer. J Pathol. 1990;162:5-6. |
| 21. | Shiao YH, Rugge M, Correa P, Lehmann HP, Scheer WD. p53 alteration in gastric precancerous lesions. Am J Pathol. 1994;144:511-517. |
| 22. | Yamamoto T, Ikawa S, Akiyama T, Semba K, Nomura N, Miyajima N, Saito T, Toyoshima K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986;319:230-234. |
| 23. | Tal M, Wetzler M, Josefberg Z, Deutch A, Gutman M, Assaf D, Kris R, Shiloh Y, Givol D, Schlessinger J. Sporadic amplification of the HER2/neu protooncogene in adenocarcinomas of various tissues. Cancer Res. 1988;48:1517-1520. |
| 24. | Park JB, Rhim JS, Park SC, Kimm SW, Kraus MH. Amplification, overexpression, and rearrangement of the erbB-2 protooncogene in primary human stomach carcinomas. Cancer Res. 1989;49:6605-6609. |
| 25. | Takehana T, Kunitomo K, Kono K, Kitahara F, Iizuka H, Matsumoto Y, Fujino MA, Ooi A. Status of c-erbB-2 in gastric adenocarcinoma: a comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int J Cancer. 2002;98:833-837. |
| 26. | Ooi A, Kobayashi M, Mai M, Nakanishi I. Amplification of c-erbB-2 in gastric cancer: detection in formalin-fixed, paraffin-embedded tissue by fluorescence in situ hybridization. Lab Invest. 1998;78:345-351. |
| 27. | Dursun A, Poyraz A, Celik B, Akyol G. Expression of c-erbB-2 oncoprotein in gastric carcinoma: correlation with histopathologic characteristics and analysis of Ki-67. Pathol Oncol Res. 1999;5:104-106. |
| 28. | Mueller J, Werner M, Siewert JR. Malignant progression in Barrett's esophagus: pathology and molecular biology. Recent Results Cancer Res. 2000;155:29-41. |
| 29. | Yu CC, Woods AL, Levison DA. The assessment of cellular proliferation by immunohistochemistry: a review of currently available methods and their applications. Histochem J. 1992;24:121-131. |
| 30. | Forones NM, Carvalho AP, Giannotti-Filho O, Lourenço LG, Oshima CT. Cell proliferation and apoptosis in gastric cancer and intestinal metaplasia. Arq Gastroenterol. 2005;42:30-34. |