Published online Aug 7, 2010. doi: 10.3748/wjg.v16.i29.3709
Revised: December 23, 2009
Accepted: December 30, 2009
Published online: August 7, 2010
AIM: To analyze the outcome of patients who received concurrent capecitabine (Xeloda) and radiation (XRT) compared to the established concurrent 5-fluorouracil (5-FU) with radiation (5FU-RT) and fluoropyrimidine-based chemotherapy alone as adjuvant treatment in gastric cancers.
METHODS: All patients with gastric cancers who received adjuvant treatment at the National Cancer Centre Singapore between 1996 and 2006 were reviewed. Treatment outcomes of patients who received XRT were compared with those who had 5FU-RT or chemotherapy alone as adjuvant therapy for gastric cancers.
RESULTS: A total of 108 patients were reviewed. Median age at diagnosis was 60. The majority of the patients (64.8%) had advanced stage III and IV disease (with no distant metastasis). All except 4 patients had D2 gastrectomy. Twenty one patients (19.4%) had positive surgical resection margins. Thirty three patients received XRT compared with 52 who had 5FU-RT and 23 who received chemotherapy alone. For the patients in the chemotherapy-only group, all had fluoropyrimidine-based therapy, with added cisplatin in 7 patients and epirubicin in 2 patients. Median recurrence-free survival was longer for the XRT group (52 mo) compared to the 5FU-RT (35 mo) and chemotherapy-only groups (25 mo) (P = 0.48). The patients in the XRT group achieved similar median overall survival (53 mo) as the 5FU-RT (54 mo) and the chemotherapy-only groups (44 mo) (P = 0.5).
CONCLUSION: Capecitabine with concurrent radiation was as effective as concurrent 5FU with radiation or fluoropyrimidine-based chemotherapy alone when used as adjuvant treatment in patients with gastric cancers.
- Citation: Tham CK, Choo SP, Poon DYH, Toh HC, Ong SYK, Tan SH, Wang MLC, Foo KF. Capecitabine with radiation is an effective adjuvant therapy in gastric cancers. World J Gastroenterol 2010; 16(29): 3709-3715
- URL: https://www.wjgnet.com/1007-9327/full/v16/i29/3709.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i29.3709
Surgery is the curative treatment for gastric cancer. However, the outcome of large T3-T4 tumors and those with lymph node involvement remains poor after surgical resection alone with high risk of local and distant recurrence[1]. Many attempts had been made to improve the prognosis of resected gastric cancers. These include postoperative adjuvant chemotherapy with or without radiation and perioperative chemotherapy.
Adjuvant therapy in gastric cancer is a field of ongoing active research. The different modalities in the adjuvant setting include chemoradiation and chemotherapy alone. There is currently no randomized phase III trial that directly compared these various modalities. Hence, the optimal adjuvant therapy in gastric cancer remains to be determined.
Capecitabine (Xeloda), an oral fluoropyrimidine, was shown to have equivalent efficacy to continuous-infusional 5-fluorouracil (5-FU) in several phase III trials in metastatic gastric cancers. The REAL-2 study compared capecitabine or 5-FU in combination with cisplatin and oxaliplatin and showed no differences in response rates or survival between all the 4 combination regimens[2]. The ML 17032 trial comparing the combination of capecitabine with cisplatin to 5-FU with cisplatin showed that capecitabine and cisplatin had a better response rate (41% vs 29%) and overall survival (10.5 mo vs 9.3 mo)[3]. Because of the convenient oral administration of capecitabine, its use in the adjuvant setting is an attractive option to explore. In fact, capecitabine with concurrent radiation as an adjuvant or neoadjuvant treatment in resected gastric cancer and other gastrointestinal malignancies, particularly rectal cancer, has been explored in several studies[4-6]. These studies demonstrated that capecitabine with concurrent radiation was feasible with manageable toxicities. However, the regimen has never been compared with the more established concurrent 5-FU and radiation (5FU-RT) or adjuvant chemotherapy alone.
Hence, in this study, we aimed to analyze the outcome of patients who received adjuvant concurrent capecitabine and radiation (XRT) compared to those who received concurrent FU-RT or fluoropyrimidine-based chemotherapy alone in the adjuvant setting in gastric cancers.
All patients diagnosed with gastric cancer who received adjuvant treatment at National Cancer Center Singapore from 1996 to 2007 were reviewed. Clinical information was collected retrospectively and included age, gender, performance status, Helicobacter pylori status, surgical outcome, tumor histology, carcinoembryonic antigen, baseline hematologic and biochemical parameters, recurrence and survival data.
The patients were divided into 3 groups based on the adjuvant treatment received. These included 5FU-RT, XRT and chemotherapy alone for analysis. The patients in the XRT group received 825 mg/m2 capecitabine daily with concurrent radiation followed by capecitabine 2000 mg/m2 every 3 wk for 6 cycles. Patients in the 5FU-RT group received chemoradiation which consisted of 5 cycles of 5-FU 400 mg/m2 plus leucovorin (20 mg/m2) given every 28 d with 2 cycles given concurrently with radiation. The total radiation dose in both the XRT and 5FU-RT groups was 45 Gy delivered over a period of 5 wk. The patients in the chemotherapy-only group received fluoropyrimidine-based chemotherapy.
The patients receiving XRT were compared with those who had 5FU-RT and fluoropyrimidine-based chemotherapy in terms of recurrence-free survival and overall survival. We also compared the patients who received radiation as part of their adjuvant therapy to those who received chemotherapy alone.
Comparison of the median age at baseline between treatment groups was done using the Mann-Whitney U test. Other baseline characteristics, such as gender, performance status, stage and grade, as shown in Table 1, were performed using Fisher’s exact test.
| All patients (n = 108) | XRT (n = 33) | 5FU-RT (n = 52) | Chemo alone (n = 23) | P-value1 for XRT vs | ||
| 5FU-RT | Chemo alone | |||||
| Age (yr) | ||||||
| Median | 60 | 64 | 57 | 56 | 0.0032 | 0.032 |
| Inter-quartile range | 49.5-66.0 | 57.7-68.8 | 48.0-63.7 | 49.3-65.7 | ||
| Gender | ||||||
| Male | 65 (60.2) | 22 (66.7) | 30 (57.7) | 13 (56.5) | 0.5 | 0.6 |
| Female | 43 (39.8) | 11 (33.3) | 22 (42.3) | 10 (43.5) | ||
| ECOG | ||||||
| 0 | 6 (5.6) | 1 (3.0) | 3 (5.8) | 2 (8.7) | 1.0 | 0.6 |
| 1 | 102 (94.4) | 32 (97.0) | 49 (94.2) | 21 (91.3) | ||
| Helicobacter pylori status | ||||||
| Yes | 36 (53.7) | 16 (66.7) | 15 (50.0) | 5 (38.5) | 0.3 | 0.2 |
| No | 31 (46.3) | 8 (33.3) | 15 (50.0) | 8 (61.5) | ||
| Surgical margins | ||||||
| Positive | 21 (19.4) | 4 (12.1) | 14 (26.9) | 3 (13.0) | 0.2 | 1.0 |
| Negative | 87 (80.6) | 29 (87.9) | 38 (73.1) | 20 (87.0) | ||
| Stage | ||||||
| 1 or 2 | 38 (35.2) | 12 (36.4) | 14 (26.9) | 12 (52.2) | 0.5 | 0.3 |
| 3 or 4 | 70 (64.8) | 21 (63.6) | 38 (73.1) | 11 (47.8) | ||
| Grade | ||||||
| 1 or 2 | 31 (28.7) | 12 (36.4) | 12 (23.1) | 7 (30.4) | 0.2 | 0.8 |
| 3 | 77 (71.3) | 21 (63.6) | 40 (76.9) | 16 (69.6) | ||
| Albumin | ||||||
| < 30 | 12 (13.3) | 4 (12.9) | 4 (10.0) | 4 (21.1) | 0.7 | 0.5 |
| ≥ 30 | 78 (86.7) | 27 (87.1) | 36 (90.0) | 15 (78.9) | ||
| CEA | ||||||
| Normal | 50 (90.9) | 17 (89.5) | 24 (92.3) | 9 (90.0) | 1.0 | 1.0 |
| High | 5 (9.1) | 2 (10.5) | 2 (7.7) | 1 (10.0) | ||
| Hemoglobin | ||||||
| < 10 | 26 (25.7) | 8 (24.2) | 14 (29.2) | 4 (20.0) | 0.8 | 1.0 |
| ≥ 10 | 75 (74.3) | 25 (75.8) | 34 (70.8) | 16 (80.0) | ||
Recurrence-free survival duration was calculated from the date of diagnosis to the date of recurrence or death (whichever occurred first) or last follow-up. Overall survival duration was calculated from the date of diagnosis to the date of death or last follow-up. The Kaplan-Meier method was used to estimate and plot the recurrence-free survival and overall survival curves for the 3 treatment groups. The log-rank test was used to test if the survival function for the treatment groups were statistically different at the 5% significance level.
For recurrence-free survival and overall survival, the Cox proportional hazards model was used to estimate the crude and age-adjusted hazard ratios between treatment groups using the XRT arm as the reference treatment. Age at diagnosis was included in multivariate Cox regression analyses to estimate the adjusted hazard ratios. Subgroup analysis of the recurrence-free survival and overall survival hazard ratios between the chemotherapy-only group and chemoradiotherapy (XRT or 5FU-RT) were performed using the Cox proportional hazard model for patients with a positive surgical margin, Stage 3 or 4, and positive nodes, respectively.
The Cox proportional hazards model and log-rank test were also used to estimate the hazard ratios to assess if any of the baseline characteristics were associated with recurrence-free survival and overall survival in each of the 3 treatment groups. Normality of the variables in the Cox model was assessed using a Q-Q plot and proportionality assumption of the Cox model was assessed using the Schoenfeld test.
Two-sided P-values of less than 5% were considered as statistically significant. The software used for the analyses was STATA version 9.1.
A total of 108 patients had received adjuvant therapy for gastric cancer at our institution in the specified period. Median follow-up duration was 23 mo.
Thirty-three of these patients received XRT, 52 received 5FU-RT and 23 received fluoropyrimidine-based chemotherapy. Of the patients who received chemotherapy alone, 11 had capecitabine alone, 6 had ECF (epirubicin, cisplatin and 5-FU), 4 had XELOX (capecitabine and oxaliplatin) and 2 had 5-FU alone. Ninety-one percent of the patients in the XRT group, 89% in the 5FU-RT and 91% in the chemotherapy-only group completed the full course of adjuvant treatment.
The characteristics of these patients are shown in Table 1. Median age at diagnosis was 60 years. Sixty-five percent of the patients had advanced stage III and IV (with no distant metastasis) disease. All except 4 patients had D2 gastrectomy (2 in the XRT group, 2 in the chemotherapy-only group). Twenty-one of these patients (19.4%) had positive surgical resection margins (14 in the 5FU-RT group, 4 in the XRT group and 3 in the chemotherapy-only group). The only significant difference in characteristics among the 3 groups of patients was median age, with the median age of the patients in the XRT group being greater than that of the other 2 groups.
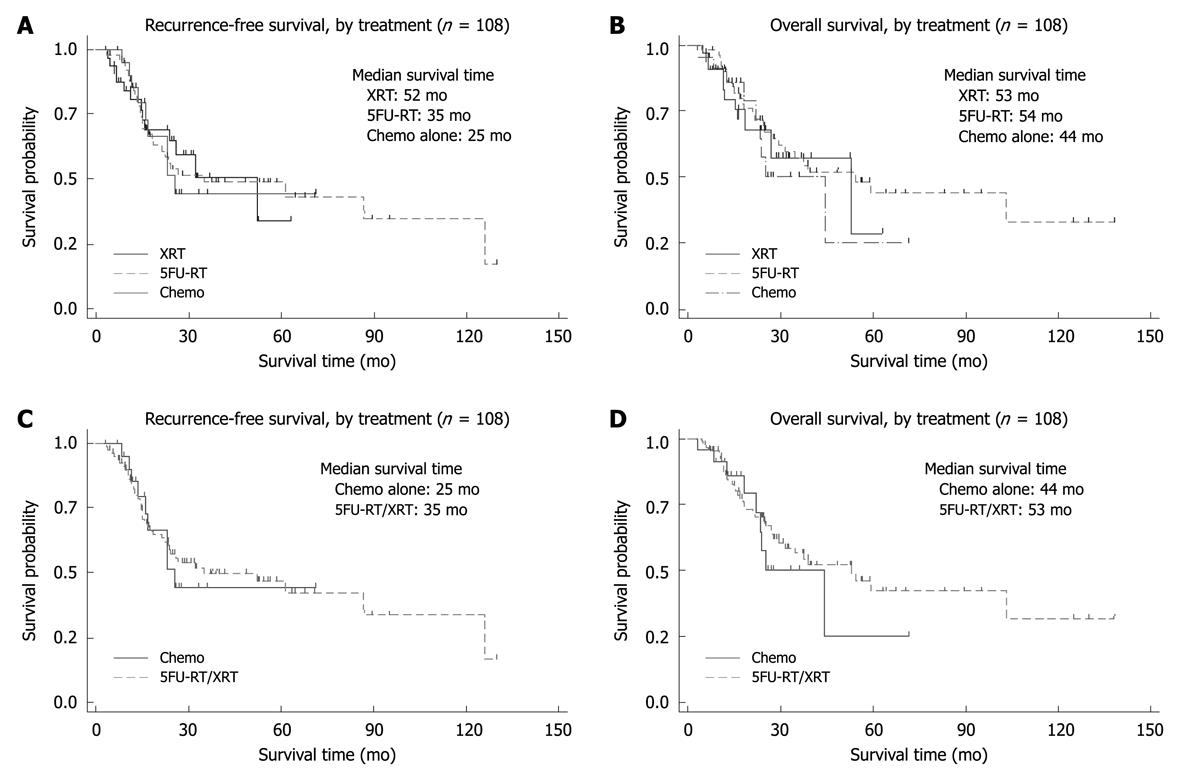
Median recurrence-free survival was longer for the XRT group (52 mo) compared with the 5FU-RT (35 mo) and chemotherapy-only groups (25 mo). However, the recurrence-free survival curves between the 3 groups were not statistically significant (P = 0.5, Figure 1A, Table 2). Patients with positive surgical margins were found to have a significantly poorer recurrence-free survival in both the XRT and 5FU-RT groups but not in the chemotherapy-only group (Table 3).
| Variable | Categories/units | n | HR (95% CI) | P-value |
| Recurrence-free survival | ||||
| Treatment | XRT | 108 | 1 | |
| 5FU-RT | 1.00 (0.508-1.959) | 1.0 | ||
| Chemo only | 1.05 (0.448-2.462) | 0.9 | ||
| Treatment | XRT | 108 | 1 | |
| 5FU-RT | 0.971 (0.477-1.966) | 0.9 | ||
| Chemo only | 1.031 (0.433-2.444) | 0.9 | ||
| Overall survival | ||||
| Treatment | XRT | 108 | 1 | |
| 5FU-RT | 0.83 (0.411-1.681) | 0.6 | ||
| Chemo only | 1.13 (0.474-2.677) | 0.8 | ||
| Treatment | XRT | 108 | 1 | |
| 5FU-RT | 0.891 (0.428-1.856) | 0.8 | ||
| Chemo only | 1.191 (0.494-2.882) | 0.7 | ||
| Variable | Categories/units | Chemo alone | 5FU-RT | XRT | |||
| HR (95% CI) | P-value1 | HR (95% CI) | P-value1 | HR (95% CI) | P-value1 | ||
| Recurrence-free survival | |||||||
| ECOG | 0 | 1 | 0.9 | 1 | 1.0 | E/N = 0/1 | 0.5 |
| 1 | 0.92 (0.115-7.422) | 1.01 (0.238-4.326) | NE | ||||
| Gender | Male | 1 | 0.9 | 1 | 0.2 | 1 | 0.5 |
| Female | 0.91 (0.244-3.419) | 1.58 (0.727-3.436) | 0.65 (0.198-2.163) | ||||
| Surgical margins | Positive | 1 | 0.5 | 1 | < 0.001 | 1 | 0.03 |
| Negative | 0.51 (0.059-4.351) | 0.16 (0.070-0.384) | 0.27 (0.081-0.918) | ||||
| Stage | 1 or 2 | 1 | 0.1 | 1 | 0.2 | 1 | 0.06 |
| 3 or 4 | 2.92 (0.724-11.773) | 1.86 (0.749-4.633) | 3.87 (0.853-17.586) | ||||
| Grade of tumor | 1, 2 | 1 | 0.3 | 1 | 0.09 | 1 | 0.3 |
| 3 | 2.17 (0.444-10.636) | 2.44 (0.836-7.099) | 1.94 (0.515-7.288) | ||||
| Overall survival | |||||||
| ECOG | 0 | 1 | 0.8 | 1 | 0.9 | E/N = 0/1 | 0.4 |
| 1 | 0.78 (0.096-6.366) | 0.95 (0.220-4.069) | NE | ||||
| Gender | Male | 1 | 1.0 | 1 | 0.2 | 8/22 | 0.7 |
| Female | 1.02 (0.253-4.148) | 1.63 (0.740-3.598) | 0.78 (0.234-2.597) | ||||
| Surgical margins | Positive | 1 | 0.004 | 1 | < 0.001 | 4/4 | 0.02 |
| Negative | 0.10 (0.013-0.700) | 0.26 (0.113-0.603) | 0.25 (0.071-0.901) | ||||
| Stage | 1 or 2 | 1 | 0.2 | 1 | 0.1 | 2/12 | 0.06 |
| 3 or 4 | 2.59 (0.646-10.412) | 2.08 (0.778-5.557) | 3.80 (0.829-17.397) | ||||
| Grade of tumor | 1, 2 | 1 | 0.4 | 1 | 0.06 | 3/12 | 0.4 |
| 3 | 2.04 (0.418-9.944) | 3.04 (0.903-10.239) | 1.83 (0.482-6.980) | ||||
The overall survival times of the patients in the 3 treatment groups were not statistically different (P = 0.5), with the median overall survival of the patients in the XRT group at 53 mo, the 5FU-RT group at 54 mo and the chemotherapy-only group at 44 mo (Figure 1B). Patients with positive surgical margins were found to have poorer survival across all the treatment groups (Table 3).
When comparing patients who received radiation as part of their adjuvant therapy to those who received chemotherapy alone, there was no statistical difference in recurrence-free survival and overall survival between these 2 groups of patients (Figure 1C and D). Subgroup analyses of patients with positive surgical margins, lymph node positive and T3 or T4 disease did not show a statistically difference in survival between patients who received radiation as part of their adjuvant therapy and those who did not (Table 4).
| Subgroup | Variable | Categories/units | n | Overall survival | Recurrence-free survival | ||
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||||
| Surgical margins = positive | Treatment | Chemo alone | 21 | 1 | 1 | ||
| 5FU-RT/XRT | 0.47 (0.100–2.191) | 0.3 | 1.58 (0.206-12.154) | 0.7 | |||
| Stage T3/4 | Treatment | Chemo alone | 66 | 1 | 1 | ||
| 5FU-RT/XRT | 0.65 (0.294–1.454) | 0.3 | 0.78 (0.352-1.709) | 0.5 | |||
| Node positive | Treatment | Chemo alone | 94 | 1 | 1 | ||
| 5FU-RT/XRT | 0.92 (0.404–2.081) | 0.8 | 1.10 (0.489-2.482) | 0.8 | |||
Treatment was generally well tolerated in the 3 treatment groups. In total only 6 patients (5%) had grade 3 or 4 toxicities, 4 in the 5FU-RT group, one in the XRT group and one in the chemotherapy-only group. Diarrhea was the most common grade 3 or 4 adverse effect. One patient in the XRT group had acute myocardial infarction during the therapy period. There was one death from non-neutropenic sepsis during the adjuvant treatment in the 5FU-RT group.
Our study showed that XRT was as effective as 5FU-RT and fluoropyrimidine-based chemotherapy when given as adjuvant treatment for locally advanced gastric cancer. XRT had comparable recurrence-free and overall survival with the other 2 adjuvant regimens.
The Intergroup-0116 study has established the role of adjuvant chemoradiation for resected locally advanced gastric cancer in the United States. Compared to surgery alone, the addition of chemoradiation after resection leads to an increased local control (30 mo vs 19 mo, P = 0.001) and better median overall survival (36 mo vs 27 mo, P = 0.005)[7]. In our study, the median overall survival for patients receiving XRT or 5FU-RT was 53 and 54 mo, respectively. This outcome when compared to the Intergroup trial is encouraging as 66% of patients in our study population had T3 or T4 disease and 86% had lymph node-positive disease, similar to the study population in the Intergroup study (68% and 85%, respectively). This difference could be explained by the differences in surgical techniques. All except 2 of our patients underwent D2 gastrectomies compared to only 10% in the Intergroup study. This may suggest that the adjuvant treatment could be a measure to compensate for inadequate surgical treatment. Nevertheless, the extent of lymph node dissection remains an ongoing debate with trials from the Dutch Gastric Cancer Group[8] and Medical Research Council[9] showing a lack of survival benefit of D2 over D1 lymph node dissection. However, the role of adjuvant chemoradiation in D2-resected gastric cancer had been studied in a Korean prospective non-randomized trial involving 544 patients receiving postoperative 5FU-radiation. This trial demonstrated significantly longer overall survival in the chemoradiation group compared to the surgery alone group (95.3 mo vs 62.6 mo, P = 0.02)[10]. Hence, there appears to be a role of adjuvant concurrent chemoradiation even in the setting of optimal surgical resection of gastric cancer.
Continuous infusion 5-FU was preferred over 5-FU bolus infusion in most of the gastrointestinal malignancies, especially colorectal cancer, and was also found to be more effective than the bolus 5-FU[11,12]. Continuous 5-FU infusion with radiation was used extensively in the neoadjuvant and adjuvant treatment of rectal cancer[13,14]. Oral capecitabine, in the metastatic and adjuvant setting, has been shown to be as effective as continuous 5-FU in gastrointestinal malignancies[2,12,15,16]. The ease of oral administration of capecitabine compared with the continuous infusion of 5-FU, which requires the placement of a central venous catheter, makes the use of capecitabine concurrent with radiotherapy an attractive option. Our results, albeit retrospective, showed an equivalent survival between XRT and 5FU-RT. Hence, XRT could be a reasonable alternative to 5FU-RT as adjuvant treatment in resected stomach cancer. Several phase I/II studies have already explored the addition of capecitabine with concurrent radiation[4,5] in adjuvant stomach cancer and shown it to be safe and tolerable.
Our analysis has shown that there was no survival difference between those who had radiation as part of their adjuvant therapy compared to those who did not. Even in patients with a positive surgical margin, the addition of radiation did not appear to significantly improve survival compared to adjuvant chemotherapy alone. The role of adjuvant chemotherapy had been studied in many phase III Western trials but the results were inconsistent in showing a survival benefit of adjuvant chemotherapy over surgery alone[17-20]. Meta-analyses of these trials suggest a potential absolute increase in 5-year survival of 2% to 4% with adjuvant chemotherapy in resected gastric cancer[21,22]. The Asian adjuvant trials had demonstrated more favorable results with a recent Japanese study on adjuvant S1 in resected stage II or III gastric cancer showing a significantly higher 3-year overall survival rate in the S1 group compared to the observation arm (80.1% vs 70.1%, P = 0.003)[23]. Hence, fluoropyrimidine-based chemotherapy may have a role in adjuvant treatment. However, there is currently no phase III trial that compared adjuvant chemoradiation and chemotherapy alone. Our study has shown that there is no survival difference between adjuvant chemoradiation and chemotherapy, suggesting that adjuvant chemotherapy alone may be a reasonable option of adjuvant therapy in resected gastric cancer. A study involving capecitabine alone for adjuvant therapy in gastric cancer will be an interesting follow-up study.
In conclusion, XRT as an adjuvant therapy in resected gastric cancer can achieve similar outcomes to that of 5FU-RT or chemotherapy. The result from our hypothesis-generating study provides the basis for a further prospective study in evaluating the role of radiation with concurrent capecitabine as adjuvant therapy in resected gastric cancers.
Gastric cancer is a major cause of cancer deaths in the world. The outcome of large gastric tumors and those with lymph node involvement remains poor after surgical resection. The optimal adjuvant therapy after surgical resection remains to be determined.
The most common strategies in the adjuvant treatment of gastric cancers include fluoropyrimidine-based chemotherapy with or without radiation. The introduction of capecitabine has largely replaced continuous-infusion 5-fluorouracil (5-FU) owing to its ease of administration. However, its efficacy is not proven in randomized phase III trials involving gastric cancers. In this retrospective review study, the authors examined the role of capecitabine with radiation and compared its efficacy to the 5-FU with radiation regimen and fluoropyrimidine-based chemotherapy alone.
This study showed that capecitabine with concurrent radiation was as effective as 5-FU with radiation or fluoropyrimidine-based chemotherapy alone without radiation when given as adjuvant treatment for locally advanced gastric cancer.
This hypothesis-generating study will provide the platform for a larger randomized study to be conducted using capecitabine as one of the study regimens in adjuvant gastric cancer trials.
Capecitabine and 5-FU are fluoropyrimidine-based chemotherapy commonly used in the treatment of gastrointestinal cancers.
This is a retrospective clinical study that looked at the effects of chemoradiation therapy by using capecitabine in patients undergoing gastric surgery for gastric cancer. The results are well presented and the discussion is well organized. The conclusions are supported by the data and the tables contain appropriate information.
Peer reviewers: Piero Marco Fisichella, MD, Assistant Professor of Surgery, Medical Director, Swallowing Center, Loyola University Medical Center, Department of Surgery, Stritch School of Medicine, 2160 South First Avenue, Room 3226, Maywood, IL 60153, United States; Dr. Takehiro Okabayashi, Department of Surgery, Kochi Medical School, Kohasu-Okocho, Nankoku-City, 783-8505, Japan
S- Editor Wang YR L- Editor Cant MR E- Editor Lin YP
| 1. | Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127-164. |
| 2. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. |
| 3. | Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666-673. |
| 4. | Jansen EP, Boot H, Saunders MP, Crosby TD, Dubbelman R, Bartelink H, Verheij M, Cats A. A phase I-II study of postoperative capecitabine-based chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2007;69:1424-1428. |
| 5. | Lee HS, Choi Y, Hur WJ, Kim HJ, Kwon HC, Kim SH, Kim JS, Lee JH, Jung GJ, Kim MC. Pilot study of postoperative adjuvant chemoradiation for advanced gastric cancer: adjuvant 5-FU/cisplatin and chemoradiation with capecitabine. World J Gastroenterol. 2006;12:603-607. |
| 6. | Kim DY, Jung KH, Kim TH, Kim DW, Chang HJ, Jeong JY, Kim YH, Son SH, Yun T, Hong CW. Comparison of 5-fluorouracil/leucovorin and capecitabine in preoperative chemoradiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2007;67:378-384. |
| 7. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. |
| 8. | Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908-914. |
| 9. | Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069-2077. |
| 10. | Kim S, Lim DH, Lee J, Kang WK, MacDonald JS, Park CH, Park SH, Lee SH, Kim K, Park JO. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279-1285. |
| 11. | Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis Group In Cancer. J Clin Oncol. 1998;16:301-308. |
| 12. | Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476-487. |
| 13. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. |
| 14. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. |
| 15. | Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, Bugat R, Findlay M, Frings S, Jahn M. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19:4097-4106. |
| 16. | Twelves C, Wong A, Nowacki MP, Abt M, Burris H 3rd, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696-2704. |
| 17. | Nitti D, Wils J, Dos Santos JG, Fountzilas G, Conte PF, Sava C, Tres A, Coombes RC, Crivellari D, Marchet A. Randomized phase III trials of adjuvant FAMTX or FEMTX compared with surgery alone in resected gastric cancer. A combined analysis of the EORTC GI Group and the ICCG. Ann Oncol. 2006;17:262-269. |
| 18. | Bajetta E, Buzzoni R, Mariani L, Beretta E, Bozzetti F, Bordogna G, Aitini E, Fava S, Schieppati G, Pinotti G. Adjuvant chemotherapy in gastric cancer: 5-year results of a randomised study by the Italian Trials in Medical Oncology (ITMO) Group. Ann Oncol. 2002;13:299-307. |
| 19. | Di Costanzo F, Gasperoni S, Manzione L, Bisagni G, Labianca R, Bravi S, Cortesi E, Carlini P, Bracci R, Tomao S. Adjuvant chemotherapy in completely resected gastric cancer: a randomized phase III trial conducted by GOIRC. J Natl Cancer Inst. 2008;100:388-398. |
| 20. | Bouché O, Ychou M, Burtin P, Bedenne L, Ducreux M, Lebreton G, Baulieux J, Nordlinger B, Martin C, Seitz JF. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801). Ann Oncol. 2005;16:1488-1497. |
| 21. | Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35:1059-1064. |
| 22. | Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, Cascinu S, Barni S, Labianca R, Torri V. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente). Ann Oncol. 2000;11:837-843. |
| 23. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. |