Published online Aug 7, 2010. doi: 10.3748/wjg.v16.i29.3680
Revised: January 7, 2010
Accepted: January 14, 2010
Published online: August 7, 2010
AIM: To investigate the interstitial cells of Cajal (ICC) number using a new rat model.
METHODS: Sprague-Dawley rats were assigned to two groups. The first group received gavage with Campylobacter jejuni (C. jejuni) 81-176. The second group was gavaged with placebo. Three months after clearance of Campylobacter from the stool, precise segments of duodenum, jejunum, and ileum were ligated in self-contained loops of bowel that were preserved in anaerobic bags. Deep muscular plexus ICC (DMP-ICC) were quantified by two blinded readers assessing the tissue in a random, coded order. The number of ICC per villus was compared among controls, Campylobacter recovered rats without small intestinal bacterial overgrowth (SIBO), and Campylobacter recovered rats with SIBO.
RESULTS: Three months after recovery, 27% of rats gavaged with C. jejuni had SIBO. The rats with SIBO had a lower number of DMP-ICC than controls in the jejunum and ileum. Additionally there appeared to be a density threshold of 0.12 DMP-ICC/villus that was associated with SIBO. If ileal density of DMP-ICC was < 0.12 ICC/villus, 54% of rats had SIBO compared to 9% among ileal sections with > 0.12 (P < 0.05). If the density of ICC was < 0.12 DMP-ICC/villus in more than one location of the bowel, 88% of these had SIBO compared to 6% in those who did not (P < 0.001).
CONCLUSION: In this post-infectious rat model, the development of SIBO appears to be associated with a reduction in DMP-ICC. Further study of this rat model might help understand the pathophysiology of post-infectious irritable bowel syndrome.
- Citation: Jee SR, Morales W, Low K, Chang C, Zhu A, Pokkunuri V, Chatterjee S, Soffer E, Conklin JL, Pimentel M. ICC density predicts bacterial overgrowth in a rat model of post-infectious IBS. World J Gastroenterol 2010; 16(29): 3680-3686
- URL: https://www.wjgnet.com/1007-9327/full/v16/i29/3680.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i29.3680
A recent series of studies suggested a link between small intestinal bacterial overgrowth (SIBO) and irritable bowel syndrome (IBS)[1,2]. The latest of these is a study that compared jejunal cultures between IBS patients and controls[3]. In that study, the number of coliform bacteria was higher in the small bowel of IBS compared to healthy controls.
Another potential bacterial pathogenesis in IBS is related to the development of IBS symptoms after acute gastroenteritis [post-infectious IBS (PI-IBS)]. In a recent meta-analysis, almost 10% of normal subjects developed IBS after an incident of bacterial gastroenteritis[4]. Initial research in this area focused on possible inflammatory events, as suggested by increased lymphocyte counts in the rectal mucosa[5-7]. Recent evidence has linked post-infectious events to the development of SIBO in a rat model of PI-IBS[8]. In this model, rats exposed to Campylobacter jejuni (C. jejuni) have persistent altered stool form that is linked to the development of SIBO in 27% of rats, based on quantitative polymerase chain reaction (PCR). This finding in rats suggests that, at least in the case of C. jejuni, a possible mechanism for PI-IBS could be the development of SIBO. How SIBO develops in this model is not known. In humans, it has been speculated that SIBO in IBS patients is related to a reduced number of fasting migrating motor complexes[9]. This has been a recognized cause of SIBO since 1977[10].
The important role of interstitial cells of Cajal (ICC) in gastrointestinal physiology has been elucidated over the past 10-20 years. ICC are required for normal intestinal motility. Their role as intestinal pacemakers has been established in a number of model systems[11-13]. Labeling ICC with anti-Kit antibodies provides an opportunity to evaluate ICC of gastrointestinal muscles in patients with various motility disorders. Reduced numbers or loss of ICC is associated with several motor dysfunctions, including achalasia, intestinal pseudoobstruction, infantile pyloric stenosis, diabetic gastroparesis, ulcerative colitis, and slow-transit constipation[14-19]. Loss of ICC also interferes with electrical pacemaker activity, slow-wave propagation, and gastrointestinal motor neurotransmission[12,13,20,21].
Given the significant role of ICC in modulating the neuromuscular activity of the gut, we sought to investigate whether the development of SIBO in the rat model infected with C. jejuni is associated with reduction in intestinal ICC.
After confirming no pre-existing presence of C. jejuni in their stools, 66 outbred Sprague-Dawley rats were randomized (in a 1:1 ratio) into two groups. One group was gavaged with a 1 mL solution containing C. jejuni 81-176 at 108 CFU/mL (C+ rats) in Brucella broth. The control rats were gavaged with an identical solution without C. jejuni (C- rats).
Following gavage, all the rats were housed at two per cage. However, the rats receiving Campylobacter (C+) were housed separately from the control (C-) rats to avoid the possibility of cross contamination of the C. jejuni infection between the two groups.
In the first 3 d after gavage, stool was collected from both groups to verify that intestinal colonization in C+ rats had occurred. It was also used to confirm the absence of infection in C- rats. Stool was later obtained in the C+ group on days seven and 14 after infection and then every 2 wk until two consecutive negative cultures for C. jejuni were confirmed.
After 90% of the C+ rats no longer had detectable C. jejuni in the stool, they were considered to be in the post-infectious time period. At this point, they were housed for three additional months. During this time, both groups were treated equally with respect to food, water, and environment. In the 3 d just prior to euthanasia (at 3 mo into the post-infectious period) stool was collected from each rat and graded blindly according to an a priori stool form grading score. This score was based on whether or not there was loose or normal stool in a blinded evaluation. Any loose stool was considered abnormal. The stool was also cultured to determine if there was any lingering case of Campylobacter (C+).
The C. jejuni 81-176 strain used in the gavage of the rats was obtained from freezer stocks, plated on selective media, and incubated for 36-48 h under microaerophilic conditions at 42°C to create a bacterial lawn. This lawn was then harvested from these plates and suspended in Brucella broth. The concentration of bacteria was estimated spectrophotometrically and confirmed via serial dilution and plating on selective media. In the 30 min prior to Campylobacter gavage, rats were gavaged with a 1 mL solution of 5% sodium bicarbonate using a ball-tipped inoculating needle. This was done to neutralize gastric acid to increase the likelihood of intestinal colonization of the pathogen. Subsequently, a 1 mL suspension of C. jejuni in Brucella broth (5 × 108 CFU/mL) was administered by gavage.
Three months after clearance of Campylobacter from the stool, fresh stool was obtained from all rats. This was used to determine the presence or absence of prolonged C. jejuni colonization. Stool was also qualitatively evaluated for stool form as described above.
Rats were then euthanized. Immediately following euthanasia, a laparotomy was performed whereby precisely determined segments of the ileum, jejunum, and duodenum were obtained, as previously described[8]. The first 2 cm segment at each location was a ligated self-contained loop of bowel. Sutures were placed on either side to prevent exposure to air. Samples were kept at 4°C in an anaerobic bag for transport. These self-contained loops were then used to extract bacterial contents for the determination of bacterial number using quantitative PCR, as previously described[8]. The quantity of bacteria in these segments was compared between control rats and rats 3 mo after recovery from Campylobacter. SIBO was considered to be present when bacterial count in the Campylobacter treated rats exceeded two standard deviations above the mean count found in the control group.
In each rat, a 2 cm segment of small bowel immediately adjacent to the closed loop of small bowel was resected and sent for bacterial quantitation. This second piece of bowel was opened longitudinally along the mesenteric border and placed in a solution of 10% formalin (VWR, West Chester, PA). After paraffin and mounting, the tissue was stained.
Rats were then divided into three groups based on the presence or absence of Campylobacter and SIBO. The three groups were a random selection of eight control rats (C-), eight Campylobacter-infected rats that were found to have bacterial overgrowth (C+/SIBO+), and eight randomly selected rats that received Campylobacter gavage but did not develop bacterial overgrowth (C+/SIBO-). Sections from each paraffin block were stained immunohistochemically using Polyclonal Rabbit Anti-Human CD117, c-kit (DakoCytomation, Carpinteria, CA). The positive control used to test the quality of the stain was a c-kit positive gastrointestinal stromal tumor. ICC were quantified by two blinded readers assessing the tissue in a random, coded order. The number of ICC was evaluated in the region of the deep muscle plexus ICC (DMP-ICC) according to the number of villi. The number of DMP-ICC per villus was compared among controls, C+/SIBO-, and C+/SIBO+.
Statistical comparisons for the number of DMP-ICC per villus were made by one-way analysis of variance among three groups and differences from controls was further analyzed using the Wilcoxon Rank-Sum Test. To compare the density threshold of ICC per villus, a Fisher’s exact test was used. A P value of <0.05 was considered significant.
Out of 66 rats used in the study, none had stool culture demonstrating C. jejuni before gavage. Of these rats, 33 were gavaged with vehicle (C-) and 33 rats received approximately 5 × 108 CFU C. jejuni 81-176 (C+). Six rats died of trauma secondary to gavage (three in each group). Among the remaining 30 rats that received C. jejuni, all had stool cultures that confirmed intestinal colonization by C. jejuni, and all but one rat cleared this colonization by 14 d.
As previously reported[8], C- rats were then used to determine the normal range of flora in the duodenum, jejunum, and ileum. Using these control data, eight C+ rats (27%) were found to have SIBO and were designated C+/SIBO+.
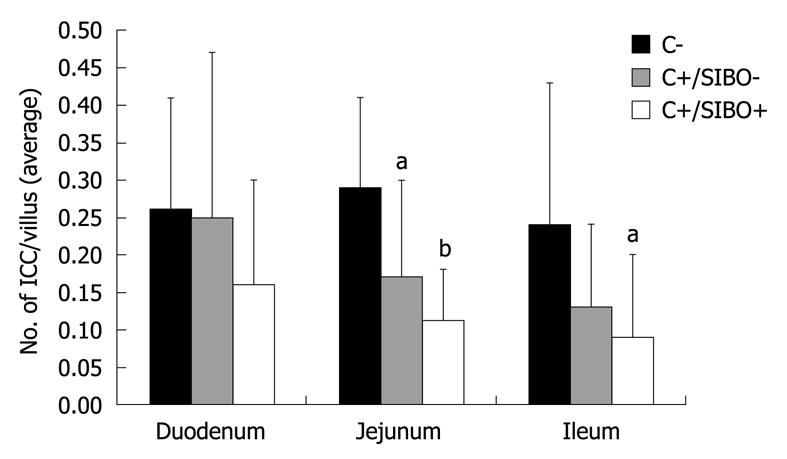
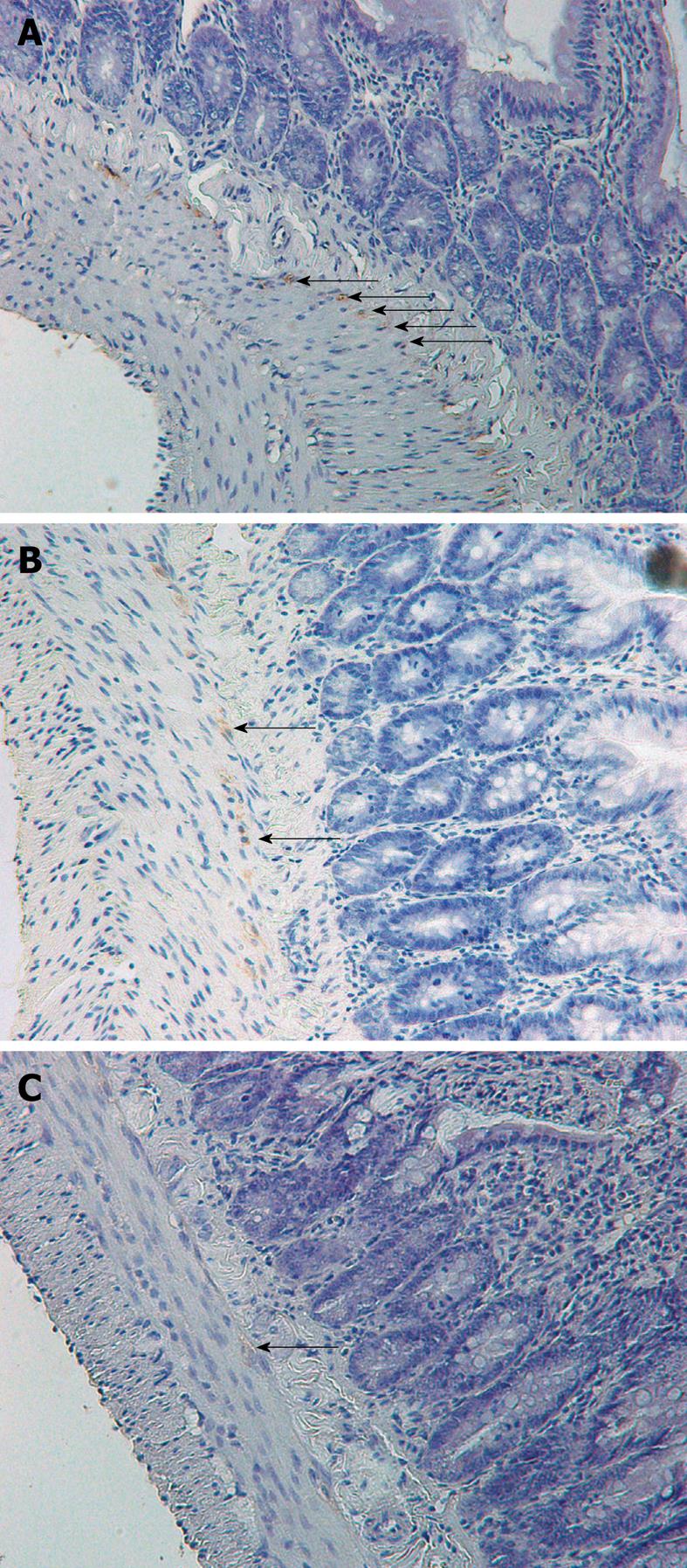
The number of DMP-ICC per villus was determined in C-, C+/SIBO-, and C+/SIBO+ rats. As shown in Figure 1, the rats with SIBO had the lowest number of DMP-ICC. This was more obvious in the jejunum and ileum than in the duodenum. Although there was a reduction of ICC in the C+/SIBO- group, this was not as great as was seen in the C+/SIBO+ group. Figure 2 shows representative examples of ileal biopsies in the C-, C+/SIBO-, and C+/SIBO+ groups, respectively. There was a reduced number of CD117 stained cells in the deep muscular plexus in the C+/SIBO+ group.
The number of DMP-ICC was then used to determine the level of ICC compared to SIBO. The data suggested that rats with < 0.12 ICC/villus were most likely to have SIBO. In fact, 54% of rats with a density < 0.12 ICC/villus in the ileum had SIBO compared to 9% in which DMP-ICC density was ≥ 0.12/villus (P < 0.05). Using all levels of bowel, the density threshold was even more relevant. If the density of DMP-ICC/villus was < 0.12/villus in more than one of the three bowel segments, 88% had SIBO compared to 6% if the figure was ≥ 0.12/villus (P < 0.001).
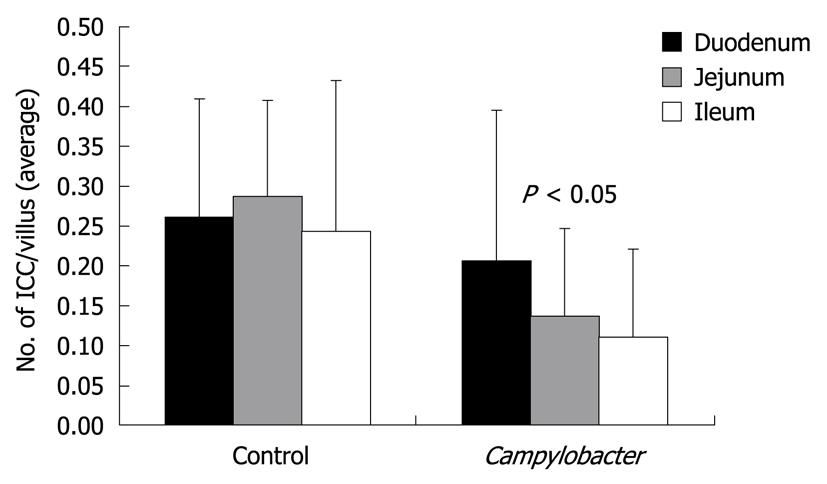
When the number of DMP-ICC was noted by segment of bowel according to whether rats received vehicle or Campylobacter, a significant difference was seen (Figure 3). There was a graded affect on DMP-ICC population, such that the greatest effect on reduction in DMP-ICC was in the distal small bowel. Reduction was also seen in the jejunum and ileum. This contrasted to the control group, whereby DMP-ICC density was uniform from proximal to distal small bowel.
In a previous study, we demonstrated that in a post-infectious rat model, altered bowel function persisted in a large number of rats even 3 mo beyond the clearance of the infection[8]. 27% of rats were found to have SIBO by quantitative PCR. SIBO correlated with those animals that had the most altered stool form. In this study, we attempted to further understand how SIBO could develop in this post-infectious rat model by studying the ICC. We found that long after Campylobacter infection had cleared, there was an apparent reduction in the number of DMP-ICC. This reduction appeared most evident in rats that developed SIBO after clearing Campylobacter. Furthermore, when the DMP-ICC density is less than 0.12/villus, SIBO is predicted to occur.
Six to seventeen percent of IBS patients believe their symptoms began after acute gastroenteritis[22], and the incidence of PI-IBS following bacterial gastroenteritis is reported to be between 4%-31%[7,23-26]. In a 6-year follow up study, 57% of subjects thought to have developed PI-IBS still had altered bowel function consistent with IBS[27]. This growing body of literature on PI-IBS has led to two recent meta-analyses[4,28]. Both studies estimated that IBS has an incidence rate of about 10% after a case of acute gastroenteritis.
Two issues make PI-IBS very important in research on IBS pathophysiology. First, acute gastroenteritis is very common, and with a 10% rate of IBS development, this phenomenon might be very important in the development of IBS as a whole. Secondly, this is the first demonstration of a direct cause and effect relationship in the precipitation of IBS. How an acute gastrointestinal infectious process produces IBS in humans remains poorly understood, but some investigators have found residual inflammatory changes in the gut among patients with PI-IBS. These include increased numbers of lymphocytes, enteroendocrine cells, and mast cells in PI-IBS[5-7,26,29,30]. It is difficult to guarantee uniformity of population and pathogen in human studies of PI-IBS as they rely on studying a group of humans who emerge from an outbreak of infectious diarrhea. As a result, the study of inflammation and other factors in PI-IBS have been moving into animal models of PI-IBS.
One model that has been studied for some time is a Trichinella spiralis (T. spiralis)-infected mouse model. Beyond inflammatory changes, research in this model has looked at the long term effect on gastrointestinal motility. A major finding in this model was that, after resolution of the initial pathogen-related inflammatory response and elimination of the nematode from the gut, there was persistent intestinal neuromuscular dysfunction[31]. T. spiralis-induced mucosal inflammation led to prolonged effects on intestinal smooth muscle, and to colonic visceral hyperalgesia[32,33]. Although models such as this are vital to our understanding of PI-IBS, no model had been developed to investigate the more common human pathogens believed to cause the bulk of PI-IBS. These include Campylobacter, Salmonella, and Shigella, among others[34].
Two existing theories of bacterial events (post-infectious and SIBO) now appear to be linked, based on a recent study suggesting that C. jejuni infection precipitates altered stool form and SIBO in a rat model[8]. How this occurs is currently unknown, although one study has demonstrated that acute C. jejuni infection alters intestinal myoelectric activity. In ileal segments of rabbits, alteration of action potential activity was seen in the small intestine infused intraluminally with C. jejuni and its cell-free filtrate[35]. In that study, the rabbits were monitored for only 24 h after exposure to C. jejuni. Unfortunately, no long term studies were done, and the cause of these effects was unknown.
An important cell in the control of gut motor function is the ICC. A growing list of animal models appears to support the notion that ICC are impaired in the presence of inflammation-induced changes in motor control. With acetic acid-induced inflammation, a reduction in resting membrane potential and the amplitude and duration of slow waves was related to the damage of the ICC in circular muscle cells in dogs[36]. In another study using the earlier described T. spiralis-model, damage to the structure of ICC networks within the region of the myenteric plexus was seen[32]. When jejunal inflammation was induced by Nippostrongylus brasiliensis in rats, changes in myenteric neurons, circular muscle cells, and ICC were observed[37].
In this study, we investigated whether the development of SIBO in the postinfectious rat model is related to the alteration of DMP-ICC populations, and whether this could in some way be linked to the development of IBS. In this study, Campylobacter gavaged rats were found to have a reduction of DMP-ICC density 3 mo after clearance of Campylobacter. More interesting was that the DMP-ICC density also corresponded to the development of SIBO. Rats with SIBO had the lowest DMP-ICC density. We could further evaluate a density threshold of DMP-ICC per villus which predicts SIBO. If the density of DMP-ICC/villus was not greater than 0.12 in more than one location among duodenum, jejunum, and ileum, 88% of the rats had SIBO. Although this finding does not examine the numerous contributions to gut motor function in addition to ICC, the possibility is raised that through some effects on ICC or neuromuscular mechanisms, acute gastroenteritis produces a long term effect that allows for the development of SIBO.
How Campylobacter affects DMP-ICC is unknown, but two possibilities include a toxin or a result of the initial acute inflammatory reaction. Although inflammation seems to be an obvious possibility, ICC’s have a significant amount of “plasticity”. Loss of ICC in pathological conditions does not always mean permanent injury. This “plasticity” of ICC was found in a murine model of partial bowel obstruction[38]. After the onset of the obstruction, hypertrophy of the smooth muscle layers and progressive loss of ICC orad to the site of obstruction were observed. Recovery of ICC and restoration of slow wave activity after removal of the obstruction were achieved within 30 d. In addition, injury to ICC due to inflammation is repaired in the course of time. Structural damage was observed in the network of ICC for 2 wk after T. spiralis infection. The structural changes were accompanied by aberrant pacemaker activity and abnormal slow waves. Sixty days after infection, motility and ICC recovered to normal values[32]. Unlike these two studies, we found a persistent decrease in the number of DMP-ICC 3 mo after clearance of infection. This was not due to any persistent Campylobacter colonization, as the pathogen was not detectable by culture in any location of the gastrointestinal tract. However, it is presumed that some event related to Campylobacter is responsible for this persistent reduction of DMP-ICC. This particular layer of ICC might be important. For example, in the W/Wv mouse, the DMP is intact and, despite loss of all other layers of ICC and loss of electrical slow wave, the migrating motor complex also remains intact[39]. Thus, DMP destruction might have an impact on mechanisms that protect against bacterial overgrowth.
An alternative explanation would be that SIBO is contributing to the reduction of ICC. Although not considered in this study, one means of determining this would be to provide antibiotic treatment to rats and count ICC after eradication of SIBO. The challenge with examining this concept is that it is difficult to identify SIBO in a live rat. This would make it difficult to know which animal should receive antibiotics.
It can be considered that ICC is just a marker of more global damage. In our study, smooth muscle was not evaluated. In a mouse model after acute nematode infection, altered neuromuscular function and long-lasting muscle contractility were noted[31,33]. The findings were considered as a model of PI-IBS. Further studies are needed to evaluate smooth muscle, toxin, and the effect of inflammation with Campylobacter infection.
In conclusion, rats with SIBO that developed 3 mo after Campylobacter gavage had a decreased number of ICC in the jejunum and ileum compared to control rats. Furthermore, the density threshold of ICC per villus appears to predict SIBO. These data suggest that a decrease in the number of ICC in the small intestine is implicated in the pathogenesis of PI-IBS. Elucidating which Campylobacter-related factor produces this decrease in the number of ICC may contribute greatly to our understanding of PI-IBS and lead to potential treatments for IBS.
There is a growing interest in understanding newly discovered bacterial mechanisms in the pathophysiology of functional bowel disease. These mechanisms might result innovative treatments for diseases such as irritable bowel syndrome (IBS).
Two bacterial hypotheses have emerged in IBS. The first hypothesis is that IBS appears to develop in humans after an episode of acute gastroenteritis. The other bacterial hypothesis is that a proportion of patients who already have IBS appear to have small intestinal bacterial overgrowth (SIBO) and improve with antibiotic therapy.
Recently, a new animal model of post-infectious IBS has been developed on the basis of a common human pathogen, Campylobacter jejuni (C. jejuni). In this model, IBS-like symptoms appear to develop in rats 3 mo after clearance of the initial infection. At this time, a proportion of these rats have bacterial overgrowth, based on quantitative polymerase chain reaction. These data now link acute gastroenteritis to the development of bacterial overgrowth and symptoms in an animal model.
This new animal model will facilitate the discovery of the cascade of events that lead to IBS and SIBO. One candidate in the cascade is likely to be an effect on the neuromuscular apparatus of the gut.
Post-infectious IBS is the development of IBS after a self-limited infection of the intestine, such as acute gastroenteritis. Interstitial cells of Cajal (ICC) are nerve cells in the intestinal lining that are important for maintaining the function of the gut.
In this manuscript, the authors investigate the potential role of a decreased number of ICC in causing SIBO after a C. jejuni infection in rats. The paper is well written and is pleasant to read. The experiments have been carefully designed and executed.
Peer reviewer: Kristin Verbeke, PhD, Professor, Laboratory Digestion and Absorption, University Hospital Leuven, E462, Herestraat 49, B-3000 Leuven, Belgium
S- Editor Wang YR L- Editor Stewart GJ E- Editor Zheng XM
| 1. | Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503-3506. |
| 2. | Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412-419. |
| 3. | Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802-808. |
| 4. | Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome--a meta-analysis. Am J Gastroenterol. 2006;101:1894-1899; quiz 1942. |
| 5. | Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400-406. |
| 6. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. |
| 7. | Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651-1659. |
| 8. | Pimentel M, Chatterjee S, Chang C, Low K, Song Y, Liu C, Morales W, Ali L, Lezcano S, Conklin J. A new rat model links two contemporary theories in irritable bowel syndrome. Dig Dis Sci. 2008;53:982-989. |
| 9. | Pimentel M, Soffer EE, Chow EJ, Kong Y, Lin HC. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci. 2002;47:2639-2643. |
| 10. | Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59:1158-1166. |
| 11. | Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci USA. 1989;86:7280-7284. |
| 12. | Ordög T, Ward SM, Sanders KM. Interstitial cells of cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518:257-269. |
| 13. | Der-Silaphet T, Malysz J, Hagel S, Larry Arsenault A, Huizinga JD. Interstitial cells of cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology. 1998;114:724-736. |
| 14. | Goldblum JR, Whyte RI, Orringer MB, Appelman HD. Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol. 1994;18:327-337. |
| 15. | Rumessen JJ. Ultrastructure of interstitial cells of Cajal at the colonic submuscular border in patients with ulcerative colitis. Gastroenterology. 1996;111:1447-1455. |
| 16. | Streutker CJ, Huizinga JD, Campbell F, Ho J, Riddell RH. Loss of CD117 (c-kit)- and CD34-positive ICC and associated CD34-positive fibroblasts defines a subpopulation of chronic intestinal pseudo-obstruction. Am J Surg Pathol. 2003;27:228-235. |
| 17. | Vanderwinden JM, Liu H, De Laet MH, Vanderhaeghen JJ. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology. 1996;111:279-288. |
| 18. | Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731-1739. |
| 19. | Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G, Salerni B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41-46. |
| 20. | Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97-111. |
| 21. | Ward SM, Harney SC, Bayguinov JR, McLaren GJ, Sanders KM. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. J Physiol. 1997;505:241-58. |
| 22. | Longstreth GF, Hawkey CJ, Mayer EA, Jones RH, Naesdal J, Wilson IK, Peacock RA, Wiklund IK. Characteristics of patients with irritable bowel syndrome recruited from three sources: implications for clinical trials. Aliment Pharmacol Ther. 2001;15:959-964. |
| 23. | Gwee KA, Graham JC, McKendrick MW, Collins SM, Marshall JS, Walters SJ, Read NW. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150-153. |
| 24. | Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ. 1997;314:779-782. |
| 25. | Thornley JP, Jenkins D, Neal K, Wright T, Brough J, Spiller RC. Relationship of Campylobacter toxigenicity in vitro to the development of postinfectious irritable bowel syndrome. J Infect Dis. 2001;184:606-609. |
| 26. | Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096-1101. |
| 27. | Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut. 2002;51:410-413. |
| 28. | Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535-544. |
| 29. | O'Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O'Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449-457. |
| 30. | Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778-1783. |
| 31. | Barbara G, Vallance BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology. 1997;113:1224-1232. |
| 32. | Der T, Bercik P, Donnelly G, Jackson T, Berezin I, Collins SM, Huizinga JD. Interstitial cells of cajal and inflammation-induced motor dysfunction in the mouse small intestine. Gastroenterology. 2000;119:1590-1599. |
| 33. | Bercík P, Wang L, Verdú EF, Mao YK, Blennerhassett P, Khan WI, Kean I, Tougas G, Collins SM. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology. 2004;127:179-187. |
| 34. | Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662-1671. |
| 35. | Sninsky CA, Ramphal R, Gaskins DJ, Goldberg DA, Mathias JR. Alterations of myoelectric activity associated with Campylobacter jejuni and its cell-free filtrate in the small intestine of rabbits. Gastroenterology. 1985;89:337-344. |
| 36. | Lu G, Qian X, Berezin I, Telford GL, Huizinga JD, Sarna SK. Inflammation modulates in vitro colonic myoelectric and contractile activity and interstitial cells of Cajal. Am J Physiol. 1997;273:G1233-G1245. |
| 37. | Faussone-Pellegrini MS, Gay J, Vannucchi MG, Corsani L, Fioramonti J. Alterations of neurokinin receptors and interstitial cells of Cajal during and after jejunal inflammation induced by Nippostrongylus brasiliensis in the rat. Neurogastroenterol Motil. 2002;14:83-95. |