Published online Jul 28, 2010. doi: 10.3748/wjg.v16.i28.3561
Revised: April 6, 2010
Accepted: April 13, 2010
Published online: July 28, 2010
AIM: To investigate survival in patients treated with FOLFOX followed by primary site resection or palliative surgery for incurable metastatic colorectal cancer.
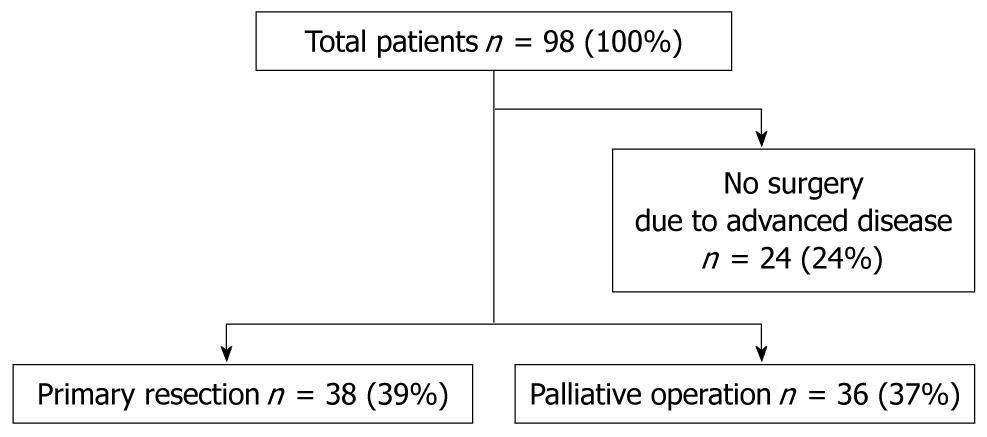
METHODS: Between 2001 and 2009, a total of 98 patients with colorectal adenocarcinoma and non-resectable metastases were diagnosed and treated with the new systemic agent chemotherapy regimen FOLFOX. Primary site resection was carried out in 38 patients, creation of a colostomy or bypass without resection was carried out in 36 patients, and 23 were not operated on because of advanced disease. The survival times of patients in different groups were analyzed.
RESULTS: There were no differences between the patients regarding their general condition, concurrent disease, or tumor stage according to AJCC classification. The median survivals of the three groups were 30.6, 20.8, and 12.7 mo (log-rank P value < 0.05), respectively. The postoperative complication rate was higher in the primary site resection group than in the palliative surgery group.
CONCLUSION: The results indicate that there are benefits from primary site resection for incurable metastatic colorectal cancer with systemic chemotherapy.
- Citation: Tanoue Y, Tanaka N, Nomura Y. Primary site resection is superior for incurable metastatic colorectal cancer. World J Gastroenterol 2010; 16(28): 3561-3566
- URL: https://www.wjgnet.com/1007-9327/full/v16/i28/3561.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i28.3561
Colorectal cancer (CRC) is the most common cancer worldwide. The estimated 5-year survival rate ranges from 90% in patients with stage 1 disease to 10% in those with metastatic CRC (mCRC). Approximately 20% of patients with primary CRC present with stage 4 metastatic disease. For many years, the standard treatment for patients with mCRC has been systemic chemotherapy with fluorouracil (FU) and combination therapy. The standard treatment remains 5-FU based. In the past five years, chemotherapy has progressed dramatically and shifted from FU to the newer agents irinotecan, oxaliplatin, bevacizumab, and cetuximab. Patients treated with oxaliplatin, 5-fluorouracil, and leucovorin (FOLFOX) displayed a median survival of 19.5 mo which is the longest survival reported in a phase 3 trial[1]. Data from this and other trials suggest that patient survival might be prolonged with adjuvant chemotherapy; therefore the role of surgical treatment in these patients is controversial.
In localized (non-metastatic) disease, surgery is the primary treatment and can be curative. On the other hand, surgical resection of the primary lesion for patients with mCRC is indicated mainly to manage symptoms such as obstruction, perforation, or bleeding, but is of uncertain benefit in the absence of these symptoms. Patient-dependent factors, such as age, medical comorbidity, extent of distant metastases, and localized invasion are among the factors that influence the decision to perform elective resection. The possible benefit of chemotherapy in conjunction with surgical management is unproven.
This study was designed to compare long-term survival and perioperative outcome in patients with incurable metastatic CRC treated with FOLFOX followed by either primary lesion resection, or palliative surgical management (colostomy or bypass).
This retrospective, observational study was done using our hospital cancer database. From 2005 to 2009, all patients who underwent systemic treatment with FOLFOX for CRC at our hospital were identified and reviewed. Patients with non-resectable CRC at the time of diagnosis or operation were included in the study. Patients were excluded if they had metachronous metastasis from previous completely resected CRC, or they could not undergo any operation due to severe comorbidity, progression of cancer, or unwillingness to accept surgery. The remaining patients were stratified into two groups. In addition to chemotherapy, patients undergoing resection of their primary lesion were included in the primary site resection group, whereas those who underwent a bypass or stoma operation without resection for relief of obstruction or bleeding were included in the palliative surgery group. Demographics, comorbidity, and cancer-specific information were evaluated for each patient, including tumor location, grade, lymph node metastasis, liver, lung and peritoneal metastasis, survival, and mortality. Comorbidity was graded using the Charlson index. The location and volume of metastatic disease were evaluated by computer tomography and by direct visualization during surgery. Each tumor stage was coded according to the TNM classification as described in the AJCC. Patients with hepatic metastatic disease were further classified according to the volume of hepatic parenchymal replacement: < 25% (H1), 25 to 50% (H2), and > 50% replacement (H3). Patients with pulmonary and peritoneal involvement were stratified according to the appearance of their CT scan’s (Table 1).
| Parameter | Total | Primary resection | Palliative surgery |
| Total cohort | 99 | 38 (100) | 36 (100) |
| Age (median, range) | 61.5 | 61 (50-79) | 61 (31-83) |
| Sex | |||
| M | 39 | 20 (53) | 19 (53) |
| F | 36 | 19 (50) | 17 (47) |
| Comorbidity1 | |||
| 0 | 57 | 28 (74) | 29 (81) |
| 1 | 12 | 8 (21) | 4 (11) |
| 2 | 5 | 2 (5) | 3 (8) |
| 3 | 1 | 1 (3) | 0 (0) |
| Primary site | |||
| Cecum | 3 | 2 (5) | 1 (3) |
| Ascending colon | 12 | 8 (21) | 4 (11) |
| Transverse colon | 6 | 2 (5) | 4 (11) |
| Descending colon | 6 | 5 (13) | 1 (3) |
| Sigmoid colon | 12 | 5 (13) | 7 (19) |
| Rectum | 35 | 16 (42) | 19 (53) |
| Stage2 | |||
| T | |||
| 1 | 0 | 0 (0) | 0 (0) |
| 2 | 16 | 10 (26) | 6 (17) |
| 3 | 29 | 20 (53) | 9 (25) |
| 4 | 29 | 8 (21) | 21 (58) |
| N | |||
| 0 | 18 | 8 (21) | 10 (28) |
| 1 | 31 | 19 (50) | 12 (33) |
| 2 | 35 | 11 (29) | 14 (39) |
| H3 | |||
| 0 | 31 | 11 (29) | 20 (56) |
| 1 | 10 | 5 (13) | 5 (14) |
| 2 | 7 | 5 (13) | 2 (6) |
| 3 | 26 | 17 (45) | 9 (25) |
| Lung metastasis | |||
| Yes | 23 | 12 (32) | 11 (31) |
| No | 51 | 26 (68) | 25 (69) |
| Peritoneal metastasis | |||
| Yes | 22 | 10 (26) | 12 (33) |
| No | 52 | 28 (74) | 24 (67) |
The FOLFOX4 protocol of systemic chemotherapy is as follows: Leucovorin (LV; 200 mg/m2 per day in a 2-h infusion), followed by bolus 5-FU (400 mg/m2 per day), and 600 mg/m2 5-FU daily in a 22-h infusion, day 1 and 2 every 2 wk, plus oxaliplatin, 85 mg/m2 (2-h infusion) on day 1, were administered through an implantable port and a disposable or electronic pump. Treatment was continued until progressive disease occurred (PD) or unacceptable toxicity occurred, or until the patient chose to discontinue treatment.
The principal outcome was survival. Survival was defined as the time from initiation of treatment, either chemotherapy or surgical operation, to the time of death. For patients who were alive at the time of analysis, data on survival were censored at the time of the last contact. The primary statistical analysis compared survival times between the two groups. Secondary analysis was conducted by comparing subgroups after excluding T4 patients. The Kaplan-Meier method was used to estimate actual control and survival curves. The log-rank test was used to evaluate the survival difference between two groups. All the P values were 2-sided and the level of significance was set at 0.05. The statistical analysis was conducted using JMP version 6.
Between 2005 and 2009, 172 patients were treated with FOLFOX in our hospital. Of these, 98 patients had metastatic, locally advanced non-resectable CRC. Of these, 38 patients underwent initial resection of only their primary lesion without concomitant hepatic or pulmonary metastasectomy. Thirty-six patients did not undergo resection of their primary lesion, but had a palliative operation to alleviate symptoms of bleeding and obstruction, and 24 patients were excluded because they were not surgical candidates. This last group had a poor prognosis, with a median survival of 12.7 mo (Figure 1). Median age of the primary site resection group was 61 years (range 50-79 years) compared to 61 years (range 31-83 years) in the palliative surgery group. Primary tumor sites in both groups are shown in Table 1. There were no significant differences in the distribution of age, sex, primary site location, comorbidity, and metastatic disease, except for the T factor. Patients in the palliative surgery group were found to have more invasive and higher T factor lesions compared with the primary site resection group.
All patients were initially treated with FOLFOX4. The median duration of FOLFOX was 10 courses in primary site resection group and 11 courses in the palliative surgery group. The corresponding rates of response were 23% and 22%, respectively (P = 0.48). A total of 26 patients changed treatment to FOLFIRI (Irinotecan, Folinic acid, and 5-FU), 12 primary site resection patients and 14 palliative surgery patients. Bevacizumab and cetuximab were available from 2008. Four patients in each group were candidates for this new agent, and received the treatment without serious complications.
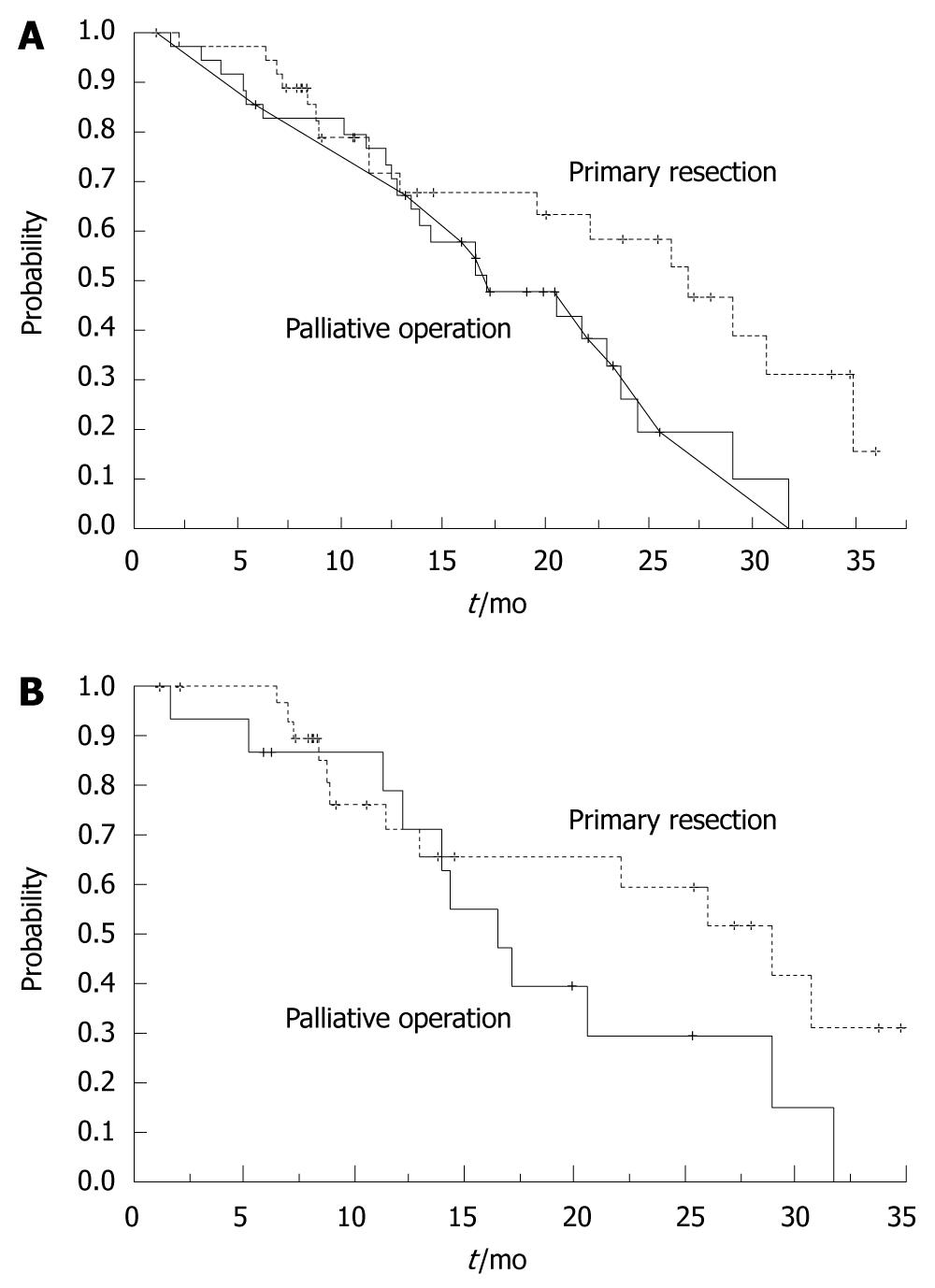
Median survival time for the primary site resection group was 30.6 ± 7.8 mo, whereas in the palliative surgery group was 20.8 ± 10.2 mo. These median survival times were significantly different (P = 0.0094, log-rank test). The two-year actuarial survival in the primary site resection group was 67.2%, compared to 31.9% in the palliative surgery group (Figure 2A). We re-examined the mortality of the two groups to exclude the influence of localized invasion. After excluding all T4 patients, we found longer survival and higher rate of two-year survival in the primary site resection group. The median survival time was 30.6 ± 10.3 mo vs 17.3 ± 8.5 mo (P = 0.0149, log-rank test). Figure 2B shown the overall survival curves by treatment of primary resection or palliative surgery with FOLFOX (Modified group of T2-3 patients).
The operations performed are listed in Table 2. Of these, eight (21%) were emergent surgery in the primary site resection group and 10 (27%) in the palliative surgery group. In the primary site resection group, 16 of 38 patients experienced one or more postoperative complications vs 12 of 36 patients in the palliative surgery group. This relatively higher complication rate resulted in longer hospitalization (22.7 ± 12.9 d vs 18.1 ± 7.9 d) in the primary site resection group (Table 3). The two most common complications were wound infection and prolonged ileus, followed by urinary tract infection, pneumonia, catheter infection, gastric hemorrhage, and liver failure. In the primary site resection group, two patients (5.3%) developed anastomotic leaks and required reoperation for drainage and diversion. Among the patients who had undergone palliative surgery, two complications (5.5%) related to the primary tumor (one perforation and one bleeding) were observed. Both were managed conservatively.
| Group | Procedure | n |
| Primary resection | Resection with anastomosis | 27 |
| Resection with anastomosis + stoma | 3 | |
| Resection without anastomosis | 8 | |
| Palliative operation | Creation of stoma | 27 |
| Stoma + bypass | 3 | |
| Bypass | 6 |
| Postoperative morbidity | Primary resection | Palliative surgery |
| Wound infection | 6 | 5 |
| Prolonged ileus | 4 | 1 |
| Intraabdominal/pelvic infection | 1 | 2 |
| Urinary tract infection | 3 | 0 |
| Anastomotic leak | 2 | 0 |
| Pneumonia | 0 | 1 |
| Catheter infection | 1 | 0 |
| Gastric hemorrhage | 1 | 0 |
| Liver failure | 0 | 1 |
| Perforation of cancer | 0 | 1 |
| Bleeding from cancer | 0 | 1 |
| Operational death | 0 | 1 |
| Total | 18 | 13 |
| Hospitalization (d) (P = 0.05) | 22.7 ± 12.9 | 18.1 ± 7.9 |
The treatment of patients who present with mCRC is controversial. Although resection has often been advocated to eliminate the source of symptoms such as bleeding, perforation and obstruction, management of asymptomatic patients has not been well defined. The purpose of our study was to define optimal primary tumor management in this subset of patients with metastatic disease. Our study demonstrated that the resection of the primary site significantly extends the median survival time in incurable CRC patients. Previous authors have addressed similar issues focusing in appropriate selection of surgical candidates, potential benefits of resection, and rates of operative morbidity and mortality. Some authors recommended resection to potentially improve survival[1-4].
Ruo et al[2] found a median survival of 16 mo in those undergoing resection and 9 mo in those never resected. Liu et al[3] also presented a retrospective series of 68 cases with longer mean survival of 10.6 mo in the resection group compared with 3.4 mo in patients who had a bypass. These findings are in agreement with our results. Others have reported similar results in terms of mortality between primary site resection and non-operative management, and recommend non-operative management of patients with metastatic CRC with synchronous metastases[5-7].
Charles et al[5] suggested that appropriately selected patients with incurable stage 4 CRC can be safely managed without primary tumor resection, with a median survival of 16.6 mo for the non-resection group. Johnson et al[7] found that in patients with rectal cancer and non-resectable liver metastases, there was no significant difference in cancer-specific survival after palliative resection or colostomy. Supporters of non-operative management argue that as metastatic tumor burden is the life-limiting factor, protracted postsurgical recovery might delay initiation of therapy targeting the disseminated disease, resulting in decreased survival. However, these studies have not addressed the effects of adjuvant chemotherapy including FOLFOX and FOLFIRI. In the study of George et al[2] of 233 patients with synchronous stage IV CRC treated by FOLFOX and FOLFIRI, 93% were managed non-operatively with 18 mo median overall survival. In comparison with our results, they might have obtained better outcomes using primary tumor resection, despite the patients’ symptoms.
We believe that the role of primary resection in mCRC in the new era of chemotherapy and in the setting of incurable metastatic disease should be re-examined. We thought that new chemotherapies, including FOLFOX and FOLFIRI, would improve patients’ prognosis and provide additional treatment options, including surgical resection. Indeed, the combination of oxaliplatin or irinotecan with 5-FU and leucovorin has proved to significantly increase response rates, prognosis, and survival compared with previous chemotherapies, such as 5-FU+LV[8-10], oxaliplatin alone[11] and IFL[1]. To compare the efficacy of FOLFOX and FOLFIRI, Tournigand et al[12] and Colucci et al[13] performed randomized trials in which patients received either FOLFOX followed by FOLFORI, or vice versa. Both investigators concluded that the regimens had similar efficacy when used as first-line therapy (median progression-free survival of 8 mo). Therefore FOLFOX followed by FOLFORI was chosen as the main chemotherapy in this study.
This study showed a significant difference in overall survival between the group of patients who underwent primary tumor resection without concomitant metastasectomy and the group of patients who underwent palliative surgery. However, patients with severe comorbidities or progressive metastasis who could not undergo an operation were excluded. We thought it was reasonable to separately compare patients who could undergo surgery. The two groups were well matched in terms of population and extent of tumor metastasis, but differed in their T factor. Thus, this observational survival benefit might be the result of patient selection. Therefore we additionally analyzed modified groups in which patients with T4 invasive CRC were excluded. Even in this analysis, the benefit of primary lesion resection was clearly shown. Postoperative complications in this study occurred in 28% of patients, which was similar to other reports[14]. Postoperative complications occurred more often after primary resection than after palliative surgery. This resulted in a significantly (P = 0.05) longer hospital stay in the primary resection group. A laparoscopic approach, when possible, might decrease morbidity[15]; however, we did not address this in our study. Laparoscopic surgery, as an alternative to open surgery, is safer with no added morbidity. In our study, two of 36 patients who were initially managed with palliative surgery developed perforation or significant gastrointestinal bleeding from their intact primary lesions and were successfully treated with no additional surgical management. Such patients should be closely observed, because recent studies suggest that up to one-third of patients without resection will require subsequent intervention for symptoms related to an intact primary lesion[4,6,16]. Mild events can be managed medically, but life-threatening events require immediate surgical intervention. The issue of these tumor-dependent complications is still important. Until now it has been thought that most patients would succumb to systemic disease before developing a complication, based on high mortality and a poor 5-year survival of patients with stage 4 CRC. However, improved multidrug regimens such as FOLFOX and FOLFIRI would probably raise the incidence of severe complications indirectly by prolonging survival. Surgical treatment of primary disease to control severe complications including bleeding, perforation, and obstruction might also become increasingly important. Furthermore, whether perforation of the primary tumor is the result of tumor progression or a side effect of chemotherapy remains to be evaluated. Bevacizumab, a monoclonal antibody against vascular endothelial growth factor, is recognized to improve survival with chemotherapy but also has serious complications, such as gastrointestinal perforation. Hedrick et al[17] and Sugrue et al[18] found that 1.7% of patients who used bevacizumab experienced GI perforation. Intra-abdominal inflammation due to tumor necrosis was thought to be the common feature of these GI perforations. In addition, both the BRiTE[14] study and the First BEATrial[17] suggested that incidence of GI perforation was higher in patients with intact primary tumors. Therefore, perforation of the primary lesion is a severe problem and requires protective treatment for patients with invasive CRC. To gain a benefit from bevacizumab treatment without concern for perforation and bleeding from the primary tumor, resection of the primary tumor, if possible, is definitely favorable. In our study, eight patients have been treated with bevacizumab without severe toxicities since 2008. However, caution must be exercised in the management of the patients requiring surgery and receiving adjuvant treatment with bevacizumab.
In conclusion, this study supports the view that resection of the primary lesions in patients with non-resectable, metastatic colorectal cancer is advisable, as there is a significant improvement in survival with primary site resection over non-resected palliative surgery. We believe that all suitable patients should undergo this combined approach to avoid primary cancer complications and improve prognosis.
Colorectal cancer (CRC) is the most common cancer worldwide. Although chemotherapy has progressed dramatically, the role of surgical treatment in patients with incurable metastasis CRC is controversial. The resection of the primary lesions in these patients is recommended due to a significant improvement in survival.
Previous studies have mentioned the comparison of primary site resection and palliative surgery. However, they have not addressed the effects of new adjuvant chemotherapy, including FOLFOX and FOLFIRI. In this study, the authors proved the superiority of primary site resection combined with FOLFOX.
This is the first study to report the combined effect of primary site resection and new chemotherapy. Furthermore, this study suggests that primary site resection might be associated with survival in patients with metastatic CRC.
This study might represent a future strategy for combined therapies for patients with CRC.
This is an institutional experience that reviews the outcomes of patients with synchronous presentation of metastatic colorectal cancer who were treated with systemic chemotherapy for which subsequent management included a resection of the primary tumor or palliative surgical procedure to manage the primary tumor.
Peer reviewer: Dr. Terence C Chua, BScMed (Hons), MB, BS, UNSW Department of Surgery, St George Hospital, Sydney, NSW 2217, Australia
S- Editor Wang JL L- Editor Stewart GJ E- Editor Ma WH
| 1. | Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30. |
| 2. | Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg. 2003;196:722-728. |
| 3. | Liu SK, Church JM, Lavery IC, Fazio VW. Operation in patients with incurable colon cancer--is it worthwhile? Dis Colon Rectum. 1997;40:11-14. |
| 4. | Mäkelä J, Haukipuro K, Laitinen S, Kairaluoma MI. Palliative operations for colorectal cancer. Dis Colon Rectum. 1990;33:846-850. |
| 5. | Scoggins CR, Meszoely IM, Blanke CD, Beauchamp RD, Leach SD. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol. 1999;6:651-657. |
| 6. | Rosen SA, Buell JF, Yoshida A, Kazsuba S, Hurst R, Michelassi F, Millis JM, Posner MC. Initial presentation with stage IV colorectal cancer: how aggressive should we be? Arch Surg. 2000;135:530-534; discussion 534-535. |
| 7. | Johnson WR, McDermott FT, Pihl E, Milne BJ, Price AB, Hughes ES. Palliative operative management in rectal carcinoma. Dis Colon Rectum. 1981;24:606-609. |
| 8. | Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, Weiser M, Temple LK, Wong WD, Paty PB. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379-3384. |
| 9. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. |
| 10. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. |
| 11. | Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, Hart LL, Gupta S, Garay CA, Burger BG. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21:2059-2069. |
| 12. | Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237. |
| 13. | Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione L. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23:4866-4875. |
| 14. | Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, Dellabona P, Di Carlo V. Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg. 2002;236:759-766; disscussion 767. |
| 15. | Hedrick E, Kozloff M, Hainsworth J, Badarinath S, Cohn A, Flynn P, Dong W, Suzuki S, Sarkar S, Sugrue M. Safety of bevacizumab plus chemotherapy as first-line treatment of patients with metastatic colorectal cancer: updated results from a large observational registry in the US (BRiTE). J Clin Oncol. 2006;24 (18 Suppl). |
| 16. | Sarela AI, Guthrie JA, Seymour MT, Ride E, Guillou PJ, O’Riordain DS. Non-operative management of the primary tumour in patients with incurable stage IV colorectal cancer. Br J Surg. 2001;88:1352-1356. |
| 17. | Kretzschmar A, Cunningham D, Berry S. Incidence of gastrointestinal perforations and bleeding in patients starting bevacizumab treatment in first-line mCRC without primary tumour resection: preliminary results from the First BEATrial. Paper presented at American Society of Clinical Oncology Gastrointestinal Symposium 2006. . |
| 18. | Sugrue M, Kozloff M, Hainsworth J, Badarinath S, Cohn A, Flynn P, Steis R, Dong W, Sarkar S, Grothey A. Risk factors for gastrointestinal perforations in patients with metastatic colorectal cancer receiving bevacizumab plus chemotherapy. Paper presented at American Society of Clinical Oncology 2006. . |