Published online Jul 14, 2010. doi: 10.3748/wjg.v16.i26.3335
Revised: April 27, 2010
Accepted: May 4, 2010
Published online: July 14, 2010
Vanishing bile duct syndrome (VBDS) refers to a group of disorders characterized by prolonged cholestasis as a result of destruction and disappearance of intrahepatic bile ducts. Multiple etiologies have been indentified including infections, neoplastic disorders, autoimmune conditions and drugs. The natural history of this condition is variable and may involve resolution of cholestasis or progression with irreversible damage. VBDS is extremely rare in human immunodeficiency virus (HIV)-infected patients and anti-retroviral therapy has never been implicated as a cause. We encountered a young pregnant female with HIV and VBDS secondary to anti-retroviral therapy. Here, we report her clinical course and outcome.
- Citation: Kochar R, Nevah MI, Lukens FJ, Fallon MB, Machicao VI. Vanishing bile duct syndrome in human immunodeficiency virus: Nevirapine hepatotoxicity revisited. World J Gastroenterol 2010; 16(26): 3335-3338
- URL: https://www.wjgnet.com/1007-9327/full/v16/i26/3335.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i26.3335
The destruction and disappearance of intrahepatic bile ducts leading to “ductopenia” and cholestasis is termed “vanishing bile duct syndrome (VBDS)”. The exact mechanism underlying this syndrome is unknown; however, common causes include autoimmune disorders such as primary biliary cirrhosis (PBC), neoplasms, infections and drug toxicities[1-7]. Several patients with VBDS respond to treatment of the underlying condition and/or removal of the offending agent. However, other patients progress to biliary cirrhosis and end stage liver disease. VBDS is rare in the human immunodeficiency virus (HIV)-infected population and to date, only one case has been reported in the literature[8]. In addition, none of the anti-retroviral medications have been implicated as a cause of ductopenia. We report the case of a young female with HIV and VBDS secondary to anti-retroviral therapy.
In August, 2009, a 28-year-old African American female in the 3rd trimester of pregnancy (G1P0, 31 wk) presented to the emergency room with jaundice for 3 d. She also complained of pruritus, light-colored stools and dark urine. She denied any abdominal pain, fever, chills, vomiting or diarrhea. The patient had a history of heavy alcohol use (approximately 5 drinks/d) for 3 years discontinued at the beginning of her pregnancy. She reported no history of intravenous drug use or unprotected sexual exposure. She had no history of taking herbal supplements or over-the-counter medications. Her past medical history was significant for HIV infection diagnosed in 2000. She had not been on highly active anti-retroviral therapy (HAART) since 2003 but recently started triple drug therapy to minimize risk of vertical transmission, with zidovudine, lamivudine and nevirapine 4 wk prior to presentation. She had no history of any opportunistic infections and was not taking any prophylactic medications. Her only other medications included folic acid and multivitamins. Her CD4 count and viral load prior to initiating therapy were 234 cells/μL (normal range: 410 to 1590 cells/μL) and 35 853 copies/mL [reverse transcriptase polymerase chain reaction (RT-PCR) lower limit of detection: 48 copies/mL], respectively.
Physical examination revealed icterus and gravid uterus consistent with duration of pregnancy. There were no stigmata of chronic liver disease. Abdomen was non-tender and without hepatosplenomegaly or ascites. Fetal exam was normal. Her liver tests revealed total bilirubin of 10.5 mg/dL (normal range: 0.2-1.3 mg/dL), direct bilirubin 6.7 mg/dL, aspartate aminotransferase 103 U/L (normal range: 0-37 U/L), alanine aminotransferase 179 U/L (normal range: 0-40 U/L), and alkaline phosphatase 496 U/L (normal range: 39-117 U/L). Hematologic and electrolyte values were within normal range, and serum ethanol and urine drug screen were negative. Serologic testing was negative for viral and autoimmune hepatitis including hepatitis E IgM, Epstein Barr virus PCR and cytomegalovirus PCR (CMV-PCR), antinuclear antibody, antismooth muscle antibody, and antimitochondrial antibody. Her CD4 cell count and HIV-1 RNA viral load were measured as 224 cells/μL and 304 copies/mL, respectively. Fungal, mycobacterial, CMV, aerobic and anaerobic blood cultures as well as tests for Cryptosporidium parvum, Microsporidium and Toxoplasma were negative.
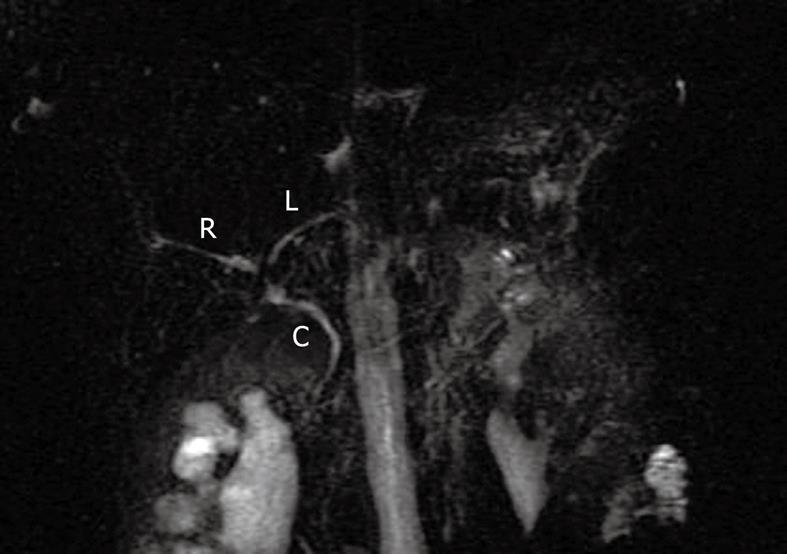
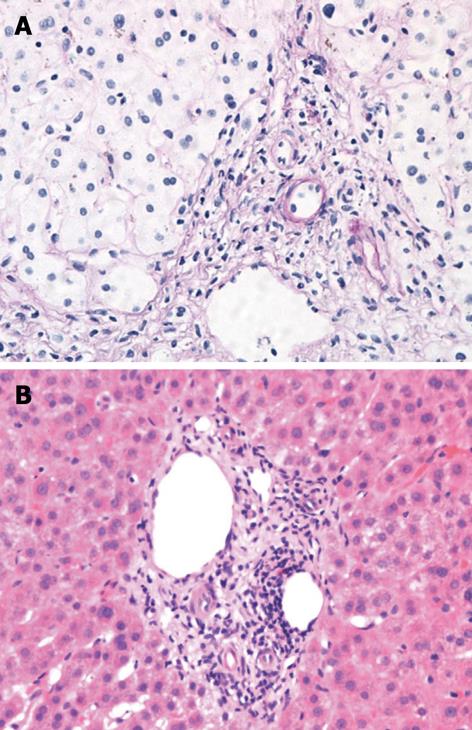
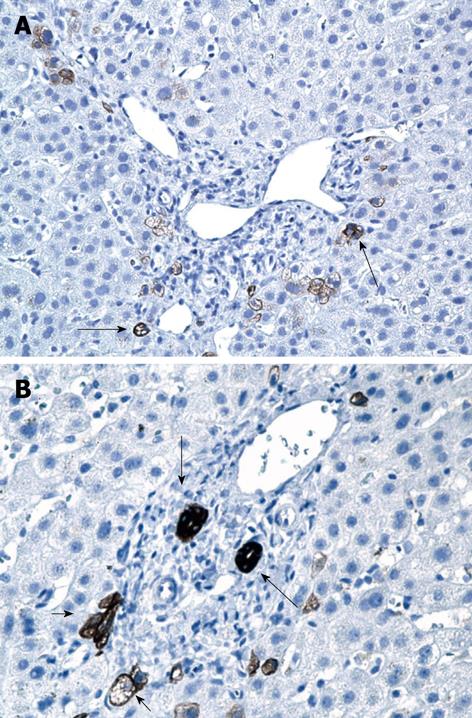
Right upper quadrant ultrasound revealed normal size liver with normal echogenicity and no focal lesions, cholelithiasis or biliary tract obstruction. Magnetic resonance cholangiopancreatography (MRCP) was performed. There was no cholangiographic evidence of extra- or intra-hepatic biliary duct dilatation (Figure 1), sclerosing cholangitis or discrete liver mass. A liver biopsy was performed which revealed marked ductopenia with cholestasis (Figure 2). Cytokeratin immunostain confirmed ductopenia and showed one irregular bile duct and scattered ductular proliferation among 11 visualized portal tracts (Figure 3). There was mild lymphocytic portal inflammation and CMV, AFB and GMS stains were negative. A diagnosis of VBDS was made.
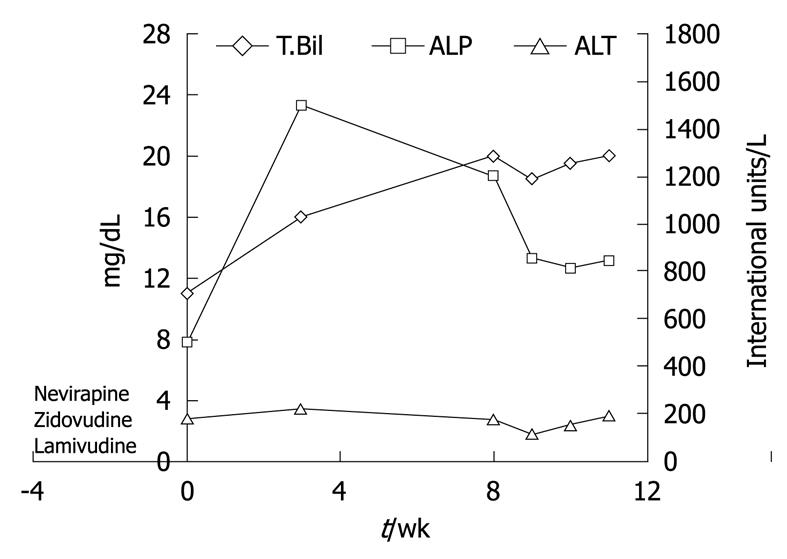
HAART medications were withdrawn and the patient was started on ursodeoxycholic acid at 15 mg/kg per day to treat cholestasis and provide symptomatic relief. Over the next few weeks, the patient continued to have severe pruritus and worsening jaundice. Her liver tests deteriorated further with peak levels as shown in Figure 4. A second liver biopsy was performed 8 wk later which showed worsening cholestasis and persistent ductopenia. Owing to the progressive nature of her disease course, liver transplant evaluation was initiated for the patient.
VBDS refers to a group of disorders resulting in cholestasis as a consequence of progressive destruction and disappearance of intrahepatic bile ducts. This condition was first described in 1988 in a case series of 3 patients by Ludwig et al[1] as “idiopathic adulthood ductopenia”. Ductopenia is defined as the absence of interlobular bile ducts in greater than 50% of small portal tracts in a liver biopsy specimen.
Several causes of VBDS have been identified with varying mechanisms of duct injury and loss. PBC is one of the most common causes of ductopenia and is likely secondary to T-cell mediated bile duct injury[1,2]. Small duct primary sclerosing cholangitis, acute and chronic hepatic cellular rejection and sarcoidosis are other causes of VBDS with an underlying immune mechanism[3,4]. Infections and neoplastic disorders commonly implicated include CMV, reovirus 3, syphilis, cryptosporidium, Hodgkin’s disease and histiocytosis X[5-7]. In addition, several drugs have been reported to cause VBDS, the most frequent ones include antibiotics and psychiatric medications (Table 1)[7].
| Antibiotics | Psychiatric drugs | Antiepileptic drugs | Oral hypoglycemics | Hormonal agents | Others |
| Ampicillin | Amitriptyline | Phenytoin | Carbutamide | Estradiol | Ibuprofen |
| Amoxicillin/clavulanic acid | Chlorpromazine | Carbamazepine | Tolbutamide | Methyltestosterone | Phenylbutazone |
| Haloperidol | Barbiturates | Glibenclamide | Norandrostenolone | Cyproheptadine | |
| Clindamycin | Imipramine | Diazepam | Cimetidine | ||
| Trimethoprim/sulfamethoxazole | Prochlorperazine | Cromolyn sodium | |||
| Trifluperazine | Chlorothiazide | ||||
| Flucloxacillin | |||||
| Tetracyclines | |||||
| Thiabendazole | |||||
| Erythromycin | |||||
| Troleandomycin |
Intrahepatic cholestasis of pregnancy was considered upon presentation as a possibility in our patient. However, this is a physiologic response to hormonal fluctuation during pregnancy and usually resolves spontaneously after delivery without persistent hyperbilirubinemia and ductopenia as seen in our patient[9]. There is no known association between pregnancy and VBDS, and the few existing case reports have been ascribed to medications such as chlorpromazine[10,11]. VBDS is even rarer in patients with HIV infection and has not been well described. Only a single case has been reported in the literature, in a patient with advanced AIDS (CD4 cell count: 7 cells/μL) and cholestasis with ductopenia presumably due to CMV infection[8]. A second case with HIV infection, low CD4 count (108 cells/μL) and idiopathic ductopenia has been reported, but does not fit the definition of VBDS since bile ducts were absent only in 6 of 15 portal tracts[12]. Cholestasis in patients with HIV infection is commonly due to sclerosing cholangitis and AIDS cholangiopathy which involves obstruction of the biliary tract due to infection-related strictures. Other causes include drug toxicity from HAART medications and antimicrobials, neoplasms (Kaposi sarcoma and lymphoma) and opportunistic infections[13]. Our patient did not have biliary obstruction on ultrasound or MRCP, and had significantly higher levels of serum bilirubin than is commonly seen in AIDS cholangiopathy. Liver biopsy was negative for malignancy and did not show any features of sclerosing cholangitis or PBC. No strictures were seen on MRCP and tests for specific opportunistic pathogens were negative, ruling out AIDS cholangiopathy and infection.
The most likely cause of cholestasis in our patient was drug-induced liver injury. Medications used by our patient prior to onset of jaundice included zidovudine, lamivudine, nevirapine, multivitamins and folic acid. Zidovudine or lamivudine toxicity is associated with lactic acidosis, elevated lactate dehydrogenase, amylase and lipase along with abnormal liver tests[14]. None of these findings were seen in our patient. In addition, these two drugs were less likely to be the culprits since their toxic effects on the liver usually manifest after 6 mo of use. Nevirapine, a non-nucleoside reverse transcriptase inhibitor, is being increasingly used in pregnant patients owing to its favorable side effect profile and lower teratogenicity compared to protease inhibitors[14]. However, hepatotoxicity is the major adverse effect of nevirapine (5%) and most often manifests as a hypersensitivity reaction with fever, rash and elevated liver tests within the first few weeks of therapy. Late onset hepatotoxicity after several weeks of nevirapine use has also been described in several cases and may be an idiosyncratic reaction to the drug[14]. Although several case reports have demonstrated a cholestatic pattern of nevirapine toxicity, VBDS has never been reported[15,16]. Our patient did not have fever and rash, but had evidence of cholestatic hepatitis and a temporal association between nevirapine use and development of biochemical abnormalities. Therefore, nevirapine toxicity was felt to be the most likely cause of cholestasis and VBDS in our patient.
In summary, VBDS can result from multiple etiologies including autoimmune disorders, infections, neoplastic disorders, genetic abnormalities and medications. The diagnosis is suggested by abnormal liver tests and paucity of interlobular bile ducts on liver biopsy. To the best of our knowledge, this is the first reported case of nevirapine-induced cholestatic hepatitis in a patient with HIV leading to severe ductopenia and VBDS. VBDS should be considered in all HIV patients with chronic cholestasis, especially those with a history of nevirapine use.
Peer reviewers: Florencia Georgina Que, MD, Department of Surgery, Mayo Clinic, 200 First Street Southwest, Rochester, MN 55905, United States; Luis Grande, Professor, Department of Surgery, Hospital del Mar, Passeig Marítim 25-29, Barcelona 08003, Spain
S- Editor Wang JL L- Editor Logan S E- Editor Zheng XM
| 1. | Ludwig J, Wiesner RH, LaRusso NF. Idiopathic adulthood ductopenia. A cause of chronic cholestatic liver disease and biliary cirrhosis. J Hepatol. 1988;7:193-199. |
| 2. | Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, Kaplan MM, Gershwin ME. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231-1240. |
| 3. | Ludwig J. Small-duct primary sclerosing cholangitis. Semin Liver Dis. 1991;11:11-17. |
| 4. | Murphy JR, Sjogren MH, Kikendall JW, Peura DA, Goodman Z. Small bile duct abnormalities in sarcoidosis. J Clin Gastroenterol. 1990;12:555-561. |
| 5. | Ballonoff A, Kavanagh B, Nash R, Drabkin H, Trotter J, Costa L, Rabinovitch R. Hodgkin lymphoma-related vanishing bile duct syndrome and idiopathic cholestasis: statistical analysis of all published cases and literature review. Acta Oncol. 2008;47:962-970. |
| 6. | Thompson HH, Pitt HA, Lewin KJ, Longmire WP Jr. Sclerosing cholangitis and histiocytosis X. Gut. 1984;25:526-530. |
| 7. | Reau NS, Jensen DM. Vanishing bile duct syndrome. Clin Liver Dis. 2008;12:203-217, x. |
| 8. | Hindupur S, Yeung M, Shroff P, Fritz J, Kirmani N. Vanishing bile duct syndrome in a patient with advanced AIDS. HIV Med. 2007;8:70-72. |
| 9. | Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066. |
| 10. | Moradpour D, Altorfer J, Flury R, Greminger P, Meyenberger C, Jost R, Schmid M. Chlorpromazine-induced vanishing bile duct syndrome leading to biliary cirrhosis. Hepatology. 1994;20:1437-1441. |
| 11. | Chlumská A, Curík R, Boudová L, Mukensnabl P, Klvana P. Chlorpromazine-induced cholestatic liver disease with ductopenia. Cesk Patol. 2001;37:118-122. |
| 12. | Aldeen T, Davies S. Vanishing bile duct syndrome in a patient with advanced AIDS. HIV Med 2007; 8: 70-72. HIV Med. 2007;8:573-574. |
| 13. | Te HS. Cholestasis in HIV-infected patients. Clin Liver Dis. 2004;8:213-228, viii-ix. |
| 14. | Medrano J, Barreiro P, Tuma P, Vispo E, Labarga P, Blanco F, Soriano V. Risk for immune-mediated liver reactions by nevirapine revisited. AIDS Rev. 2008;10:110-115. |
| 15. | Prakash M, Poreddy V, Tiyyagura L, Bonacini M. Jaundice and hepatocellular damage associated with nevirapine therapy. Am J Gastroenterol. 2001;96:1571-1574. |
| 16. | Clarke S, Harrington P, Condon C, Kelleher D, Smith OP, Mulcahy F. Late onset hepatitis and prolonged deterioration in hepatic function associated with nevirapine therapy. Int J STD AIDS. 2000;11:336-337. |