Published online Jul 14, 2010. doi: 10.3748/wjg.v16.i26.3258
Revised: January 24, 2010
Accepted: January 31, 2010
Published online: July 14, 2010
AIM: To investigate the expression and function of CD74 in normal murine colon epithelial cells (CEC) and colon carcinoma cells.
METHODS: Expression of CD74 mRNA and protein were measured by reverse transcriptase-polymerase chain reaction (RT-PCR), Western blotting and fluorescence-activated cell sorter (FACS). The effect of migration inhibitory factor (MIF) on the survival of normal CEC from C57BL/6, NOD/SCID, and CD74 deficient mice both in vitro and in vivo, and on the CT26 carcinoma cell line was analyzed by (quantitative) qRT-PCR, RT-PCR, Western blotting and FACS.
RESULTS: CD74 was found to be expressed on normal CEC. Stimulation of CD74 by MIF induced a signaling cascade leading to up-regulation of Bcl-2 expression, resulting in a significant increased survival of CEC. CD74 was also expressed on the CT26 colon carcinoma cell line and its stimulation by MIF resulted in enhanced cell survival, up-regulation of Akt phosphorylation and Bcl-2 expression.
CONCLUSION: CD74 is expressed on CEC and colon carcinoma cells and serves as a survival receptor in these cells. These results may have implications on colorectal cancer research.
- Citation: Maharshak N, Cohen S, Lantner F, Hart G, Leng L, Bucala R, Shachar I. CD74 is a survival receptor on colon epithelial cells. World J Gastroenterol 2010; 16(26): 3258-3266
- URL: https://www.wjgnet.com/1007-9327/full/v16/i26/3258.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i26.3258
CD74 (Invariant chain; Ii) is a type II integral membrane protein, which acts as a chaperone for major histocompatibility complex (MHC) class II protein expression[1]. It is a non-polymorphic type II integral membrane protein; the mouse protein is comprised of a 30 amino acid (aa) N-terminal cytoplasmic tail, followed by a single 24 aa transmembrane region and an approximately 150 aa long lumenal domain. The CD74 chain was initially thought to function mainly as an MHC class II chaperone, which promotes endoplasmic reticulum (ER) exit of MHC class II molecules, directs them to endocytic compartments, prevents peptide binding in the ER, and contributes to peptide editing in the MHC class II compartment[1]. A small proportion of CD74 is modified by the addition of chondroitin sulfate (CD74-CS), and this form of CD74 is expressed on the surface of antigen presenting cells (APCs), including monocytes and B cells. In addition to APCs, other cells of the gastrointestinal tract, such as epithelial cells, express class II MHC proteins and CD74 and act as APCs[2]. The role of CD74 in the epithelium is not fully defined. It was formerly accepted that the functions of CD74 in these cells have been correlated with its role in antigen processing and presentation in conventional APCs. However, CD74 may play additional roles in epithelial cells. CD74 expression on normal human colon epithelial cells (CEC) is still controversial, as the presence of this molecule was demonstrated using immunohistochemistry by some groups[3], but not by others[4,5]. On human colonic epithelial cell lines it was found to be expressed only after interferon-γ treatment[6]. In mice, CD74 expression on CEC is also not clear, although CD74 was not demonstrated on normal murine CEC[7]. In one study, CLIP (class II invariant chain peptide) was shown to be expressed in a complex with MHC class II, suggesting that CD74 is expressed on these cells as well[8]. Recently, it was demonstrated that CD74 is expressed on CEC of APCMin/+ mice. However, these mice bear a point mutation in the murine homolog of the APC gene and develop multiple intestinal adenomas[9]. In humans, on the contrary, there was no CD74 expression on normal CEC, but it was expressed on CEC of sporadic colorectal adenomas[10].
It was shown previously that macrophage migration inhibitory factor (MIF) binds to the CD74 extracellular domain, a process that results in the initiation of a signaling pathway in a CD44 dependent manner[11-13].
In our previous studies, we showed that CD74 expressed on B cells is directly involved in shaping the B cell repertoire[14-16] through a pathway leading to the activation of transcription mediated by the nuclear factor-κB (NF-κB) p65/RelA homodimer and its co-activator, TAFII105[17]. We demonstrated that CD74 stimulation with anti-CD74 antibody or MIF leads to NF-κB activation, enabling entry of the stimulated B cells into the S phase, an increase in DNA synthesis, cell division, and augmented expression of anti-apoptotic proteins. These findings indicated that surface CD74 functions as a survival receptor[13,18,19].
In addition, CD74 is expressed at high levels from an early stage of the B cell leukemia, B-CLL. The activation of CD74 on human B-CLL cells by MIF, initiates a signaling cascade that contributes to tumor progression. This pathway induces NF-κB activation, resulting in the secretion of interleukin 8, which in turn promotes cell survival. Blocking of this pathway leads to decreased cell survival. Thus, CD74 expressed on the surface of B-CLL cells plays a critical role in regulating the survival of these malignant cells[20].
MHC class II expression was initially thought to be limited to a restricted set of cells collectively known as APCs. However, in addition to conventional APCs, other cell types, including mucosal epithelial cells, were subsequently reported to express class II MHC molecules and to present antigens[21].
Surface expression of newly synthesized CD74 is followed by rapid internalization to the endosomal pathway. Experiments that investigate cell surface CD74 are complicated by the fact that CD74 remains on the cell surface for a very short time. The surface half-life of CD74 was calculated to be less than 10 min[22,23].
In this article, we followed CD74 expression in colonic intestinal epithelial cells in the mouse. We show that CD74 is expressed on CEC derived from C57BL/6 and on the CT26 colon carcinoma cell line and serves as a survival receptor on these cells. This finding may suggest a role for CD74 in colon cancer development.
C57BL/6, C57BL/6 CD74 deficient[24], C57BL/6 MIF deficient[25], CD44 deficient NOD/SCID mice (Jackson Lab), were used. All animals were used at 6-10 wk of age.
All animal procedures were approved by the Animal Research Committee at the Weizmann Institute of Science.
Recombinant murine MIF was purified from an expression system as previously described and contaminating endotoxin removed by C8 chromatography[26]. Mice were injected daily ip with MIF (400 ng) or with PBS, as indicated. Mice were sacrificed after 3.5 or 24 h and CEC were isolated.
CEC were isolated using a modification of the method described previously[27]. Briefly, mice were sacrificed, colons were immediately removed and washed with phosphate buffered saline (PBS) until all content was removed. Colons were inverted and washed gently with Roswell Park Memorial Institute solution. Mucus was removed by incubation for 10 min in 1 mmol/L DTT. Specimens were treated with Dispase II (Roche Diagnostics; 3 mg/mL) in DMEM for 30 min (vortexing every 5 min) at 37°C. CEC were isolated from the remaining tissue by passage through a metal filter. In order to purify CEC, cells were centrifuged on a discontinuous Percoll gradient for 30 min, 2000 r/min. Cells found on the top 0%-30% gradient are CEC[28]. Isolated cells were washed in PBS. CEC isolated in this fashion contained over 90% viable cells as determined by Trypan blue exclusion. A total of 92%-95% of the cells stained with the anti-epithelial cell marker anti-pan cytokeratin-26 (FITC conjugated) (Sigma-Aldrich). The remaining contaminating cells represented CD3+ and B220+ cells.
CT26 murine colon carcinoma cells were grown as monolayer cultures in DMEM-10%, fetal bovine serum (FBS) (Invitrogen) supplemented with 100 IU/mL penicillin and 100 μg/mL streptomycin. Cells were maintained in a 37°C incubator with 5% CO2-humidified air.
CECs (3-6 × 106 cells/well) isolated from CD57BL/6 mice, were cultured in 12-well plates at 37°C in DMEM medium supplemented with 10% FCS, 2 mmol/L glutamate, 300 U/mL penicillin, 300 μg/mL streptomycin, with or without 400 ng/mL of MIF for 17 h.
1.5 × 106 cells (CT26 cell line) were plated in complete medium into dishes of 6-well cell culture plates, and were allowed to adhere for 24 h. After washing the cells twice with PBS, they were incubated with MIF (400 ng/mL) for 3 or 6 h (as indicated in the text) in serum-free medium.
Total RNA was isolated from cells using the Tri Reagent Kit (MRC), according to the manufacturer’s instructions. Reverse transcription was carried out using Superscript II RT (Gibco-BRL).
Primers that were used in polymerase chain reaction (PCR) reactions: Bcl-2: 5'-CACCGAACACTTGATTCTG, 3'-AGATCTCTGGTTGGGATTC; Cyclin E: 5'-GAAAATCAGACCACCCAGAGCC, 3'-GAAATGATACAAAGCAGAAGCAGCG; CD74: 5'-GGAGTACCCGCAGCTGAAGGGG, 3'-GAAGATAGGTCTTCCATGTCCAGTG; HPRT: 5'-GAGGGTAGGCTGGCCTATGCCT, 3'-GTTGGATACAGGCCAGACTTTGTTG.
Levels of mRNA of Actin, Bcl-2 and Cyclin E, were analyzed by quantitative real-time reverse transcriptase (RT)-PCR using a Light-Cycler instrument (Roche Diagnostics, Mannheim, Germany). Total RNA was isolated from cells using the Tri Reagent Kit (MRC). Reverse transcription was carried out using Superscript II RT (Gibco-BRL). The reaction volume (10 mL) contained 3 mmol/L MgCl2, LightCycler HotStart DNA SYBR Green I mix (Roche Diagnostics), specific primer pairs, and 2.5 mL of cDNA. Conditions for PCR were as follows: 10 min at 95°C followed by 60 cycles of 15 s at 95°C, 15 s at 60°C, and 15 s at 72°C. PCR was performed in triplicates. Primer sequences were as follows: Bcl-2: 5'-GCTACCGTCGTGACTT-3', 5'-GCCGGTTCAGGTACTC-3'; Cyclin E: 5'-GTAACATAAGCAAACTG-3', 5'-TTCTTCTGGATTGGCTAA-3'; Actin: 5'-CAGTAACAGTCCGCCT-3', 5'-GTGACGTTGACATCCG-3'; β-actin levels were used to normalize samples for calculation of the relative expression levels of the genes.
To detect whether CD74 protein is expressed by CEC and to examine levels of Bcl-2 protein, cells were lysed in RIPA lysis buffer (10 mmol/L Tris, pH 7.2, 150 mmol/L NaCl, 1% deoxycholate, 1% Triton X-100, 0.1% SDS, 5 mmol/L EDTA) containing complete protease inhibitor cocktail [10 μg/mL leupeptin, 10 μg/mL aprotinin, 10 μg/mL pepstatin, 10 μg/mL chymostatin (Roche), 1 mmol/L PMSF (Sigma), and 20 mmol/L N-etheyl-melamide (Sigma)], for 30 min on ice and then centrifuged at 14 000 g at 4°C to remove cell debris. Lysates were separated by 12% (w/v) SDS-PAGE. The proteins were then transferred onto a nitrocellulose membrane and probed with anti-CD74 (FL-293; Santa Cruz) or anti-Bcl-2 (C-2; Santa Cruz), followed by horseradish peroxidase-conjugated anti-mouse (Jackson Laboratories).
To detect changes in Akt phosphorylation, stimulated cells were lysed in buffer containing: 25 mmol/L Tris, pH 7.4; 2 mmol/L vanadate; 75 mmol/L glycophosphate, pH 7.2; 2 mmol/L EDTA; 2 mmol/L EGTA; 10 mmol/L NaPPi; and 0.5% NP-40 in the presence of protease inhibitors. Lysates were separated by 10% (w/v) SDS-PAGE. The proteins were transferred onto a nitrocellulose membrane and probed with anti-p-Akt antibody (Cell Signaling Technology) followed by peroxidase-conjugated anti-mouse (Jackson Labs). The membrane was then stripped and reprobed with anti-tubulin antibody (Sigma), followed by peroxidase-conjugated anti-mouse (Jackson Laboratories).
Staining of IECs was performed using anti-CD74 (Santa Cruz), anti pan-cytokeratin (Sigma-Aldrich).
Annexin and PI staining: Cells were centrifuged, washed, and stained with annexin (BD Biosciences) and propidium iodide (PI) for 15 min at room temperature. The extent of annexin and PI staining was analyzed by FACS. Unstained cells were classified as living cells; annexin stained cells are apoptotic, and PI stained cells were considered necrotic.
FLICA staining: Analysis of apoptosis. To detect earlier stages of apoptosis, intracellular caspase 3 and 7 activity was analyzed using the FLICA (Fluorochrome Inhibitors of Caspases) Apoptosis Detection kit from Immunochemistry Technologies (Bloomington, MN). The kit contains carboxyfluorescein-labeled valylalanylaspartic acid fluoromethyl ketone, which tightly binds activated caspases. Cells were harvested and incubated with a FLICA solution in the complete medium at 37°C for 1 h. After washing twice cells were analyzed by FACS.
Results are represented as averages of several experiments (as indicated) ± SE. Comparison between groups was done by Student’s t-test.
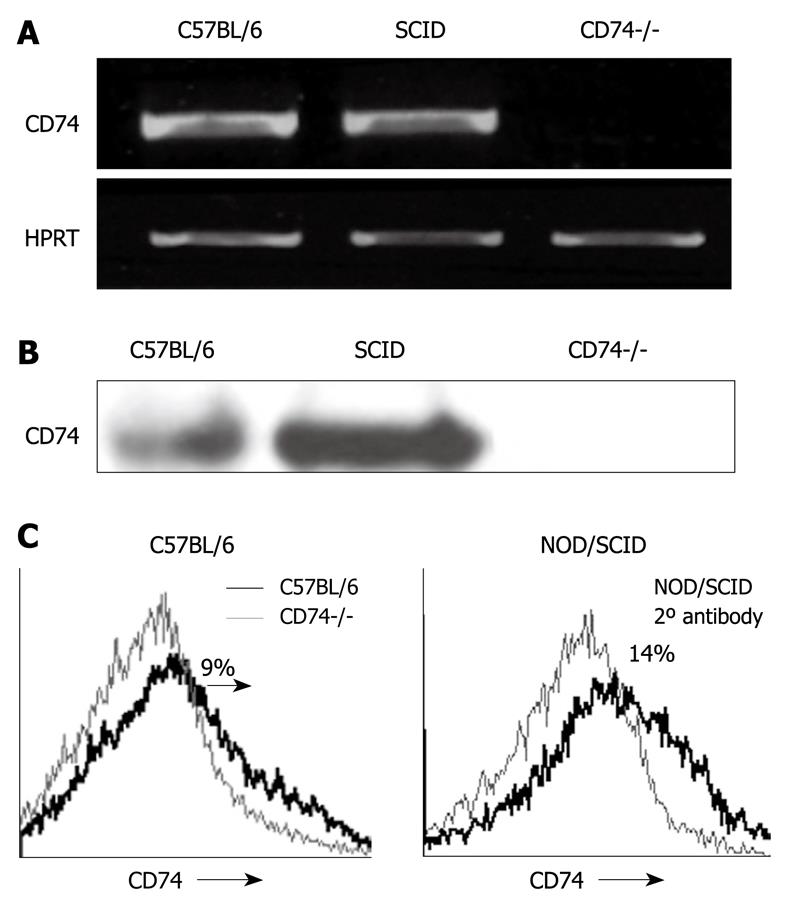
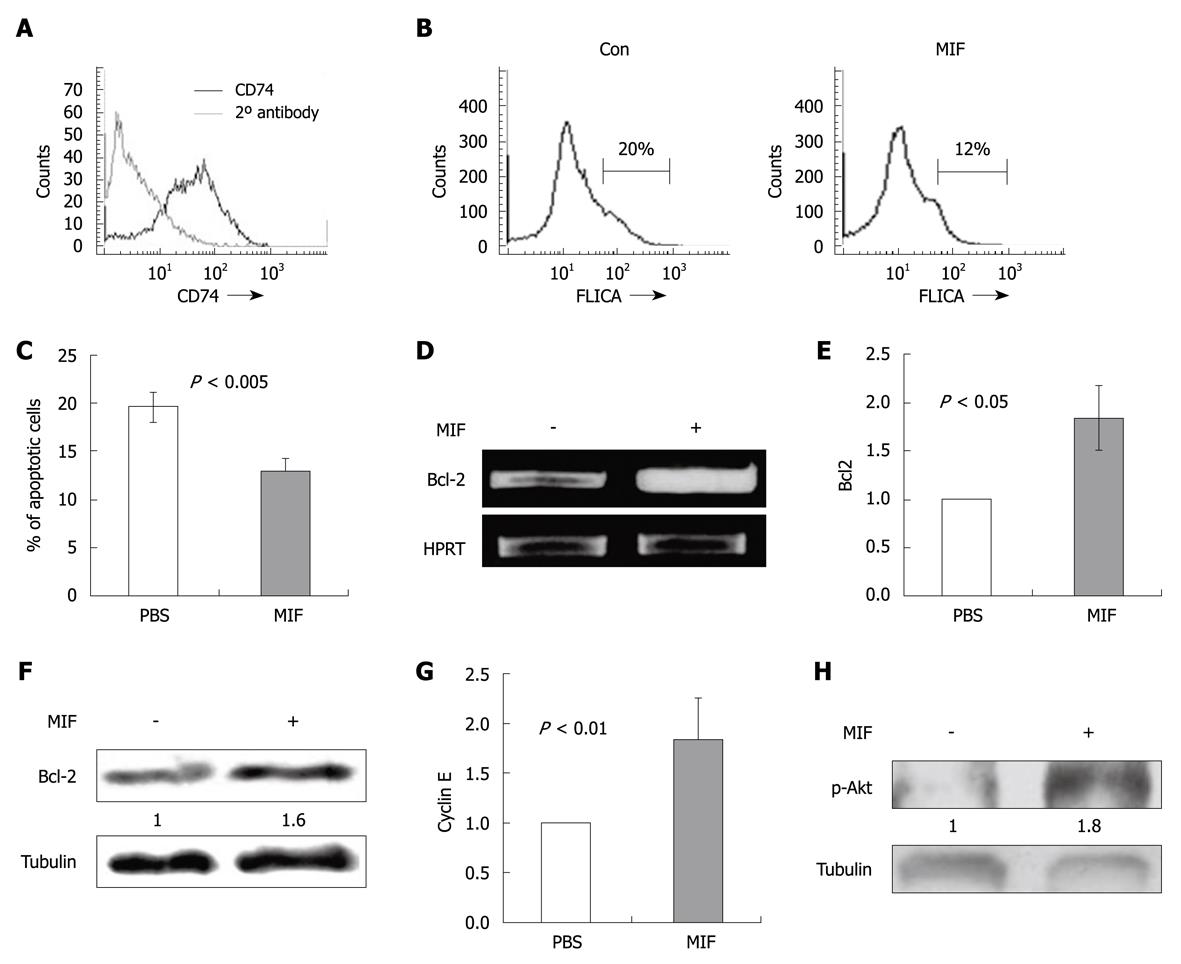
To determine whether CD74 is expressed in normal CEC, we first analyzed its mRNA and protein levels in control and CD74 deficient (CD74-/-) CEC. As shown in Figure 1, CD74 mRNA (Figure 1A) and protein (Figure 1B) were detected in CEC, while they were not observed in the CD74 deficient cells. Isolation of CEC from C57BL/6 resulted in a population that was more than 90% pure, containing less than 5% B cells (data not shown), which expressed relatively high levels CD74. To eliminate contamination by B cell CD74, we analyzed CD74 mRNA and protein levels in CEC isolated from NOD/SCID mice, which lack B and T cells. As demonstrated in Figure 1, CD74 mRNA (Figure 1A) and protein (Figure 1B) were detected in CEC derived from NOD/SCID mice as well, showing that normal CEC express CD74. We next analyzed, by FACS analysis, CD74 cell surface expression on normal CEC. As shown in Figure 1C, CD74 is specifically expressed on normal CEC derived from C57BL/6 or NOD/SCID mice. Thus, CD74 is expressed on normal CEC.
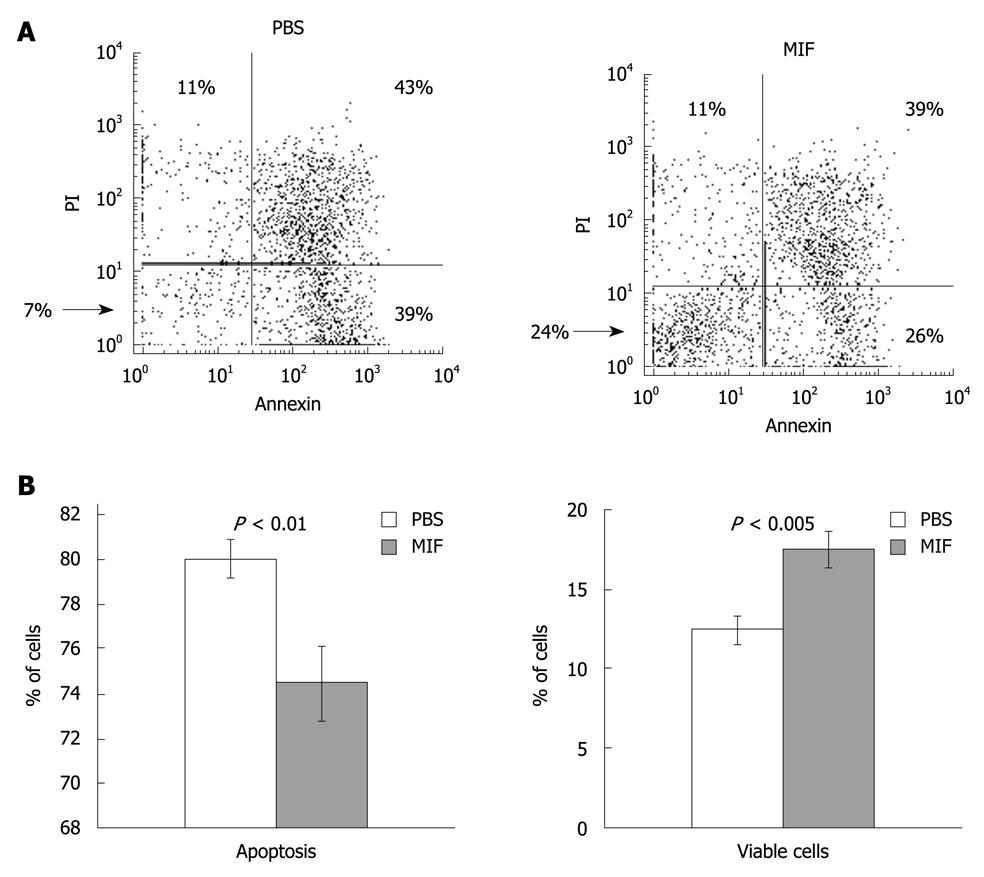
We have previously shown that CD74 stimulation results in augmented expression of anti-apoptotic proteins, resulting in induction of cell survival[13,18]. In order to examine whether CD74 serves as a survival receptor on CEC, we followed the downstream cascade initiated by MIF in CEC. CEC from C57BL/6 mice were incubated in vitro for 17 h with MIF or with PBS, and the percentage of live and apoptotic cells in each group was analyzed using annexin-PI staining. As shown in Figure 2, MIF stimulation resulted in elevation of the proportion of live CEC (42%, n = 4, P = 0.003) and reduction in the apoptotic population.
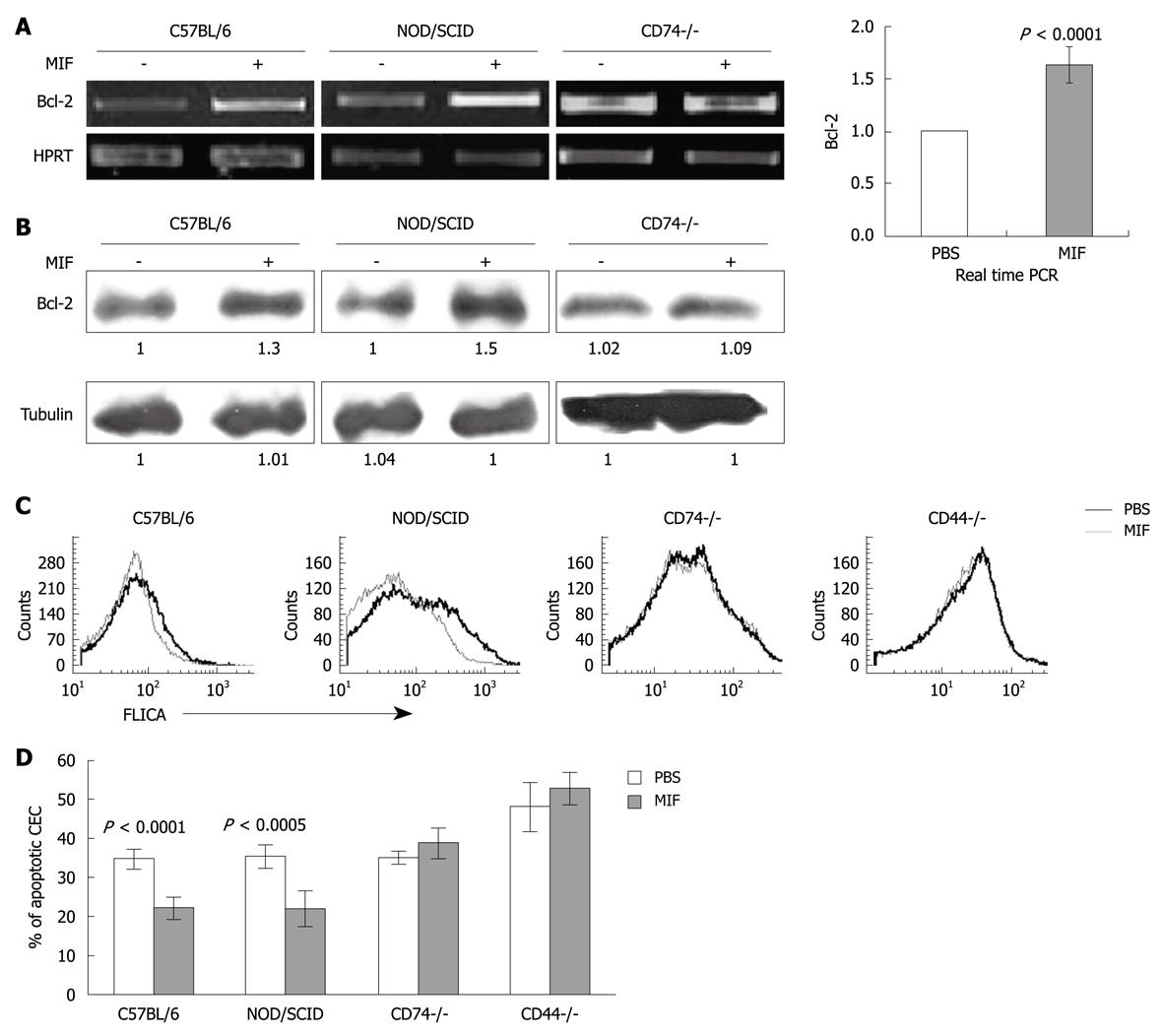
To further show the in vivo role of MIF and CD74 in CEC survival, MIF was injected ip to control C57BL/6 and NOD/SCID mice and Bcl-2 mRNA and protein levels were determined. As can be seen in Figure 3A and B, a significant elevation in Bcl-2 mRNA and protein levels were detected following MIF stimulation compared to PBS-treated cells. The MIF-induced cascade was CD74 dependent, since in its absence, MIF was not able to increase Bcl-2 levels.
Since CEC survive poorly in vitro, and following 17 h incubation a large proportion of the cells are dead, we followed CEC survival immediately after their isolation. Thus, 24 h after MIF injection to C57BL/6, NOD/SCID and CD74-/- mice, cells were isolated and their cell death was analyzed using FLICA, which detects early stages of apoptosis by analyzing intracellular caspase 3 and 7 activity. The percentage of apoptotic cells was significantly higher in the PBS treated cells compared with the MIF treated cells derived from C57BL/6 and NOD/SCID mice (Figure 3C), while the CD74-/- cells were insensitive to MIF treatment. Results of four experiments are summarized in Figure 3D.
It has been recently shown that CD44 forms a complex with CD74 which regulates MIF-induced B cell survival[11,13]. CD44 expressed on CEC was previously characterized as a negative regulator of apoptosis as well[29]. Accordingly, CD44 deficient cells (CD44-/-) demonstrated an elevated basal apoptotic rate compared to control mice. In addition, there was no increase in CEC survival upon MIF stimulation (Figure 3C and D), suggesting that CD44 is a crucial component in the CD74-induced survival cascade.
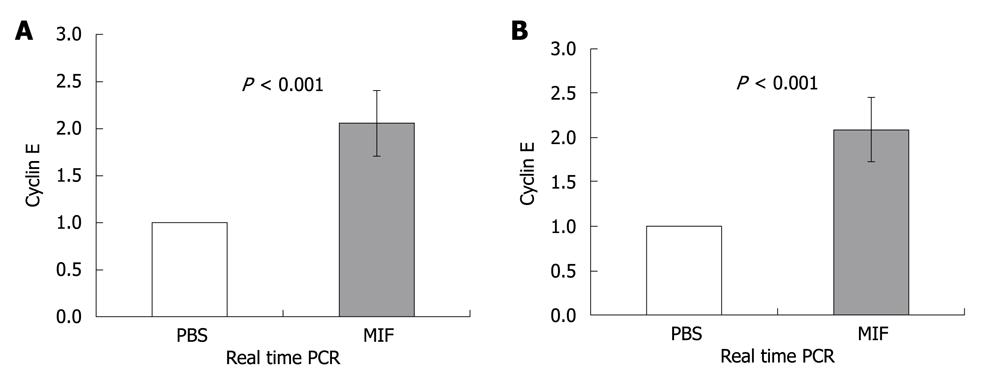
Cell cycle progression is regulated by cyclin dependent kinases (Cdks). Cdks are constitutively expressed during the cell cycle and are activated upon specific cyclin binding. Different cyclins are differentially expressed during various stages of the cell cycle. This transient expression activates Cdks and regulates cell cycle progression. To further determine whether CD74 regulates cell entry to the S-phase, we followed cyclin E, which is expressed upon S-phase initiation. MIF (400 ng) was injected to C57BL/6 and NOD/SCID mice. 3.5 h later, CEC were isolated and their cyclin E mRNA levels were analyzed. As demonstrated in Figure 4A and B, cyclin E mRNA levels were upregulated in both C57BL/6 and NOD/SCID derived cells. These results demonstrate that following CD74 stimulation, CEC cells synthesize DNA, enter S phase, and probably divide.
Taken together, our results demonstrate that CD74 serves as a survival receptor on CEC. MIF binding to CD74 induces a signaling cascade resulting in Bcl-2 and cyclin E expression and survival in normal CEC.
In order to evaluate whether our finding may have implications on malignant cells, we further assessed the expression and function of CD74 in the colon carcinoma cell line CT26. We found that CD74 is expressed on the CT26 cell line (Figure 5A) and MIF stimulation reduces their apoptosis (Figure 5B and C), by upregulating Bcl-2 mRNA (Figure 5D and E), protein (Figure 5F) and cyclin E (Figure 5G) mRNA levels.
We have previously shown that in B cells the CD74 induced survival cascade involves Akt phosphorylation[18]. In intestinal epithelial cells, it has been shown that phosphorylation of Akt enhances cell survival as well[30] and is closely associated with CRC[31,32]. We therefore analyzed Akt phosphorylation in CT26 cells. As shown in Figure 5H, MIF elevated Akt phosphorylation 1 min following stimulation.
Together, our results suggest that MIF and CD74 regulate colon tumor cell proliferation and survival.
The importance of CD74 as a survival receptor on B cells, and its role in the pathogenesis of certain malignancies is a subject of intense study. In the colon, CD74 is expressed on malignant cells, and its expression correlates with tumor grade[5]. Recent evidence suggests that up-regulation of CD74 and MIF on human colon adenomas is in correlation with dysplasia of the epithelial cells[10]. However, contradictory results regarding the presence of CD74 on normal human CEC were published through the years. In one report, the authors were able to detect the presence of CD74 by using immunohistochemistry, whereas, in a more recent study, using similar methodology, CD74 was not detected. In this article, we show CD74 expression in mouse normal CEC at the levels of mRNA, protein, and cell surface expression. Furthermore, we found that stimulation of CD74 by its natural ligand, MIF, results in increased survival of CEC. This can be at least partially attributed to enhanced expression of the survival gene, Bcl-2, which was found to be up-regulated in MIF-stimulated CEC. These findings suggest a role for CD74 on normal CEC.
Demonstration of CD74 on CEC is complicated by two main phenomena. First, CD74 has a very short half-life on these cells, and secondly, CEC are difficult to handle. These cells typically undergo apoptosis and/or necrosis, shortly after isolation[33]. Therefore, experiments involving incubation of these cells for more than a few hours usually result in death of most of the cells. In addition, isolates of CEC are never free of contaminating cells, specifically B cells from the lamina propria that also express CD74. In order to overcome these limitations, we performed our experiments shortly after CEC isolation. In experiments where we tested stimulation by MIF, we used mainly in vivo stimulation by ip injections of MIF for various amounts of time. To avoid contamination with B cells, we also repeated most of the experiments in NOD/SCID mice, which lack B cells. Our results show that CD74 is expressed on normal CEC cells.
Due to the difficulties discussed above, we used multiple models in order to demonstrate the positive effect CD74 has on CEC survival. In these experiments, we analyzed by FACS the percentage of living and apoptotic CEC. We demonstrated that stimulation by MIF either by ip injection in vivo or by incubation of isolated CEC with MIF in vitro, resulted in elevated CEC survival and decreased apoptosis. These results strengthen our hypothesis that CD74 is a survival receptor on CEC and suggests that MIF is the natural ligand of CD74 on CEC, as described for B lymphocytes[13,18,19].
MIF is expressed throughout the human gastrointestinal tract, and has previously been implicated in control of apoptosis of non-epithelial and epithelial cells[34,35]. MIF induces Bcl-2 expression and cell survival. It was previously shown that CD74 forms a complex with CD44 in normal[11,12] and tumor cells[36]. We show here that CD44 is crucial for the MIF/CD74 induced survival cascade, since stimulation of CD44 deficient cells by MIF did not affect cell death.
Evidence for a role of CD74 in colorectal cancer was suggested by its expression on carcinoma cell lines of the colon[37], and by the observation that in human colorectal carcinomas, the grade of the tumor correlated with the level of CD74 expression on the transformed CEC[5]. Interestingly, both MIF and CD74 have been associated with tumor progression and metastasis. It was reported that MIF mRNA is over-expressed in various tumors[38,39] and MIF has also been associated with the growth of malignant cells[40]. Numerous studies have demonstrated the overexpression of CD74 in various cancers[20,41-46]. CD74 expression may also serve as a prognostic factor in many of these cancers, with higher relative expression of CD74 behaving as a marker of tumor progression[47]. Our findings further support a possible role for CD74 in CRC. Additionally, CD44, which we showed essential in the MIF-CD74 survival cascade, is overexpressed in CRC, and has an anti-apoptotic effect on these cells, and probably involved in disease progression and metastases[29,48]. Moreover, we show that CD74 is expressed on the CT26 cell line and its stimulation by MIF enhances Akt phosphorylation, cyclin E and Bcl-2 expression resulting in their survival.
Our work clearly shows that CD74 is expressed on mouse intestinal epithelial cells, and serves as a survival receptor on these cells. Our results further show that CD74 may regulate survival of colorectal carcinoma tumor cells. Since we found that CD74 is a survival receptor on CEC, any change in gene expression that causes enhanced expression of CD74 or MIF may result in increased risk of colorectal cancer. Thus, CD74 may be found in the future to serve not only as a marker of colorectal cancer, but also as a therapeutic target.
CD74 is a protein that is expressed in and on some of the cells of the immune system, such as B lymphocytes and antigen presenting cells. This protein is known for its function in helping the immune cells to process and present foreign bodies. During recent years it was found that CD74 serves additionally as a survival receptor on cells of the immune system, and that its stimulation by its natural ligand - migration inhibitory factor (MIF) - prevents apoptosis (self destruction) of the cells.
CD74 was found to be markedly expressed on numerous tumors - hematologic as well as epithelial; in some of these tumors it can serve as a tumor marker and its level of expression may be related to the prognosis. Moreover, activation of CD74 on B lymphocytes in B cell leukaemia, results in tumor progression, and blocking this pathway leads to decreased cell survival, and thus may be applicable as a therapeutic intervention. Whether CD74 is expressed on colon intestinal epithelial cells is controversial, although it was shown to be expressed on epithelial cells of colorectal adenomas.
The authors’ work clearly shows that CD74 is expressed on mouse intestinal epithelial cells and serves as a novel survival receptor on these cells. They show that CD74 may regulate survival of colorectal carcinoma as well. Since they found that CD74 is a survival receptor on colonic epithelial cells, any change in gene expression that causes enhanced expression of CD74 or MIF may result in increased risk of colorectal cancer.
As CD74 is a survival receptor on colon epithelial cells and on malignant cell lines from mice, CD74 may be found in the future to serve not only as a marker of colorectal cancer, but also as a therapeutic target. Future research should focus on the role of CD74 in colorectal cancer models in animals and on human tissues.
This paper is well written and documents expression on CD74 receptor mRNA and protein in colon epithelial cells. The study is very well done and straightforward. The experiments are of good quality.
Peer reviewer: Shanthi V Sitaraman, MD, Division of Digestive Diseases, Room 201-F, 615, Michael Street, Whitehead Research Building, Emory University, Atlanta, Georgia, GA 30322, United States
S- Editor Wang YR L- Editor O'Neill M E- Editor Lin YP
| 1. | Stumptner-Cuvelette P, Benaroch P. Multiple roles of the invariant chain in MHC class II function. Biochim Biophys Acta. 2002;1542:1-13. |
| 2. | Beswick EJ, Reyes VE. CD74 in antigen presentation, inflammation, and cancers of the gastrointestinal tract. World J Gastroenterol. 2009;15:2855-2861. |
| 3. | Momburg F, Koretz K, Von Herbay A, Möller P. Nonimmune human cells can express MHC class II antigens in the absence of invariant chain--an immunohistological study on normal and chronically inflamed small intestine. Clin Exp Immunol. 1988;72:367-372. |
| 4. | Möller P, Momburg F, Koretz K, Moldenhauer G, Herfarth C, Otto HF, Hämmerling GJ, Schlag P. Influence of major histocompatibility complex class I and II antigens on survival in colorectal carcinoma. Cancer Res. 1991;51:729-736. |
| 5. | Jiang Z, Xu M, Savas L, LeClair P, Banner BF. Invariant chain expression in colon neoplasms. Virchows Arch. 1999;435:32-36. |
| 6. | Hershberg RM, Framson PE, Cho DH, Lee LY, Kovats S, Beitz J, Blum JS, Nepom GT. Intestinal epithelial cells use two distinct pathways for HLA class II antigen processing. J Clin Invest. 1997;100:204-215. |
| 7. | Vidal K, Samarut C, Magaud JP, Revillard JP, Kaiserlian D. Unexpected lack of reactivity of allogeneic anti-Ia monoclonal antibodies with MHC class II molecules expressed by mouse intestinal epithelial cells. J Immunol. 1993;151:4642-4650. |
| 8. | Beers C, Burich A, Kleijmeer MJ, Griffith JM, Wong P, Rudensky AY. Cathepsin S controls MHC class II-mediated antigen presentation by epithelial cells in vivo. J Immunol. 2005;174:1205-1212. |
| 9. | Moser AR, Mattes EM, Dove WF, Lindstrom MJ, Haag JD, Gould MN. ApcMin, a mutation in the murine Apc gene, predisposes to mammary carcinomas and focal alveolar hyperplasias. Proc Natl Acad Sci USA. 1993;90:8977-8981. |
| 10. | Cuthbert RJ, Wilson JM, Scott N, Coletta PL, Hull MA. Differential CD74 (major histocompatibility complex Class II invariant chain) expression in mouse and human intestinal adenomas. Eur J Cancer. 2009;45:1654-1663. |
| 11. | Shi X, Leng L, Wang T, Wang W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595-606. |
| 12. | Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467-1476. |
| 13. | Gore Y, Starlets D, Maharshak N, Becker-Herman S, Kaneyuki U, Leng L, Bucala R, Shachar I. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283:2784-2792. |
| 14. | Shachar I, Flavell RA. Requirement for invariant chain in B cell maturation and function. Science. 1996;274:106-108. |
| 15. | Matza D, Lantner F, Bogoch Y, Flaishon L, Hershkoviz R, Shachar I. Invariant chain induces B cell maturation in a process that is independent of its chaperonic activity. Proc Natl Acad Sci USA. 2002;99:3018-3023. |
| 16. | Matza D, Kerem A, Shachar I. Invariant chain, a chain of command. Trends Immunol. 2003;24:264-268. |
| 17. | Matza D, Wolstein O, Dikstein R, Shachar I. Invariant chain induces B cell maturation by activating a TAF(II)105-NF-kappaB-dependent transcription program. J Biol Chem. 2001;276:27203-27206. |
| 18. | Starlets D, Gore Y, Binsky I, Haran M, Harpaz N, Shvidel L, Becker-Herman S, Berrebi A, Shachar I. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807-4816. |
| 19. | Lantner F, Starlets D, Gore Y, Flaishon L, Yamit-Hezi A, Dikstein R, Leng L, Bucala R, Machluf Y, Oren M. CD74 induces TAp63 expression leading to B-cell survival. Blood. 2007;110:4303-4311. |
| 20. | Binsky I, Haran M, Starlets D, Gore Y, Lantner F, Harpaz N, Leng L, Goldenberg DM, Shvidel L, Berrebi A. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci USA. 2007;104:13408-13413. |
| 21. | Barrera CA, Almanza RJ, Ogra PL, Reyes VE. The role of the invariant chain in mucosal immunity. Int Arch Allergy Immunol. 1998;117:85-93. |
| 22. | Ghosh P, Amaya M, Mellins E, Wiley DC. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457-462. |
| 23. | Freisewinkel IM, Schenck K, Koch N. The segment of invariant chain that is critical for association with major histocompatibility complex class II molecules contains the sequence of a peptide eluted from class II polypeptides. Proc Natl Acad Sci USA. 1993;90:9703-9706. |
| 24. | Elliott EA, Drake JR, Amigorena S, Elsemore J, Webster P, Mellman I, Flavell RA. The invariant chain is required for intracellular transport and function of major histocompatibility complex class II molecules. J Exp Med. 1994;179:681-694. |
| 25. | Fingerle-Rowson G, Petrenko O, Metz CN, Forsthuber TG, Mitchell R, Huss R, Moll U, Müller W, Bucala R. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc Natl Acad Sci USA. 2003;100:9354-9359. |
| 26. | Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry. 1994;33:14144-14155. |
| 27. | van de Wal Y, Corazza N, Allez M, Mayer LF, Iijima H, Ryan M, Cornwall S, Kaiserlian D, Hershberg R, Koezuka Y. Delineation of a CD1d-restricted antigen presentation pathway associated with human and mouse intestinal epithelial cells. Gastroenterology. 2003;124:1420-1431. |
| 28. | Panja A, Barone A, Mayer L. Stimulation of lamina propria lymphocytes by intestinal epithelial cells: evidence for recognition of nonclassical restriction elements. J Exp Med. 1994;179:943-950. |
| 29. | Lakshman M, Subramaniam V, Jothy S. CD44 negatively regulates apoptosis in murine colonic epithelium via the mitochondrial pathway. Exp Mol Pathol. 2004;76:196-204. |
| 30. | Zhang HM, Rao JN, Guo X, Liu L, Zou T, Turner DJ, Wang JY. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J Biol Chem. 2004;279:22539-22547. |
| 31. | Chen J. Is Src the key to understanding metastasis and developing new treatments for colon cancer? Nat Clin Pract Gastroenterol Hepatol. 2008;5:306-307. |
| 32. | Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27:5511-5526. |
| 33. | Hofmann C, Obermeier F, Artinger M, Hausmann M, Falk W, Schoelmerich J, Rogler G, Grossmann J. Cell-cell contacts prevent anoikis in primary human colonic epithelial cells. Gastroenterology. 2007;132:587-600. |
| 34. | Wilson JM, Coletta PL, Cuthbert RJ, Scott N, MacLennan K, Hawcroft G, Leng L, Lubetsky JB, Jin KK, Lolis E. Macrophage migration inhibitory factor promotes intestinal tumorigenesis. Gastroenterology. 2005;129:1485-1503. |
| 35. | Cordon-Cardo C, Prives C. At the crossroads of inflammation and tumorigenesis. J Exp Med. 1999;190:1367-1370. |
| 36. | Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177:8730-8739. |
| 37. | Ong GL, Goldenberg DM, Hansen HJ, Mattes MJ. Cell surface expression and metabolism of major histocompatibility complex class II invariant chain (CD74) by diverse cell lines. Immunology. 1999;98:296-302. |
| 38. | Meyer-Siegler K, Hudson PB. Enhanced expression of macrophage migration inhibitory factor in prostatic adenocarcinoma metastases. Urology. 1996;48:448-452. |
| 39. | Bando H, Matsumoto G, Bando M, Muta M, Ogawa T, Funata N, Nishihira J, Koike M, Toi M. Expression of macrophage migration inhibitory factor in human breast cancer: association with nodal spread. Jpn J Cancer Res. 2002;93:389-396. |
| 40. | Nishihira J, Ishibashi T, Fukushima T, Sun B, Sato Y, Todo S. Macrophage migration inhibitory factor (MIF): Its potential role in tumor growth and tumor-associated angiogenesis. Ann N Y Acad Sci. 2003;995:171-182. |
| 41. | Young AN, Amin MB, Moreno CS, Lim SD, Cohen C, Petros JA, Marshall FF, Neish AS. Expression profiling of renal epithelial neoplasms: a method for tumor classification and discovery of diagnostic molecular markers. Am J Pathol. 2001;158:1639-1651. |
| 42. | Meyer-Siegler KL, Leifheit EC, Vera PL. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer. 2004;4:34. |
| 43. | Lazova R, Moynes R, May D, Scott G. LN-2 (CD74). A marker to distinguish atypical fibroxanthoma from malignant fibrous histiocytoma. Cancer. 1997;79:2115-2124. |
| 44. | Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Iwashige H, Aridome K, Hokita S, Aikou T. Invariant chain expression in gastric cancer. Cancer Lett. 2001;168:87-91. |
| 45. | Ioachim HL, Pambuccian SE, Hekimgil M, Giancotti FR, Dorsett BH. Lymphoid monoclonal antibodies reactive with lung tumors. Diagnostic applications. Am J Surg Pathol. 1996;20:64-71. |
| 46. | Datta MW, Shahsafaei A, Nadler LM, Freeman GJ, Dorfman DM. Expression of MHC class II-associated invariant chain (Ii;CD74) in thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol. 2000;8:210-215. |
| 47. | Mizue Y, Nishihira J, Miyazaki T, Fujiwara S, Chida M, Nakamura K, Kikuchi K, Mukai M. Quantitation of macrophage migration inhibitory factor (MIF) using the one-step sandwich enzyme immunosorbent assay: elevated serum MIF concentrations in patients with autoimmune diseases and identification of MIF in erythrocytes. Int J Mol Med. 2000;5:397-403. |
| 48. | Subramaniam V, Vincent IR, Gardner H, Chan E, Dhamko H, Jothy S. CD44 regulates cell migration in human colon cancer cells via Lyn kinase and AKT phosphorylation. Exp Mol Pathol. 2007;83:207-215. |