INTRODUCTION
Successful transplantation depends on multiple factors including the prevention of preservation injuries incurred by cold storage and reperfusion[1,2]. A shortage of donor organs has an impact on the development of preservation methods. We developed the two-layer cold storage method (TLM) using perfluorochemicals (PFC) to reduce ischemic injury and maintain cellular integrity during preservation.
In 1966, it was reported that mice could breathe and survive in oxygen-saturated PFC[3]. PFC is a hydrocarbon, comprising a colorless and odorless solution with specific gravity approximately twice that of water. The most interesting property of PFC is a very high capacity for dissolving respiratory and other nonpolar gases[4]. A negligible O2-binding constant of PFC allows them to release O2 more effectively than hemoglobin into the surrounding tissue. As a result of this unique property, PFC-based solutions have been examined as oxygen carriers for blood substitutes, myocardial protection, respiratory support[4], and organ preservation before transplantation[5].
In this editorial, the role of the TLM in pancreas and islet transplantation is discussed.
DEVELOPMENT OF TWO-LAYER COLD STORAGE METHOD
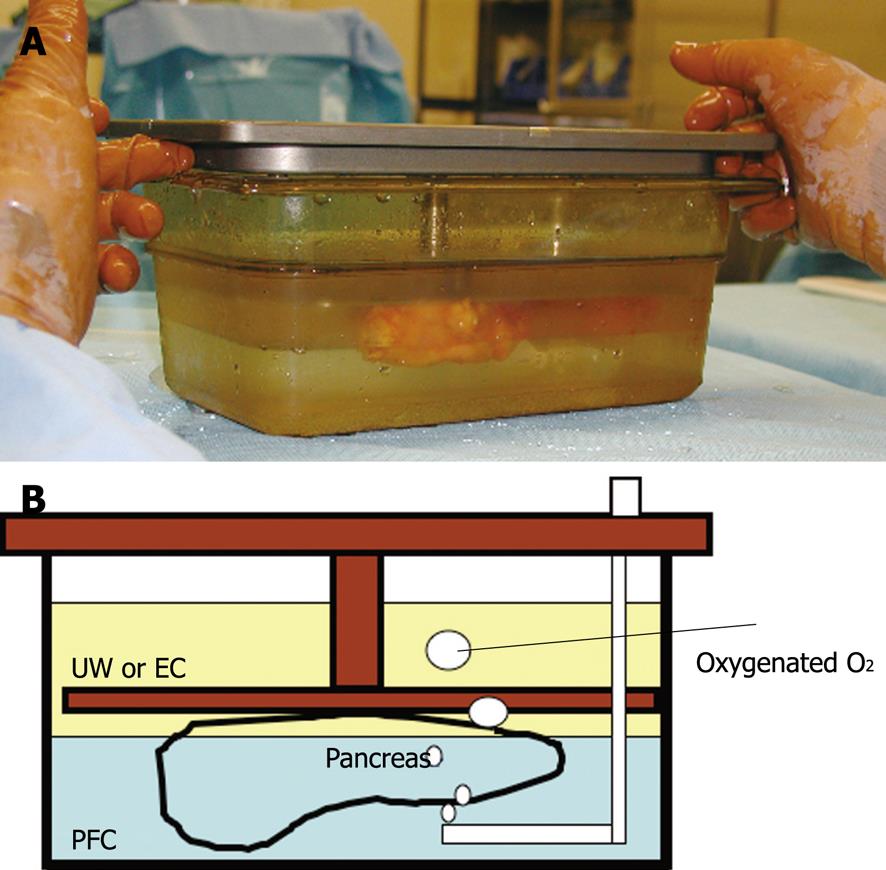
The TLM was first reported in 1988 at Kobe University as a pancreas cold storage method[5]. The TLM first consisted of a PFC and initially Euro-Collins’ solution (EC), which was later replaced by University of Wisconsin solution (UW)[6]. Because the PFC is lipophilic and has a high density, the PFC and EC or UW separate into layers. This allows for the pancreas graft to float on the oxygenated PFC surrounded by EC or UW (Figure 1).
Figure 1 The two-layer method.
The pancreas graft is surrounded by UW or EC and floated on the oxygenated PFC. A: The photograph; B: The schema. UW: University of Wisconsin solution; EC: Euro-Collin’s solution; PFC: Perfluorochemical.
Before UW was developed, EC was the standard solution for pancreas preservation before whole-pancreas transplantation. EC could preserve human pancreata for up to 10 h[7] and canine pancreata for up to 24 h[5]. The original TLM (EC/ PFC) could preserve canine pancreata for up to 72 h[5]. UW solution could preserve canine pancreata for up to 72 h[6], and the modified TLM (UW/ PFC) could preserve canine pancreata for up to 96 h[6]. Although PFC alone may provide an excellent preservation solution, this solution only preserved canine pancreata for 48 h[8]. Despite evidence for the superiority of the TLM, simple cold storage using UW has become the standard organ preservation method in clinical pancreas transplantation. This is likely to be because UW effectively preserves human pancreata for more than 24 h and the TLM is relatively more complicated compared with UW cold storage.
MECHANISM OF THE TWO-LAYER METHOD
The TLM reduced cold ischemic[6], warm ischemic[9], re-warming ischemic[10], and reperfusion[11] injuries in pancreas and islet transplantation. The mechanisms of the TLM have been vigorously examined using experimental transplantation models. During preservation by the TLM, the pancreas is directly oxygenated through PFC and maintains an oxygen tension at about 60% of the normal physiologic level[12].
During ischemia, tissue adenosine triphosphate (ATP) degrades to hypoxanthine following an increase of xanthine oxidase. Next, reactive oxygen radicals are generated and involved in a complex interaction of immediate cellular damage in ischemia-reperfusion injury[13,14]. On the other hand, tissue ATP is maintained in grafts preserved by the two-layer method and is rapidly recovered after reperfusion, which may be a better prophylaxis and treatment for the first step of ischemia-reperfusion injuries. During preservation by the TLM, pancreas grafts continuously generate ATP for up to 96 h[6]. ATP levels are also enhanced in human pancreas stored using the TLM. This proves the ability of the TLM to oxygenate human pancreas[15,16]. The ATP generated is used to drive a sodium-potassium pump to maintain cell integrity; thus, the TLM prevents pancreas swelling more effectively than UW solution[17]. Furthermore, the TLM improves the viability of the vascular endothelium and microcirculation[18]. Although the mechanisms involved in reducing ischemic reperfusion injury are unclear, one possibility is the induction of heat shock proteins during preservation[11]. ATP is essential for protein synthesis, and we demonstrated that protein synthesis was likely to be involved in the process of postischemic cellular recovery during preservation by the two-layer method[11,19]. These heat shock proteins may work to provide an anti-reperfusion or cell repair property.
The TLM also has an excellent ability to resuscitate pancreas grafts that have suffered from warm ischemic injury and to prolong the preservation time of ischemically damaged pancreata[20]. After 90 min of warm ischemic injury, the canine pancreas grafts lost ATP and were no longer viable. However, when the damaged pancreata were preserved and resuscitated by the TLM for 24 to 48 h at 4°C, the grafts regained tissue ATP and became viable[19]. The resuscitation effect is associated with active protein synthesis, suggesting active cell repair, and may involve heat shock proteins[19]. This suggested the possibility of pancreas transplantation from ischemically damaged or non-heart-beating donors.
Resuscitation by the TLM before pancreas transplantation correlates with the pancreatic tissue ATP levels after the TLM[21]. This is important because it predicts the outcomes of marginal donor grafts before transplantation. Recently, the TLM has shown promise in small intestine[22] and heart preservation[23].
PANCREAS PRESERVATION BEFORE ISLET ISOLATION
The most common exclusion criteria of pancreata for islet isolation are an unstable blood pressure, significant downtime, and high-level vasopressor usage for donor management. All of these criteria are related to warm ischemic injury and can be alleviated using the TLM; thus, the TLM could potentially make the majority of the donor pancreata usable for islet isolation.
Tanioka et al[24] first reported islet isolation after preservation by the TLM in a canine pancreas model. After 24 h storage of the canine pancreas in UW solution, islet yields were significantly decreased and the posttransplant outcome was further deteriorated. However, after 24 h of preservation by the TLM, the islet yield and posttransplant outcome were essentially equal to the immediate isolation.
After 60 min of warm ischemia, the islet yield after islet isolation was significantly decreased compared with the islet yield from the pancreas without warm ischemia[25]. When pancreata ischemically damaged for 60 min were preserved using the TLM for 24 h, the level of islet yield became similar to that from pancreata without warm ischemia[26]. Tanaka et al[27] also demonstrated that pancreata damaged on 30 min warm ischemia were restored by 3 h TLM preservation. The TLM may facilitate the selective use of non-heart-beating donors as an alternative source for islet transplantation.
In the rat model, the TLM for pancreas preservation prior to islet isolation resulted in an excellent islet function in addition to improved islet yield, which was almost comparable to freshly isolated islets[28].
At the University of Alberta (Edmonton, Canada), it was observed that seven of seven patients with type 1 diabetes receiving allogeneic islet transplants became insulin independent[29]. Two major drawbacks of the Edmonton protocol are that it requires pancreata to have short preservation periods before islet isolation, and almost always requires two or more donor pancreata to cure one diabetic patient.
On the basis of these data, Matsumoto et al[30] examined the effect of the TLM on the pancreas before islet isolation using preclinical, nonhuman primate pancreata and human pancreata. In the primate model, storing pancreata in UW solution for 5 h yielded a similar islet number, viability, and in vitro function compared with immediate isolation. However, 5 h of TLM preservation significantly increased the islet yield, viability, and in vitro function. They also suggested that the TLM maintains or repairs exocrine cell integrity and prevents trypsin activation, which enables effective collagenase delivery and protects islets from enzymatic digestion.
Zhang et al[31] also demonstrated that the islet yield from pancreata preserved using the TLM was more than that from pancreata stored in UW solution. Islet viability was significantly higher with the TLM vs UW solution. This experiment confirmed the superiority of the TLM-based preservation of pancreata for human islet isolation compared with UW solution. Although the beneficial effect of the TLM is controversial, the overall performance of the TLM could improve the outcome of islet isolation and transplantation[32].
CLINICAL APPLICATION OF THE TWO-LAYER METHOD
The TLM was clinically employed for pancreas transplantation at the University of Minnesota for the first time in 1999[33]. In this first clinical trial involving 10 cases, the TLM had no adverse effect on the recipients after transplantation. Furthermore, the morphologic quality of the human pancreas grafts after reperfusion was excellent compared with the pancreata stored in UW solution. There was no episode of acute rejection of pancreata preserved using the TLM. This is interesting because the immunosuppressive property of PFCs was one of the reasons not to use them as a blood substitute[34]. This immunosuppressive property could be beneficial for allogeneic organ preservation, and the role is under investigation.
Pancreas preservation using the TLM before islet isolation for clinical islet transplantation began at the University of Minnesota[35]. Hering et al[35] reported the cure of four patients with islets from a single donor. In this report, cadaver pancreata were subjected to the TLM immediately after retrieval at the procurement site without UW solution storage before islet isolation. Their islet yield was sufficient to cure one patient from one donor. Their experiment suggests that pancreata should be preserved by the TLM immediately at the time of procurement.
CONCLUSION
Irrespective of these promising data, the role of the TLM requires further investigation in pancreas and islet transplantation.
Peer reviewer: Norbert Senninger, Professor, Department of General Surgery, University Clinics, Westphalian-Wilhelm’s-University, Waldeyerstrasse 1, D-48149 Muenster, Germany
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH