Published online Jul 7, 2010. doi: 10.3748/wjg.v16.i25.3215
Revised: March 30, 2010
Accepted: April 6, 2010
Published online: July 7, 2010
We report a case of hepatocellular carcinoma (HCC) occurring in a patient with Crohn’s disease (CD) without chronic hepatitis or liver cirrhosis, and review the clinicopathological features of HCC in CD patients. A 37-year-old Japanese man with an 8-year history of CD and a medication history of azathioprine underwent resection of a liver tumor. The histopathology of the liver tumor was pseudoglandular type HCC. In the non-neoplastic liver, focal hepatocyte glycogenosis (FHG) was observed, however, there was no evidence of liver cirrhosis or primary sclerosing cholangitis. Only nine cases of HCC in CD patients have been reported previously in the English-language literature. Eight of 10 cases (including the present case) had received azathioprine treatment, and four of these cases also showed FHG, which is considered a preneoplastic liver lesion, within the non-neoplastic liver. Although the precise mechanism of the development of HCC in CD patients is controversial, these results suggest that azathioprine therapy and FHG in the non-neoplastic liver contribute to the development of HCC. These findings also indicate that it is important to survey CD patients treated with prolonged azathioprine therapy for potential liver tumors.
- Citation: Ishida M, Naka S, Shiomi H, Tsujikawa T, Andoh A, Nakahara T, Saito Y, Kurumi Y, Takikita-Suzuki M, Kojima F, Hotta M, Tani T, Fujiyama Y, Okabe H. Hepatocellular carcinoma occurring in a Crohn’s disease patient. World J Gastroenterol 2010; 16(25): 3215-3218
- URL: https://www.wjgnet.com/1007-9327/full/v16/i25/3215.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i25.3215
Fatty liver and primary sclerosing cholangitis are known to be associated with Crohn’s disease (CD), and patients with CD also experience an increased risk of malignant lymphoma and cancers of the small intestine or colon[1,2]. Nonetheless, hepatocellular carcinoma (HCC) in CD patients is extremely rare, with only nine cases reported in the English-language literature[3-11]. Here, we report one additional case of HCC in a CD patient without established chronic liver disease, and review the clinicopathological features of HCC in CD patients. In addition, we discuss the tumorigenesis of HCC in CD patients and the relationship between HCC and azathioprine treatment.
CASE REPORT
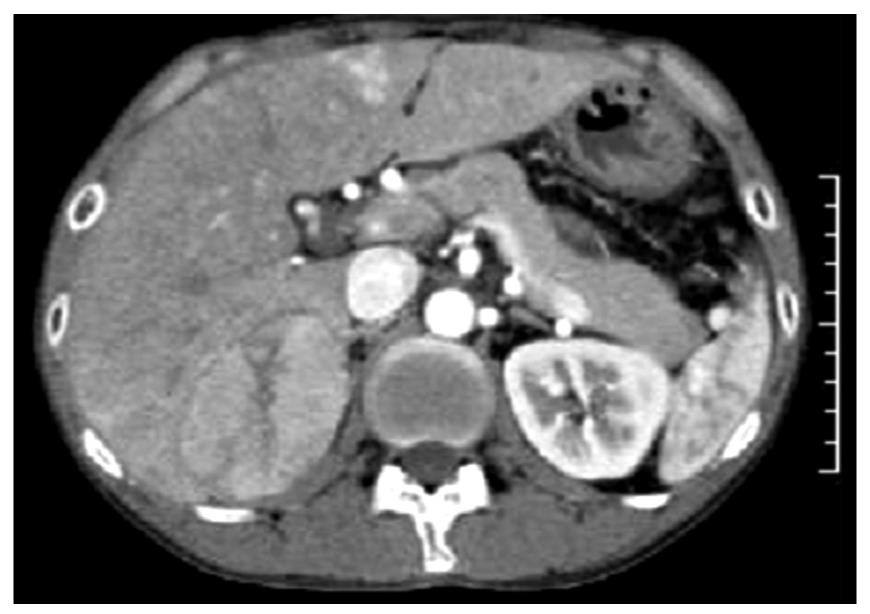
A 37-year-old Japanese man with an 8-year history of CD was admitted to our hospital for examination of a liver tumor. He had been diagnosed with CD at age 29 years, when he required surgery for a bowel fistula. He had been treated with elemental diet, prednisolone, azathioprine, and 5-aminosalicylic acid. Two years prior to this admission, magnetic resonance imaging (MRI) showed a liver tumor in S7, which measured 4 cm × 3 cm. The liver tumor enlarged gradually in follow-up computed tomography (CT) and MRI. Preoperative abdominal contrast-enhanced CT disclosed the S7 tumor that measured 8 cm × 5 cm, which showed early arterial enhancement (Figure 1).
Upon admission, biopsy of the S7 tumor was performed, and histopathological study showed HCC. Then, the patient underwent hepatic resection of the posterior segment.
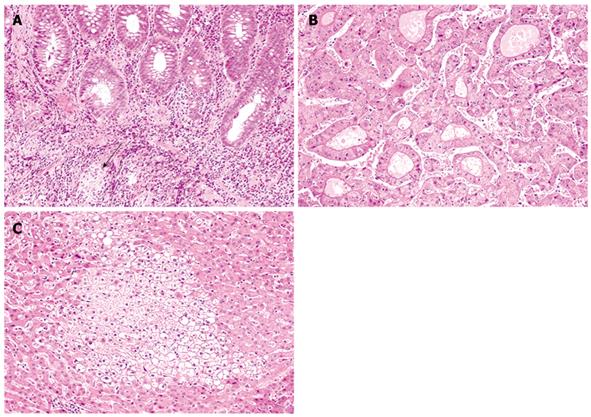
Preoperative colorectal endoscopic examination revealed mucosal redness and pseudopolyposis throughout the entire colorectum. Histopathological findings of the colorectal mucosa corresponded to CD, with the presence of discontinuous lymphoplasmacytic infiltrate in the lamina propria and a few non-caseating granulomas, unrelated to crypt rupture (Figure 2A).
Preoperative laboratory data revealed mild anemia (hemoglobin 11.1 g/dL; range 12.4-17.0). Liver enzymes were within normal limits (aspartate aminotransferase 14 IU/L; range 7-38, and alanine aminotransferase 15 IU/L; range 4-43). C-reactive protein was slightly elevated (2.57 mg/dL; range < 0.3). Although serum alpha-fetoprotein level was normal (7.7 ng/mL; range < 20), protein induced by vitamin K absence II (PIVKA II) level was markedly elevated (757 mAU/mL; range < 40). Serology was negative for hepatitis B surface antigen, hepatitis B surface antibody, hepatitis B core antibody, and hepatitis C antibody. In addition, he had no history of alcohol consumption.
Microscopically, the resected specimen of the S7 tumor was almost well-circumscribed by a fibrous capsule, but focal extracapsular invasion was observed. The tumor displayed pseudoglandular to focal trabecular growth of tumor cells with rich eosinophilic cytoplasm and enlarged, round to oval nuclei with a nucleolus (Figure 2B). These histopathological findings were typical of pseudoglandular type HCC.
Non-neoplastic resected liver tissue showed no evidence of liver cirrhosis, chronic hepatitis, or primary sclerosing cholangitis. However, some foci of benign-appearing clear hepatocytes were observed (Figure 2C). These clear hepatocytes were confirmed to have glycogen accumulation (focal hepatocyte glycogenosis; FHG), because they stained positive for periodic acid-Schiff and were digested by diastase. In addition, no histopathological evidence suggestive of non-alcoholic steatohepatitis, such as macrovesicular steatosis, pericellular fibrosis, and neutrophils infiltration, was observed in the non-neoplastic liver tissue.
The postoperative course was uneventful, and no tumor recurrence has been observed during 2 years follow-up.
HCC generally occurs in patients with established chronic liver disease, such as liver cirrhosis and viral hepatitis. HCC in CD patients is extremely rare; to the best of our knowledge, only nine cases have been reported previously in the English-language literature[3-11]. The Table 1 summarizes the clinicopathological features of HCC in CD patients. The mean duration from the onset of CD to the development of HCC is 15.1 years (range: 0-36). Although no patients, including the present case, demonstrated apparent risk factors for developing HCC, such as liver cirrhosis or viral hepatitis, two patients had primary sclerosing cholangitis (Table 1)[6,8]. In the five cases in which histological typing of HCC was available, there were three cases of pleomorphic type HCC and two of trabecular type HCC (Table 1). The present case is believed to be the first case of pseudoglandular type HCC occurring in CD.
| Case No. | Sex | Age at: onset of CD/discovery of HCC (yr) | Medication for CD | Serum AFP/PIVKA II (ng/mL, mAU/mL) | Histology of HCC | Histology ofnon-neoplastic liver | Outcome | Ref. |
| 1 | F | 29/43 | AZA, PSL | NA/NA | NA | No cirrhosis | DOD with intraabdominal and intrathoracic metastases | [3] |
| 2 | F | 9/22 | 5-ASA, AZA | 55 000/NA | Trabecular | No cirrhosis FHG (+) | Recurrence at 6 mo, liver transplantation performed | [4] |
| 3 | M | 13/33 | 5-ASA, AZA | NA/NA | NA | No cirrhosis | Lung metastases | [5] |
| 4 | F | 63/63 | 5-ASA | NA/1100 | NA | PSC | No evidence of recurrence | [6] |
| 5 | F | 14/28 | AZA, IFx | 26.9/NA | Trabecular, pleomorphic | No cirrhosis FHG (+) | NA | [7] |
| 6 | M | 17/33 | AZA | Normal range/NA | NA | No cirrhosis PSC | Mediastinal and abdominal metastases; died 1 year after | [8] |
| 7 | M | 19/37 | 5-ASA, AZA, PSL | 15/NA | Trabecular to sinusoidal, pleomorphic | No cirrhosis (only CT imaging) | DOD 3 mo after surgery | [9] |
| 8 | M | 16/52 | 5-ASA | 13.9/16 300 | Trabecular | Chronic liver damage | No recurrence was found on CT imaging, but PIVKA II was 1980 | [10] |
| 9 | M | 13/25 | AZA, IFx, PSL | 78/NA | Pleomorphic | No cirrhosis FHG (+) | No metastasis or recurrence 1 yr after surgery | [11] |
| Present report | M | 29/37 | 5-ASA, AZA, PSL | 7.7/757 | Pseudoglandular | No cirrhosis FHG (+) | No recurrence 2 yr after surgery |
Most of the CD patients showed a history of medication with one or more drugs (Table 1). Eight of 10 cases had been treated with azathioprine and six had a medication history with 5-aminosalicylic acid. Azathioprine is an inhibitor of purine synthesis, and is prescribed widely in patients with organ transplantation or inflammatory bowel disease[12]. Patients who undergo long-term azathioprine-based immunosuppressive treatment have been reported to show an increased risk of non-Hodgkin lymphoma and cutaneous squamous cell carcinoma[13,14].
The occurrence of HCC in patients who received azathioprine therapy in the absence of liver cirrhosis and viral hepatitis remains exceptional; however, one case of HCC in a renal transplant patient without apparent risk factors other than prolonged azathioprine therapy has been reported (no hepatitis C studies were available at that time)[15], and one case of HCC after azathioprine therapy has also been reported in a patient with ulcerative colitis but no hepatitis B and C[16]. In addition, 80% of CD patients who developed HCC had a medication history of azathioprine (Table 1).
The precise mechanism involved in the development of HCC in CD patients is controversial. In one experimental study, azathioprine was shown to increase hepatocyte turnover[17]. It was hypothesized that the degree of immunosuppression by prolonged azathioprine therapy was associated with the incidence of neoplasia[15]. These results suggest that azathioprine therapy is associated with the development of HCC in CD patients.
FHG was observed in the present case, as well as in three other CD patients previously reported to have developed HCC[4,7,11]. Although the cause and significance of FHG are not well established, glycogenated foci can be considered preneoplastic lesions, because FHG and lesions observed during drug-induced hepatocarcinogenesis have histological and biochemical similarities, and the presence of FHG within non-malignant hepatocellular nodules of cirrhotic liver can predict their malignant transformation[18]. Cattan et al[4] have reported previously on a CD patient with HCC who had both a medication history of azathioprine as well as disseminated FHG in the non-neoplastic liver. They suspected the pathogenic link between long-term azathioprine therapy and FHG. These results suggest that FHG could be related to hepatocarcinogenesis in CD patients.
In conclusion, we reported a case of pseudoglandular type HCC in a CD patient without established chronic liver disease. The patient had a medication history of azathioprine and FHG in the non-neoplastic liver. Although the precise mechanism of the development of HCC in CD patients is controversial, azathioprine therapy and FHG are likely to be involved. Therefore, accumulation of cases is necessary to clarify the precise mechanism of the development of HCC in CD patients, and we stress the importance of surveying CD patients with prolonged azathioprine therapy for liver tumors.
Peer reviewer: Satoru Kakizaki, MD, PhD, Assistant Professor, Department of Medicine and Molecular Science, Gunma University, Graduate School of Medicine, 3-39-15 Showa-machi, Maebashi, Gunma 371-8511, Japan
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Juillerat P, Mottet C, Pittet V, Froehlich F, Felley C, Gonvers JJ, Vader JP, Michetti P. Extraintestinal manifestations of Crohn’s disease. Digestion. 2007;76:141-148. |
| 2. | von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The risk of cancer in patients with Crohn’s disease. Dis Colon Rectum. 2007;50:839-855. |
| 3. | Lee FL, Murray SM, Prior J, Shreeve DR. Primary liver cell cancer occurring in association with Crohn’s disease treated with prednisolone and azathioprine. Hepatogastroenterology. 1983;30:188. |
| 4. | Cattan S, Wendum D, Chazouilleres O, Schmitz J, Gendre JP. Hepatocellular carcinoma and focal hepatic glycogenosis after prolonged azathioprine therapy. Hum Pathol. 2000;31:874-876. |
| 5. | Borum ML. Unusual development of hepatocellular carcinoma in a patient with Crohn’s disease. Dig Dis Sci. 2001;46:2199-2200. |
| 6. | Oya H, Sato Y, Yamamoto S, Takeishi T, Kobayashi T, Hatakeyama K. Living related donor liver transplantation for primary sclerosing cholangitis with hepatocellular carcinoma and Crohn’s disease: a case report. Transplant Proc. 2004;36:2297-2298. |
| 7. | Chen SC, Cummings OW, Hartley MP, Filomena CA, Cho WK. Hepatocellular carcinoma occurring in a patient with Crohn's disease treated with both azathioprine and infliximab. Dig Dis Sci. 2006;51:952-955. |
| 8. | Demarchi B, Bresso F, Novero D, Palestro G, Sapone N, Pellicano R, Bonardi R, Smedile A, Rizzetto M, Astegiano M. Hepatocellular carcinoma complicating primary sclerosing cholangitis in Crohn’s disease. A case report. Minerva Gastroenterol Dietol. 2007;53:279-283. |
| 9. | Samarasena J, Borgaonkar M. Development of hepatocellular carcinoma in a patient with Crohn’s disease treated with azathioprine. Dig Dis Sci. 2007;52:2748-2750. |
| 10. | Miura H, Kawaguchi T, Takazoe M, Kitamura S, Yamada H. Hepatocellular carcinoma and Crohn’s disease: a case report and review. Intern Med. 2009;48:815-819. |
| 11. | Murakami A, Tanaka Y, Ueda M, Nagano Y, Kunisaki R, Morimoto M, Enaka M, Tanabe M, Kawachi K, Sasaki T. Hepatocellular carcinoma occurring in a young Crohn’s disease patient. Pathol Int. 2009;59:492-496. |
| 12. | Etchevers MJ, Aceituno M, Sans M. Are we giving azathioprine too late? The case for early immunomodulation in inflammatory bowel disease. World J Gastroenterol. 2008;14:5512-5518. |
| 13. | Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121-1125. |
| 14. | Ingvar A, Smedby KE, Lindelöf B, Fernberg P, Bellocco R, Tufveson G, Höglund P, Adami J. Immunosuppressive treatment after solid organ transplantation and risk of post-transplant cutaneous squamous cell carcinoma. Nephrol Dial Transplant. 2009;Epub ahead of print. |
| 15. | Gruber S, Dehner LP, Simmons RL. De novo hepatocellular carcinoma without chronic liver disease but with 17 years of azathioprine immunosuppression. Transplantation. 1987;43:597-600. |
| 16. | Russmann S, Zimmermann A, Krähenbühl S, Kern B, Reichen J. Veno-occlusive disease, nodular regenerative hyperplasia and hepatocellular carcinoma after azathioprine treatment in a patient with ulcerative colitis. Eur J Gastroenterol Hepatol. 2001;13:287-290. |
| 17. | Arber N, Zajicek G, Nordenberg J, Sidi Y. Azathioprine treatment increases hepatocyte turnover. Gastroenterology. 1991;101:1083-1086. |
| 18. | Terasaki S, Kaneko S, Kobayashi K, Nonomura A, Nakanuma Y. Histological features predicting malignant transformation of nonmalignant hepatocellular nodules: a prospective study. Gastroenterology. 1998;115:1216-1222. |