Published online Jul 7, 2010. doi: 10.3748/wjg.v16.i25.3196
Revised: March 17, 2010
Accepted: March 24, 2010
Published online: July 7, 2010
AIM: To determine the clinical value of diffusion-weighted imaging (DWI) for the diagnosis of extrahepatic cholangiocarcinoma (EHCC) by comparing the diagnostic sensitivity of DWI and magnetic resonance cholangiopancreatography (MRCP).
METHODS: Magnetic resonance imaging examination was performed in 56 patients with suspected EHCC. T1-weighted imaging, T2-weighted imaging, MRCP and DWI sequence, DWI using single-shot spin-echo echo-planar imaging sequence with different b values (100, 300, 500, 800 and 1000 s/mm2), were performed. All cases were further confirmed by surgery or histopathological diagnosis. Two radiologists jointly performed the analysis of the DWI and MRCP images. Apparent diffusion coefficient (ADC) value and signal-noise ratio were calculated for EHCC. Sensitivity, specificity, accuracy, positive predictive value and negative predictive value were tested using DWI with a b value of 500 s/mm2 and MRCP images, respectively.
RESULTS: Histopathological diagnosis confirmed that among the 56 cases, 35 were EHCC (20 hilar and 15 distal extrahepatic), 16 were cholangitis, and 5 were calculus of bile duct. Thirty-three out of the 35 EHCC cases were detected by DWI. EHCC exhibited differential levels of high signal intensity in DWI and low signal intensity in the ADC map. The mean value for ADC was (1.31 ± 0.29) × 10-3 mm2/s. The detection rate of EHCC was significantly higher by DWI (94.3%) than by MRCP (74.3%) (P < 0.05). There was a significant difference in sensitivity (94.3% vs 74.3%), specificity (100% vs 71.4%), accuracy (96.4% vs 73.2%), positive predictive value (100% vs 81.3%), and negative predictive value (91.3% vs 62.5%) between DWI and MRCP in diagnosing EHCC.
CONCLUSION: DWI has a high sensitivity for the detection of EHCC as it shows the EHCC lesion more unambiguously than MRCP does. DWI can also provide additional clinically important information in EHCC patients when added to routine bile duct MR imaging protocols.
- Citation: Cui XY, Chen HW. Role of diffusion-weighted magnetic resonance imaging in the diagnosis of extrahepatic cholangiocarcinoma. World J Gastroenterol 2010; 16(25): 3196-3201
- URL: https://www.wjgnet.com/1007-9327/full/v16/i25/3196.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i25.3196
Extrahepatic cholangiocarcinoma (EHCC) refers to cancers occurring at the left or right hepatic duct to the ampulla. In clinical practice, the lack of typical early symptoms often results in a delayed or incorrect diagnosis. In recent years, advances in imaging technology have significantly improved the detection rate and accuracy of definitive diagnosis for EHCC.
With the continuous development and improvement of magnetic resonance imaging (MRI) hardware and software, the use of parallel acquisition techniques to reduce scan time and improve spatial resolution, and the wide application of the shortened echo chain technique in spin-echo echo-planar imaging (SE-EPI), diffusion-weighted imaging (DWI) has been increasingly used in a wide range of clinical applications[1,2]. Although the application of DWI to the diagnosis of numerous conditions has attracted increased attention and is the focus of many researches, to our knowledge, there exists no literature on the clinical application of DWI to the diagnosis of EHCC.
A total of 56 patients (34 male and 22 female) were examined between March 2008 and December 2009. The patients had a suspected diagnosis of EHCC, and the main clinical symptom was jaundice. Patient ages ranged from 38 to 75, with an average age of 61.0. Thirty-three patients had an elevated level of carbohydrate antigen 19-9 (CA19-9).
All imaging was performed on a 1.5T MRI scanner (Signa Excite HD, General Electric Healthcare) and a body phased array coil. Patients were asked to fast 4-6 h before imaging, were coached in breathing and breath-hold and were imaged in the supine position. All patients underwent MR axial fast spoiled gradient echo (FSPGR) T1-weighted (T1W), fast recovery fast spin echo (FRFSE) fat-suppressed T2-weighted (T2W), coronal fast imaging employing steady-state acquisition (FIESTA), 3D MRCP and DWI axial scans. 3D MRCP using fast recovery fast spin echo sequence (FRFSE-XL), heavily T2W with respiratory gating, triggered at exhale, and thin multi-plane collection. The scan parameters were: TR and TE time adjusted according to the respiratory rate, and set at approximately 2000-4500 ms and 550-750 ms, respectively; bandwidth 62.5 kHz, echo chain length (ETL) 8; field of view (FOV) 360-400 mm, matrix 320 × 256; slice thickness 3 mm, without interval (-1.5 mm), 80 slices, NEX 1, acquisition time was 2 min and 20 s to 3 min. The original 2D multi-plane formation image was reconstructed into 3D MRCP images using the maximum intensity projection (MIP) and volume-rendering (VR) functions in the workstation, thereby allowing the reconstructed 3D MRCP images to be rotated in any direction. Axial DWI used a single-shot SE-EPI sequence, space parallel acquisition and array spatial sensitivity encoding technique (ASSET), and the scan was performed under breath-hold. Diffusion gradients were applied along three orthogonal directions: frequency encoding (X), phase encoding (Y) and slice-select (Z). The diffusion sensitivity coefficients were b = 100, 300, 500, 800, and 1000 s/mm2. The DWI scan parameters were: TR 1300 ms, effective TE 41.0-69.2 ms; bandwidth 166.67 kHz, flip angle 90°, slice thickness 5 mm, slice gap 1 mm, single acquisition, FOV 340-400 mm, matrix 128 × 128, NEX 4, acceleration factor 2.
All MR images were jointly viewed and analyzed by two senior radiologists with more than 5 years of combined experience in abdominal MRI diagnosis. All image post-processing was performed using the Advantage Workstation 4.3 (AW4.3, General Electric Healthcare) and the FuncTool software package.
The apparent diffusion coefficient (ADC) values and signal-noise ratio (SNR) of the lesions imaged in the DWI sequence were calculated for different b values. The formula[3] for calculating the value of ADC is given by: ADC = [ln (Slow/Shigh)]/(bhigh-blow). Where Slow and Shigh represent low signal intensity and high signal intensity, respectively, and bhigh, and blow represent the high b value and low b value, respectively.
After calculating the ADC map using AW4.3, the ADC values can be automatically measured by mapping regions of interest (ROI). The ROI is mapped by selecting lesion areas with high signal intensity in DWI and low signal intensity in ADC. Preferably, the size of ROI is between 8-20 mm2. Two radiologists performed these tests three times each to derive the mean value. The SNR represents the ratio of lesion signal intensity to the standard deviation of background noise along the phase-encoding direction.
For DWI-based detection and confirmation of diagnosis of EHCC, the b value used was 500 s/mm2. The evaluation criteria for EHCC were the status of the malignant lesions, the intensity of the malignant lesion signal, the degree of reduction in the malignant lesion signal with the increase of b value, and the ADC value of the malignant lesion. In detail, the following descriptions were assigned[4]: malignant lesions demonstrate mild to moderate high signal intensity in DWI with a b value of 0 s/mm2; compared with the surrounding tissue, malignant lesions continue to demonstrate high signal intensity in DWI with a b value of 500 s/mm2; ADC map demonstrates low signal intensity and has a relatively low ADC value. Otherwise, the lesion was considered benign.
In MRCP, the malignant stricture is characterized by an irregular narrow edge, non-symmetry of the narrow and an abrupt cut-off[5,6].
All data were processed using the SPSS 13.0 statistical package. Measurement data are represented by mean ± SD. To compare the ADC value and SNR under different b values, the Friedman test was performed over multiple relevant samples, and the detection sensitivity of DWI and MRCP for EHCC was determined using the χ2 test. A P value of < 0.05 was used as the statistical significance level of the observed differences.
All 56 cases collected in the study were confirmed by histopathological diagnosis. Of the 56 cases, 35 were EHCC (20 located near the hepatic hilum, 15 located at the far end of the extrahepatic bile duct), 16 were cholangitis, and 5 were bile duct stones.
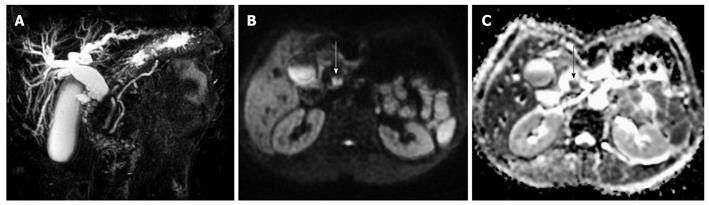
Out of the 35 histopathologically-confirmed EHCC cases, 33 cases were detected by DWI. Compared with the surrounding tissues, these cases of EHCC exhibited differential levels of high signal intensity in the DWI image and exhibited low signal intensity in the ADC map (Figure 1). The average size of the EHCC was 28.5 mm (range, 5.0-67.2 mm). For EHCC, the mean ADC value (n = 33) was (1.31 ± 0.29) × 10-3 mm2/s (95% CI, 1.20-1.47). For bile duct stones, the mean ADC value was (0.48 ± 0.22) × 10-3 mm2/s (95% CI, 0.21-0.75). The two cases of EHCC undetected by DWI were due to the interference of artifacts around the bile duct and the small size of the malignant transformation of the bile duct adenoma (5 mm), respectively.
For the DWI sequence, the SNR gradually decreased in the lesion of EHCC as the b value was increased (Table 1). The difference was statistically significant (P < 0.01). With the increase in b value, the ADC value for the lesion gradually decreased (Table 1), and a P value of less than 0.05 was considered statistically significant.
| B value (s/mm2) | ADC (× 10-3 mm2/s) | SNR |
| 100 | 2.73 ± 0.55 | 135.81 ± 33.27 |
| 300 | 1.84 ± 0.42 | 99.17 ± 22.63 |
| 500 | 1.31 ± 0.29 | 69.31 ± 16.36 |
| 800 | 1.22 ± 0.29 | 47.90 ± 12.35 |
| 1000 | 0.89 ± 0.23 | 29.48 ± 9.72 |
| M | 136.23 | 142.79 |
| P | < 0.01 | < 0.01 |
MRCP correctly diagnosed 4 out of 5 cases of bile duct stones, 11 out of 16 cases of cholangitis (5 cases were incorrectly diagnosed as EHCC), and 26 out of 35 cases of EHCC (2 cases were not detected and 7 were incorrectly diagnosed). The overall rate of correct diagnosis was 73.2% (41/56) (Table 2). DWI correctly diagnosed all 5 cases of calculi, all 16 cases of inflammation and 33 out of 35 cases of EHCC (1 case was not detected and 1 was incorrectly diagnosed). The overall rate of correct diagnosis was 96.4% (54/56) (Table 2). The detection sensitivity and specificity of MRCP was 74.3% and 71.4%, respectively. For DWI, the sensitivity and specificity was 94.3% and 100%, respectively. The differences in detection sensitivity, specificity, and accuracy between the two examination methods for EHCC were of statistical significance (P < 0.05). The positive predictive value and negative predictive value of DWI (100%, 91.3%) were higher than those of MRCP (81.3%, 62.5%). The differences between the two sets of values were of statistical significance (P < 0.05).
| Confirmed pathological diagnosis | Total | ||
| EHCC | Non-EHCC | ||
| DWI | |||
| EHCC | 33 | 0 | 33 |
| Non-EHCC | 2 | 21 | 23 |
| MRCP | |||
| EHCC | 26 | 6 | 32 |
| Non-EHCC | 9 | 15 | 24 |
| Total | 35 | 21 | 56 |
DWI reflects the micro-movements of water molecules. It produces images by comparing the inter-tissue diffusion coefficient. It can be used to investigate tissue organization, structure and functional status of a biological object at the cellular and molecular level. Unlike conventional MRI examination, DWI enables imaging of the human body to be performed at a microscopic level, thereby allowing for accurate, rapid and reliable characterization of the spatial organization and composition of tissues, and the functional status of water exchange between tissue components affected by pathological or physiological status.
The extent of water molecule diffusion ability in a tissue is negatively correlated to that tissue’s cell density and integrity of the cell membranes. The degree of water molecule movement was found to positively correlate with signal reductions in DWI. The movement of water molecules is more restricted as the cell membrane becomes more intact or if the cell density is higher (i.e. tumor tissue). Therefore, the observation of a relative reduction of signal in tissue imaged with DWI can assist in the detection and confirmation of tumor. The solid portion of the tumor has a higher cell density than normal tissues, and thus should continuously exhibit relatively higher signal intensity on DWI. However, given the fact that DWI is inherently T2-weighted, an extra-long T2 relaxation time can also show as continuously higher signal intensity in DWI, which may then be misdiagnosed as restricted diffusion. This is known as the T2 shine-through effect[7,8]. To avoid this effect, ADC values can be calculated using two images obtained under different b values. The ADC map can then be used to overcome the T2 shine-through effect by observing the relative reduction in signal in images obtained under different b values. Confirmation of tumor through differences in tissue water molecule diffusion is thus made possible. Higher cell densities restrict diffusion and exhibit low ADC values, otherwise, high ADC values are present. The b value is the sensitivity coefficient for diffusion; it can be used to determine the sensitivity for detection of diffusion of water molecules in the tissue under examination. The larger the b value used in DWI, the greater the dispersion of water molecules, and the more obvious the signal intensity reduction. This work focused on observing different ADC values for EHCC under different b values, followed by a comparison of the impact of b value on the ADC value measurement. The ADC value for EHCC was (1.84 ± 0.42) × 10-3 mm2/s under a b value of 300 smm2; the ADC value was (0.89 ± 0.23) × 10-3mm2/s under a b value of 1000 s/mm2. Using a smaller b value can produce a relatively higher ADC value, and with the increase in b value, the ADC value for lesions decreased. This correlation was of statistical significance. In biological tissues, the signal reduction in DWI is determined not only by the diffusion effect of water molecules, but also by reperfusion from the capillary microcirculation. In the work by Yamada et al[9], when a low b value was used, the derived ADC value mainly reflected the blood perfusion status of the tissue. Hence, large b values should be used to reflect the true diffusion ability of water molecules in the lesion. The larger the b value used, the better the image will reflect the true diffusion ability of water molecules in the lesion. In theory, when deriving the ADC value, a larger b value should cause the lesion to exhibit relatively higher signal intensity in DWI, producing greater lesion contrast in the ADC map, and increasing the accuracy of the derived ADC value. However, it is worth noting that increasing the b value will likely result in an increase in the diffusion gradient pulse time. The increased TE value can reduce the SNR of DWI and impact on the image quality. Our results show that the SNR of the lesion in DWI decreases following an increase in b value. Hence, the b value should be set between 500 and 800 s/mm2 to ensure good image quality and an accurate ADC value, which is relatively close to the true diffusion value.
Previously, image quality for abdominal imaging has been relatively poor due to the impact of such factors as abdominal breathing, cardiac motion and chemical shifts. With the development of fast imaging techniques, i.e. single-shot echo planar imaging[10-14], the technology for performing abdominal DWI has improved rapidly[15,16]. DWI has been widely applied to the detection and confirmation of cancer in liver, pancreas, kidney, colon and prostate[17-21]. It has been reported that the characterization sensitivity of MRCP combined with an enhanced 3D MRI scan for EHCC is 87%, and the characterization specificity is 51%[22]. Our results demonstrate that DWI has a greater detection sensitivity and specificity than MRCP in terms of detection of EHCC. DWI reflects microstructural changes in tumor cells, where increases in the DWI signal are mainly caused by diffusion contrast, while T2W and T1W imaging cause signal increases by relaxation time differences. EHCC leads to increased cell density, diminished extracellular space, and restricted movement of water molecules due to an increase of macromolecular materials, all of which increase the EHCC signal. DWI possesses good background suppression effects; blood vessels, the bile duct and intra-abdominal fat all exhibit obvious low signal intensities. This increases tumor contrast to surrounding tissues, which improves lesion detection and facilitates observation of the size and scope of EHCC. DWI also provides a good image-based reference for clinical treatment. By contrast, MRCP only performs analysis over the site and shape of the bile duct stricture, and does not provide intuitive data by which to judge the characteristics and scope of the disease. When the cholangiocarcinoma is of an infiltrating growth along the bile wall, MRCP shows the lesion as funnel-shaped or a gradually narrowing cone shape, which is difficult to differentiate from chronic inflammatory bile duct stricture. It is also hard to distinguish non-typical cholangiolithiasis and cholangiocarcinoma growing in the bile cavity, as both exhibit low signal intensity filling defects in the high signal intensity bile of MRCP. In theory, different compositions and spatial distributions of lesion tissue and cells lead to different diffusion coefficients, which can contribute to the identification of bile duct lesions. This work shows that with a b value of 500 s/mm2, the ADC value for EHCC was (1.31 ± 0.29) × 10-3mm2/s, while the ADC value for cholangiolithiasis was (0.48 ± 0.22) × 10-3 mm2/s. Hence, the measurement of lesion ADC values is useful for distinguishing non-typical cholangiolithiasis and cholangiocarcinoma growing in the bile cavity. However, due to the fact that the size of the ROI often exceeds the size of the thickened bile wall, it is difficult to derive an accurate ADC value for cholangitis. Distinguishing between cholangitis and EHCC only depends on changes in DWI signal but lacks quantitative indicators.
There remain challenges in the use of DWI for examination of the bile duct. The EPI-DWI technique currently in use employs small matrix scanning methods, which produce a low spatial resolution and SNR. The high frequency switching of the high-intensity gradient field can easily produce eddy currents and is highly sensitive to magnetic field inhomogeneities, which will result in unavoidable reverse geometry of space artifacts and partial image signal loss. These artifacts and signal loss become more obvious at air-tissue interfaces. As the b value increases, these artifacts worsen. In this study, one instance of undetected EHCC was attributed to the interference of artifacts around the common bile duct. In addition, the use of fat-suppression techniques to avoid artifacts due to chemical shifts can further degrade image quality. The other exception in the study was due to the partial malignant transformation of bile duct adenoma, where the lesion was too small for the DWI signal change to be obvious. Improvements to MR technology may further enhance image quality in DWI. Comparing with MRCP, DWI is a more intuitive approach to detect and confirm the diagnosis for EHCC. The cholangiocarcinoma DWI results are still preliminary and more research should be conducted to determine its value in clinical applications.
Extrahepatic cholangiocarcinomas account for 65% of primary neoplasms of the biliary tract. In recent years, improvement in imaging technology has significantly improved the detection rate and accuracy of definitive diagnosis for extrahepatic cholangiocarcinoma (EHCC). Diffusion-weighted imaging (DWI) has recently emerged as a method for detecting cancers in the abdomen. This study aimed to evaluate the usefulness of DWI in the differential diagnosis of extrahepatic cholangiocarcinomas.
DWI has been increasingly used in a wide range of clinical applications. Although the application of DWI in the diagnosis of various conditions has attracted increased attention and has become the focus of many researches, literature on clinical application of DWI for the diagnosis of EHCC has not been observed.
The authors suggest that all patients presenting with an EHCC or who are suspected to have EHCC, should have an abdominal DWI scan as a regular diagnostic test. DWI can also provide additional clinically important information in EHCC patients when added to routine bile duct MR imaging protocols.
DWI is used to observe the random motion of water molecules in the body. The degree of restriction to water diffusion in biologic tissue is inversely correlated to the tissue cellularity and integrity of cell membranes. The motion of water molecules is more restricted in tissues with a high cellular density, associated with numerous intact cell membranes (e.g. tumor tissues). The degree of water motion has been found to be proportional to the degree of signal attenuation in DWI. Visual assessment of the relative tissue signal attenuation in DWI has been applied to tumor detection and tumor characterization. By observing the relative attenuation of signal intensity on images obtained at different b values, tissue characterization based on differences in water diffusion becomes possible. More cellular solid tumor areas will continue to show relatively high signal intensity in DWI. Therefore, the observation of the relative reduction of tissue signal in DWI can help with the detection and confirmation of tumors.
The authors compare DWI with magnetic resonance cholangiopancreatography in 56 patients with suspected extrahepatic cholangiocarcinoma. Basically this is an extremely interesting study for assessing the new DWI in more tumor entities. It is nicely performed and provides a histopathological correlation.
Peer reviewers: Dr. Andreas G Schreyer, Professor, Department of Radiology, University Hospital Regensburg, Franz-Josef-Strauss-Allee 11, Regensburg 93053, Germany; Kevin Robertson, MB, ChB, FRCS (Gen surg), MD, 8 Belmont Crescent, Glasgow, G12 9QP, United Kingdom
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
| 1. | Bammer R, Schoenberg SO. Current concepts and advances in clinical parallel magnetic resonance imaging. Top Magn Reson Imaging. 2004;15:129-158. |
| 2. | Koike N, Cho A, Nasu K, Seto K, Nagaya S, Ohshima Y, Ohkohchi N. Role of diffusion-weighted magnetic resonance imaging in the differential diagnosis of focal hepatic lesions. World J Gastroenterol. 2009;15:5805-5812. |
| 3. | Le Bihan D, Turner R, Douek P, Patronas N. Diffusion MR imaging: clinical applications. AJR Am J Roentgenol. 1992;159:591-599. |
| 4. | Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622-1635. |
| 5. | Stroszczynski C, Hunerbein M. Malignant biliary obstruction: value of imaging findings. Abdom Imaging. 2005;30:314-323. |
| 6. | Zhong L, Yao QY, Li L, Xu JR. Imaging diagnosis of pancreato-biliary diseases: a control study. World J Gastroenterol. 2003;9:2824-2827. |
| 7. | Roberts TP, Schwartz ES. Principles and implementation of diffusion-weighted and diffusion tensor imaging. Pediatr Radiol. 2007;37:739-748. |
| 8. | Castillo M. Diffusion-weighted imaging of the spine: is it reliable? AJNR Am J Neuroradiol. 2003;24:1251-1253. |
| 9. | Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology. 1999;210:617-623. |
| 10. | Jiang ZX, Peng WJ, Li WT, Tang F, Liu SY, Qu XD, Wang JH, Lu HF. Effect of b value on monitoring therapeutic response by diffusion-weighted imaging. World J Gastroenterol. 2008;14:5893-5899. |
| 11. | Manenti G, Squillaci E, Di Roma M, Carlani M, Mancino S, Simonetti G. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissue using thin-slice echo-planar imaging. Radiol Med. 2006;111:1124-1133. |
| 12. | Abdel Razek AA, Kandeel AY, Soliman N, El-shenshawy HM, Kamel Y, Nada N, Denewar A. Role of diffusion-weighted echo-planar MR imaging in differentiation of residual or recurrent head and neck tumors and posttreatment changes. AJNR Am J Neuroradiol. 2007;28:1146-1152. |
| 13. | Koh DM, Scurr E, Collins DJ, Pirgon A, Kanber B, Karanjia N, Brown G, Leach MO, Husband JE. Colorectal hepatic metastases: quantitative measurements using single-shot echo-planar diffusion-weighted MR imaging. Eur Radiol. 2006;16:1898-1905. |
| 14. | Taouli B, Vilgrain V, Dumont E, Daire JL, Fan B, Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003;226:71-78. |
| 15. | Low RN, Gurney J. Diffusion-weighted MRI (DWI) in the oncology patient: value of breathhold DWI compared to unenhanced and gadolinium-enhanced MRI. J Magn Reson Imaging. 2007;25:848-858. |
| 16. | Oner AY, Celik H, Oktar SO, Tali T. Single breath-hold diffusion-weighted MRI of the liver with parallel imaging: initial experience. Clin Radiol. 2006;61:959-965. |
| 17. | Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, Taouli B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246:812-822. |
| 18. | Matsuki M, Inada Y, Nakai G, Tatsugami F, Tanikake M, Narabayashi I, Masuda D, Arisaka Y, Takaori K, Tanigawa N. Diffusion-weighed MR imaging of pancreatic carcinoma. Abdom Imaging. 2007;32:481-483. |
| 19. | Zhang J, Tehrani YM, Wang L, Ishill NM, Schwartz LH, Hricak H. Renal masses: characterization with diffusion-weighted MR imaging--a preliminary experience. Radiology. 2008;247:458-464. |
| 20. | Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, Fujii H. High-B-value diffusion-weighted MRI in colorectal cancer. AJR Am J Roentgenol. 2006;187:181-184. |
| 21. | Issa B. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissues using echo-planar imaging. J Magn Reson Imaging. 2002;16:196-200. |
| 22. | Guarise A, Venturini S, Faccioli N, Pinali L, Morana G. Role of magnetic resonance in characterising extrahepatic cholangiocarcinomas. Radiol Med. 2006;111:526-538. |