Published online Jul 7, 2010. doi: 10.3748/wjg.v16.i25.3133
Revised: April 15, 2010
Accepted: April 22, 2010
Published online: July 7, 2010
AIM: To investigate efficacy and safety of cetuximab combined with two chemotherapy regimens in patients with unresectable metastatic colorectal cancer (mCRC).
METHODS: Randomized patients received cetuximab with 5-fluorouracil (5-FU), folinic acid (FA) and oxaliplatin (FOLFOX) 6 (arm A, n = 74) or 5-FU, FA and irinotecan (FOLFIRI) (arm B, n = 77). KRAS mutation status was determined retrospectively in a subset of tumors (n = 117).
RESULTS: No significant difference was found between treatment arms A and B in the progression-free survival (PFS) rate at 9 mo, 45% vs 34%; median PFS, 8.6 mo vs 8.3 mo [hazard ratio (HR) = 1.06]; overall response rate (ORR) 43% vs 45% [odds ratio (OR) = 0.93] and median overall survival (OS), 17.4 mo vs 18.9 mo (HR = 0.98). Patients with KRAS wild-type tumors demonstrated improved PFS (HR = 0.55, P = 0.0051), OS, (HR = 0.62, P = 0.0296) and ORR (53% vs 36%) and in arm A, improved PFS (HR = 0.49, P = 0.0196), OS (HR = 0.48, P = 0.0201) and ORR (56% vs 30%), compared with patients with KRAS mutated tumors. In arm B no significant differences were found in efficacy by KRAS mutation status. Treatment in arms A and B was generally well tolerated.
CONCLUSION: This study confirms that combinations of cetuximab with FOLFOX6 or FOLFIRI are effective and significantly improve clinical outcome in KRAS wild-type compared with KRAS mutated mCRC.
- Citation: Ocvirk J, Brodowicz T, Wrba F, Ciuleanu TE, Kurteva G, Beslija S, Koza I, Pápai Z, Messinger D, Yilmaz U, Faluhelyi Z, Yalcin S, Papamichael D, Wenczl M, Mrsic-Krmpotic Z, Shacham-Shmueli E, Vrbanec D, Esser R, Scheithauer W, Zielinski CC. Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World J Gastroenterol 2010; 16(25): 3133-3143
- URL: https://www.wjgnet.com/1007-9327/full/v16/i25/3133.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i25.3133
Colorectal cancer (CRC) accounted for 529 000 deaths worldwide in 2002[1]. Up to 25% of CRC patients present with metastatic disease (mCRC) with five-year survival rates of approximately 10% reported[2,3]. The standard treatment for unresectable mCRC has been to administer first-line 5-fluorouracil (5-FU) with folinic acid (FA)[2,4,5], with improvements in clinical outcome being demonstrated for infusional 5-FU/FA combined with oxaliplatin (FOLFOX)[6,7] or irinotecan (FOLFIRI)[8,9]. However safety profiles differ, with grade 3/4 neutropenia and neurotoxicity more common with FOLFOX, and grade 3/4 mucositis and nausea/vomiting more common with FOLFIRI.
Cetuximab [Erbitux, developed by Merck KGaA Darmstadt, Germany (under license from Imclone, NY USA)] is an immunoglobulin G1 monoclonal antibody that specifically targets the epidermal growth factor receptor (EGFR), competitively inhibiting ligand binding and ligand-dependent downstream signaling[10,11]. Cetuximab first gained approval for use in Europe and the United States in the treatment of EGFR-expressing mCRC following failure of irinotecan-containing regimens[12]. More recently, the randomized CRYSTAL study demonstrated improved progression-free survival (PFS) in EGFR-expressing mCRC patients receiving FOLFIRI plus cetuximab compared with FOLFIRI alone[13]. In addition, the phase II OPUS trial reported a trend towards improved overall response rate (ORR) in EGFR-expressing mCRC patients receiving FOLFOX4 plus cetuximab compared with FOLFOX4 alone[14].
An accumulating body of data from studies of chemorefractory mCRC patients receiving cetuximab as monotherapy or in combination with chemotherapy suggests that clinical responses are confined to those patients whose tumors do not harbor mutations in codons 12 or 13 of the KRAS gene (KRAS wild-type)[15-19]. The KRAS gene encodes a GDP/GTP binding protein which, following ligand binding to receptor tyrosine kinases including EGFR, activates downstream intracellular signaling cascades promoting cellular growth and proliferation[20,21]. KRAS mutations (in codons 12 or 13) occur in 40%-50% of CRCs and circumvent the cellular requirement for receptor activation of the KRAS protein[20,21]. Metastatic colorectal tumors harboring KRAS mutations are therefore hypothesized to be refractory to EGFR-targeting monoclonal antibodies. Data from retrospective analyses of the CRYSTAL and OPUS studies confirmed that the efficacy of cetuximab in combination with FOLFIRI or FOLFOX was restricted to patients with KRAS wild-type tumors[13,14], indicating tumor KRAS mutation status to be a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy.
In the current Central European Co-operative Oncology Group (CECOG)-sponsored randomized phase II trial, the efficacy and safety of cetuximab in combination with either FOLFOX6 or FOLFIRI was investigated first-line in patients with mCRC. In addition, a retrospective subgroup analysis of clinical outcome according to tumor KRAS mutation status was performed.
Patients (≥ 18 years old) with histologically confirmed adenocarcinoma of the colon or rectum, with metastatic disease unsuitable for resection with curative-intent, an Eastern Co-operative Oncology Group (ECOG) performance status < 2, and adequate organ function were eligible for inclusion.
Exclusion criteria included: previous chemotherapy for metastatic disease; prior EGFR-targeted therapy; adjuvant chemotherapy with oxaliplatin or irinotecan (5-FU-based adjuvant chemotherapy was allowed provided the chemotherapy treatment-free interval was > 6 mo). Patients with brain metastases; concurrent malignancy and those with a previous malignancy within the last 5 years (excluding non melanoma skin cancer and in situ carcinoma of cervix); coronary artery disease or a history of myocardial infarction within 12 mo of study entry; pre-existing neuropathy > grade 1; intestinal occlusion or a history of inflammatory bowel disease; a ≥ grade 3 allergic reaction to study treatment components; those undergoing surgery (excluding biopsy) or irradiation within 4 wk of study entry were also excluded, as were pregnant or lactating patients.
The study was approved by independent ethics committees at each center and was conducted in accordance with the principles of the Declaration of Helsinki and the Note for Guidance on Good Clinical Practice. All patients provided written informed consent.
This was a two-arm randomized multicenter, open-label, parallel-group phase II study involving 28 participating centers across 13 countries (CECOG/CORE1.2.001). Eligible patients were centrally randomized 1:1, using a minimization technique, stratifying patients according to study site, the number of organs involved and prior neoadjuvant/adjuvant therapy. Patients received cetuximab (400 mg/m2 initial infusion day 1, then 250 mg/m2 weekly), then either in arm A: oxaliplatin (day 1, 100 mg/m2) with FA [400 mg/m2 (racemic) or 200 mg/m2 (L-form)] plus 5-FU (400 mg/m2 bolus plus 2400 mg/m2 as a 46-h continuous infusion) every 2 wk (FOLFOX6), or in arm B: irinotecan (180 mg/m2) with the 5-FU/FA regimen described (FOLFIRI). Patients received 6 mo of combination therapy, after which cetuximab was continued. Study treatment was discontinued in the case of progressive disease (PD). Patient follow-up was every 12 wk until treatment end or clinical cut-off date. The primary endpoint was PFS at 9 mo, secondary endpoints included ORR, PFS at 3, 6 and 12 mo, overall survival and safety.
Dose reductions, treatment delays and the omission of a maximum of two consecutive doses of cetuximab were permitted in cases of grade 3 skin reactions. Two dose reductions for irinotecan or oxaliplatin were permitted after which the drug was discontinued (in either case cetuximab could be continued). Dose reductions were permanent.
Computed tomography or magnetic resonance imaging of chest, abdomen and pelvis was performed at baseline and weeks 6, 12, and every 12 wk thereafter during treatment, and at the end of the study or upon PD. PFS rate was defined as the percentage of patients in each arm alive and free of tumor progression at analysis from the time of randomization, using response evaluation criteria in solid tumors (RECIST). Tumor response was evaluated according to RECIST guidelines. Survival was defined as time from randomization until death (patients lost to follow-up were censored at the time they were last determined to be alive). Adverse events (AEs) were assessed at treatment visits using National Cancer Institute Common Terminology Criteria for Adverse Events (version 3) and coded using the Medical Dictionary for Regulatory Activities (MedDRA; version 10.1).
Tumor DNA was extracted and purified from formalin-fixed paraffin-embedded tissues as previously described[14]. The presence of KRAS mutations in codons 12 and 13 was determined by allele-specific real time polymerase chain reaction assays using validated methodology (DxS Ltd Manchester UK)[22,23]. EGFR expression was determined using the DAKO EGFR pharmDx™ test. Tumor KRAS mutation status and EGFR expression were assessed centrally by one pathologist.
The primary objective of the study was to estimate the difference in 9-mo PFS rates between the treatment arms. In accordance with the objective of this phase II study, the planned sample size was fixed to 2 × 75 patients to achieve appropriate precision for the estimate of the difference in 9-mo PFS rates. With 75 patients in each treatment arm evaluable for 9-mo PFS, the two-sided 95% confidence intervals (CI) for the difference in PFS rates had a range of not more than ± 16% assuming a low number of censored cases (up to 5%) and PFS rates in both treatment arms being approximately 50%-60%.
Statistical analyses were performed on data accrued up until the clinical cut-off date (January 31, 2008). The efficacy analyses were performed on the intention to treat (ITT) population defined as all randomized patients who received at least one dose of study medication, which was the same as the safety population. Time to event data were analyzed using the Kaplan-Meier method[24]. Standard errors were calculated using Greenwoods formula[25] and the hazard ratio (HR) for PFS between both treatment groups and corresponding 95% CIs was calculated using an unadjusted Cox proportional hazard model. Differences in survival were tested using the logrank test. Estimates of ORR in each treatment group, odds ratios and associated 95% CIs were calculated using the Cochran-Mantel-Haenszel procedure.
The study was initiated and patient recruitment finished (2006) before the evidence from a randomized trial that KRAS tumor mutation was associated with clinical outcome in patients treated with cetuximab in combination with chemotherapy was first presented[26]. Subsequently a retrospective analysis of efficacy and safety was performed in the subgroup of patients with available tumor material that was evaluable for KRAS mutation status (wild-type vs mutant). Exploratory Cox proportional hazard models and logistic regression models were used to investigate the impact of KRAS mutation status on PFS, overall survival and ORR across the treatment groups adjusted for other significant confounding factors. A significance level of 0.2 was used to enter a factor into the model and a significance level of 0.10 was used for removing a factor from the model. Following an update of survival time, all information available by December 16th 2008 was considered for survival analyses. All calculations were performed with SAS release 8.2 (SAS Institute, Cary, NC USA).
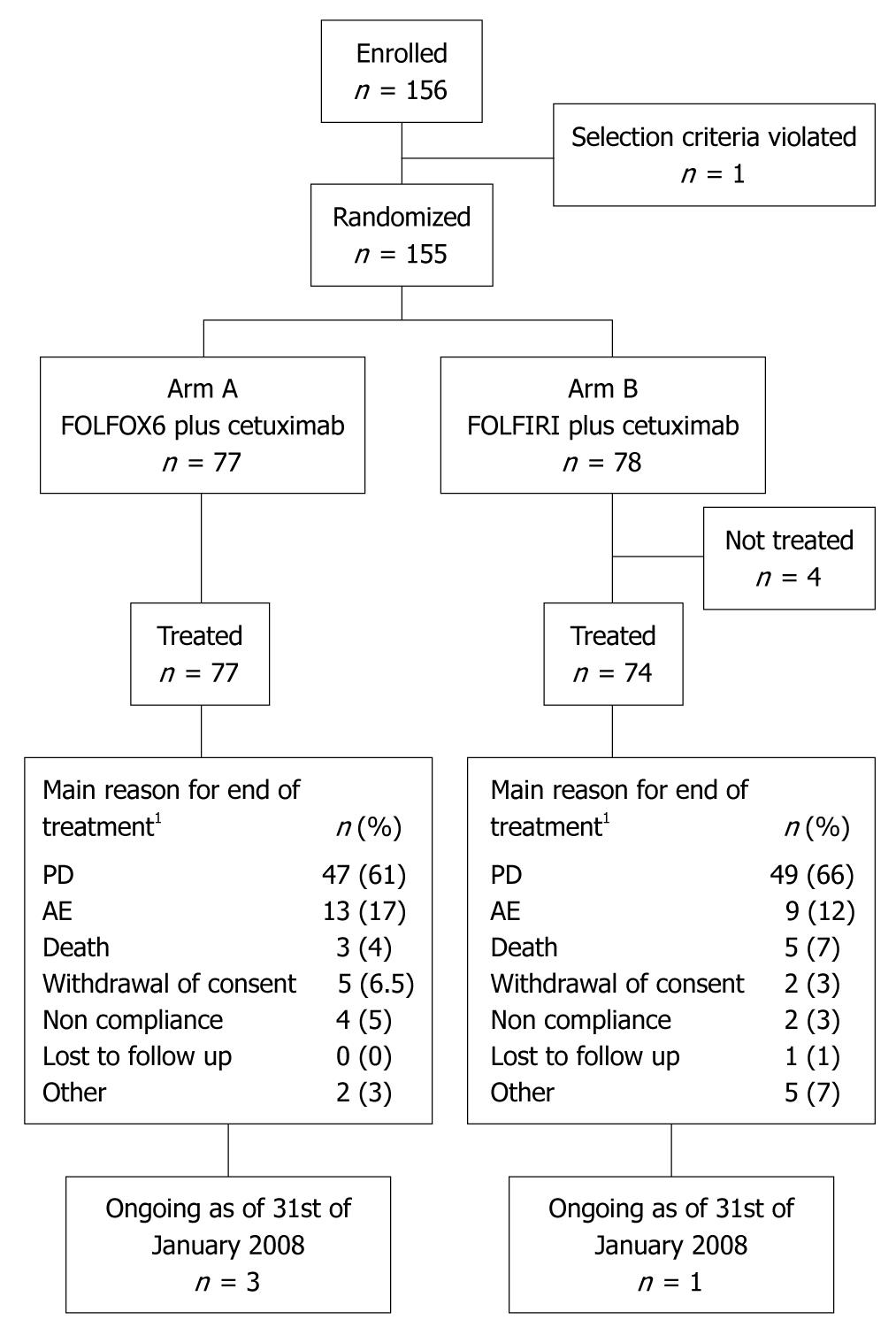
Patients were enrolled between July 2005 and July 2006; patient disposition is shown in Figure 1. Four patients randomized to receive cetuximab plus FOLFIRI withdrew their consent prior to treatment, with 151 patients subsequently receiving treatment. Reasons for discontinuing the study treatment were similar for both treatment arms.
Patient characteristics at baseline were generally well balanced between treatment groups (Table 1). KRAS mutation status was evaluable in 117/151 (77%) patient tumors; of these, KRAS mutations were detected in 55/117 (47%) patient tumors. Baseline characteristics of the KRAS subpopulation were representative of those of the ITT population (Table 1).
| Characteristic | ITT population | KRAS population | ||||
| KRAS wild-type | KRAS mutant | |||||
| FOLFOX6 plus cetuximab (n = 77) | FOLFIRI plus cetuximab (n = 74) | FOLFOX6 plus cetuximab (n = 34) | FOLFIRI plus cetuximab (n = 28) | FOLFOX6 plus cetuximab (n = 23) | FOLFIRI plus cetuximab (n = 32) | |
| Gender, n (%) | ||||||
| Male | 43 (56) | 45 (61) | 22 (65) | 17 (61) | 11 (48) | 21 (66) |
| Female | 34 (44) | 29 (39) | 12 (35) | 11 (39) | 12 (52) | 11 (34) |
| Age (yr) | ||||||
| Median (Q1-Q3) | 62.0 (54-67) | 62.5 (54-68) | 62.5 (55-67) | 64.0 (56-68) | 63.0 (49-68) | 62.5 (54-70) |
| < 65, n (%) | 46 (60) | 46 (62) | 19 (56) | 17 (61) | 13 (57) | 19 (59) |
| > 65, n (%) | 31 (40) | 28 (38) | 15 (44) | 11 (39) | 10 (43) | 13 (41) |
| ECOG PS, n (%) | ||||||
| 0 | 46 (60) | 38 (51) | 20 (59) | 17 (61) | 13 (57) | 14 (44) |
| 1 | 31 (40) | 36 (49) | 14 (41) | 11 (39) | 10 (43) | 18 (56) |
| Primary tumor location, n (%) | ||||||
| Colon | 52 (68) | 47 (64) | 26 (76) | 15 (54) | 13 (57) | 22 (69) |
| Rectum | 25 (32) | 27 (36) | 8 (24) | 13 (46) | 10 (43) | 10 (31) |
| Metastasis1, n (%) | 45 (58)a | 46 (62) | 17 (50) | 18 (64) | 16 (70) | 18 (56) |
| Organs with metastases, n (%) | ||||||
| 1-2 | 59 (77) | 56 (76) | 28 (82) | 23 (82) | 17 (74) | 26 (81) |
| > 2 | 18 (23) | 18 (24) | 6 (18) | 5 (18) | 6 (26) | 6 (19) |
| Metastatic sites2, n (%) | ||||||
| Intestine/bowel | 12 (16) | 12 (16) | 3 (9) | 6 (21) | 6 (26) | 5 (16) |
| Liver | 66 (86) | 63 (85) | 30 (88) | 24 (86) | 20 (87) | 26 (81) |
| Lung | 27 (35) | 28 (38) | 11 (32) | 10 (36) | 8 (35) | 10 (31) |
| Lymph nodes | ||||||
| Chest | 7 (9) | 5 (7) | 2 (6) | 2 (7) | 3 (13) | 2 (6) |
| Abdomen | 22 (29) | 24 (32) | 9 (26) | 8 (29) | 5 (22) | 8 (25) |
| Bone | 2 (3) | 4 (5) | 0 (0) | 1 (4) | 2 (9) | 1 (3) |
| Other | 10 (13) | 10 (14) | 5 (15) | 3 (11) | 2 (9) | 4 (13) |
| Duration of disease, mo | ||||||
| CRC, median (Q1-Q3) | 2.1a (1-15) | 1.9 (1-14) | 2.2 (1-18) | 1.8 (1-6) | 1.8 (1-3) | 2.4 (1-18) |
| mCRC median (Q1-Q3) | 1.4 (1-2) | 1.2 (1-2) | 1.1 (1-2) | 1.0 (1-2) | 1.3 (1-2) | 1.4 (1-2) |
| EGFR status, n (%) | ||||||
| Detectable | 43 (56) | 46 (62) | 21 (62) | 20 (71) | 17 (74) | 24 (75) |
| Undetectable | 17 (22) | 12 (16) | 10 (29) | 4 (14) | 5 (22) | 7 (22) |
| Non evaluable | 17 (22) | 16 (22) | 3 (9) | 4 (14) | 1 (4) | 1 (3) |
| Prior treatment, n (%) | ||||||
| At least 1 therapy | 63 (82) | 59 (80) | 31 (91) | 22 (79) | 19 (83) | 29 (91) |
| Adjuvant chemotherapy3 | 14 (18) | 10 (14) | 9 (26) | 2 (7) | 2 (9) | 6 (19) |
| Surgery | 61 (79) | 58 (78) | 30 (88) | 22 (79) | 18 (78) | 29 (91) |
| Other | 8 (10) | 5 (7) | 3 (9) | 2 (7) | 3 (13) | 2 (6) |
Patient exposure to cetuximab and chemotherapy was similar for both treatment arms (Table 2). The proportion of dose reductions and treatment delays for cetuximab was slightly higher in arm A than arm B. Treatment delays were more commonly due to diarrhea in the FOLFIRI arm and to neuropathy in the FOLFOX6 arm. In treatment arm A, median exposure to cetuximab in patients with KRAS wild-type patients was 35.4 wk and KRAS mutant tumors 23.3 wk, compared with 24.5 and 32.4 wk, respectively, in arm B. Exposure to chemotherapy in each treatment arm by KRAS mutation status was similar to that for the safety population. The proportion of patients experiencing dose reductions, delays in treatment, and treatment discontinuations for cetuximab or chemotherapy in each treatment arm by KRAS mutation status was not markedly different and was comparable with that found in the safety population.
| Characteristic | FOLFOX6 plus cetuximab (arm A, n = 77) | FOLFIRI plus cetuximab (arm B, n = 74) |
| Exposure to cetuximab (Q1-Q3) | ||
| Median duration, wk | 28.0 (17-46) | 29.1 (13-46) |
| Median number of infusions | 26.0 (14-40) | 26.0 (12-42) |
| Relative dose intensity, n (%) | ||
| Only initial dose | 4 (5) | 3 (4) |
| < 60% | 2 (3) | 3 (4) |
| 60% to < 80% | 15 (19) | 8 (11) |
| 80% to < 90% | 21 (27) | 20 (27) |
| ≥ 90% | 35 (45) | 40 (54) |
| Exposure to chemotherapy (Q1-Q3) | ||
| Median duration, wk | 25.1 (19-28) | 25.5 (14-28) |
| Median number of cycles | 12 (7-12) | 12 (6-12) |
| Relative dose intensity, n (%) | ||
| Oxaliplatin | ||
| No dose | 1 (1) | 74 (100) |
| < 60% | 4 (5) | - |
| 60% to < 80% | 24 (31) | - |
| 80% to < 90% | 22 (29) | - |
| ≥ 90% | 26 (34) | - |
| Irinotecan | ||
| No dose | 77 (100) | 2 (3) |
| < 60% | - | 3 (4) |
| 60% to < 80% | - | 18 (24) |
| 80% to < 90% | - | 13 (18) |
| ≥ 90% | - | 38 (51) |
| Bolus 5-FU | ||
| No dose | 1 (1) | 2 (3) |
| < 60% | 1 (1) | 2 (3) |
| 60% to < 80% | 28 (36) | 19 (26) |
| 80% to < 90% | 19 (25) | 14 (19) |
| ≥ 90% | 28 (36) | 37 (50) |
| Continuous infusion 5-FU | ||
| No dose | 1 (1) | 2 (3) |
| < 60% | 1 (1) | 3 (4) |
| 60% to < 80% | 21 (27) | 14 (19) |
| 80% to < 90% | 13 (17) | 11 (15) |
| ≥ 90% | 41 (53) | 44 (59) |
| Dose reductions1, n (%) | ||
| Cetuximab | 9 (12) | 5 (7) |
| Chemotherapy | 25 (32) | 17 (23) |
| Treatment delays1, n (%) | ||
| Any cetuximab | ||
| ≥ 3 d | 59 (77) | 47 (64) |
| ≥ 16 d | 12 (16) | 8 (11) |
| Any chemotherapy | ||
| ≥ 3 d | 59 (77) | 51 (69) |
| ≥ 14 d | 25 (32) | 15 (20) |
| Treatment discontinuation1, n (%) | ||
| Cetuximab | 13 (17) | 9 (12) |
| Chemotherapy | 9 (12) | 4 (5) |
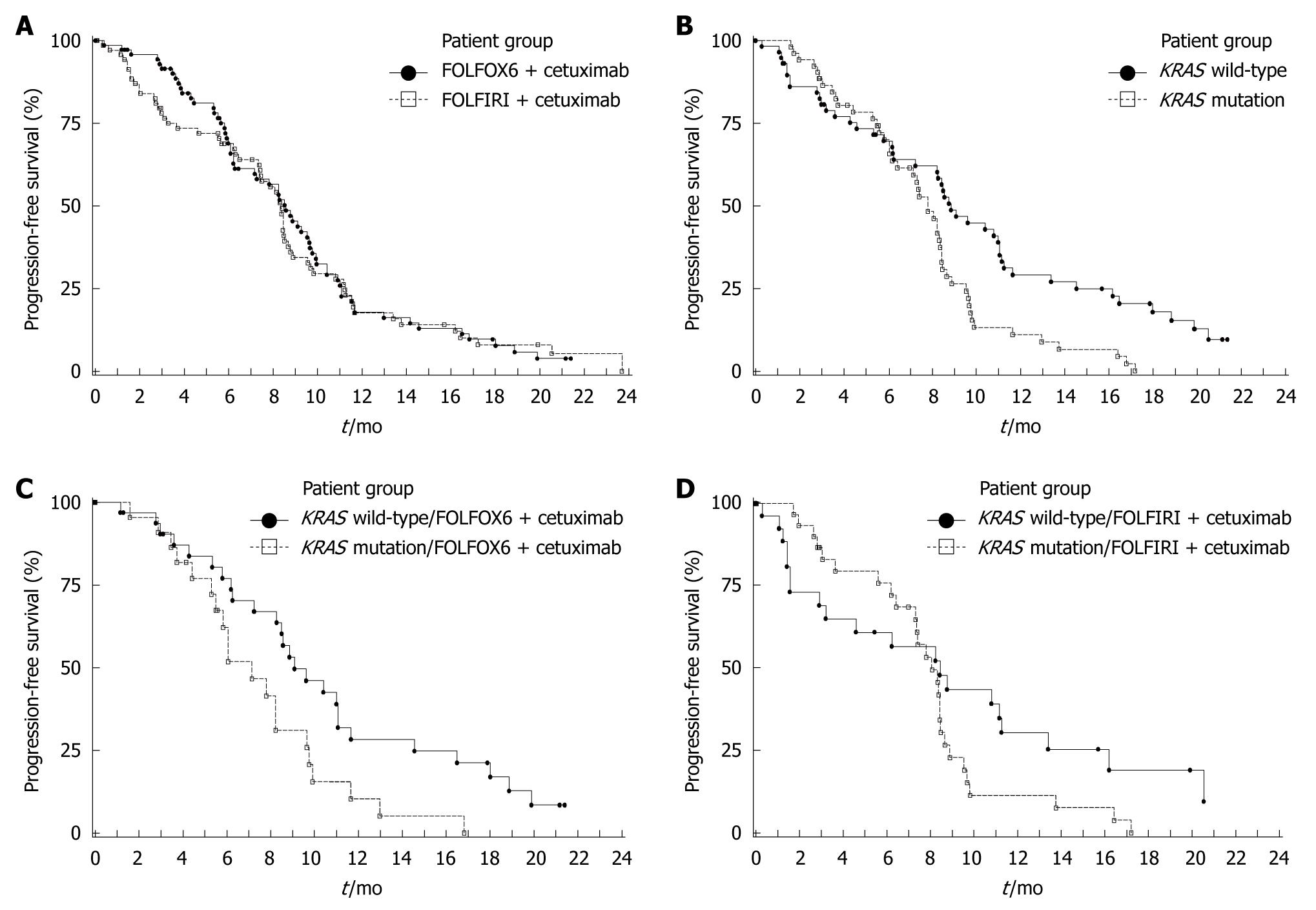
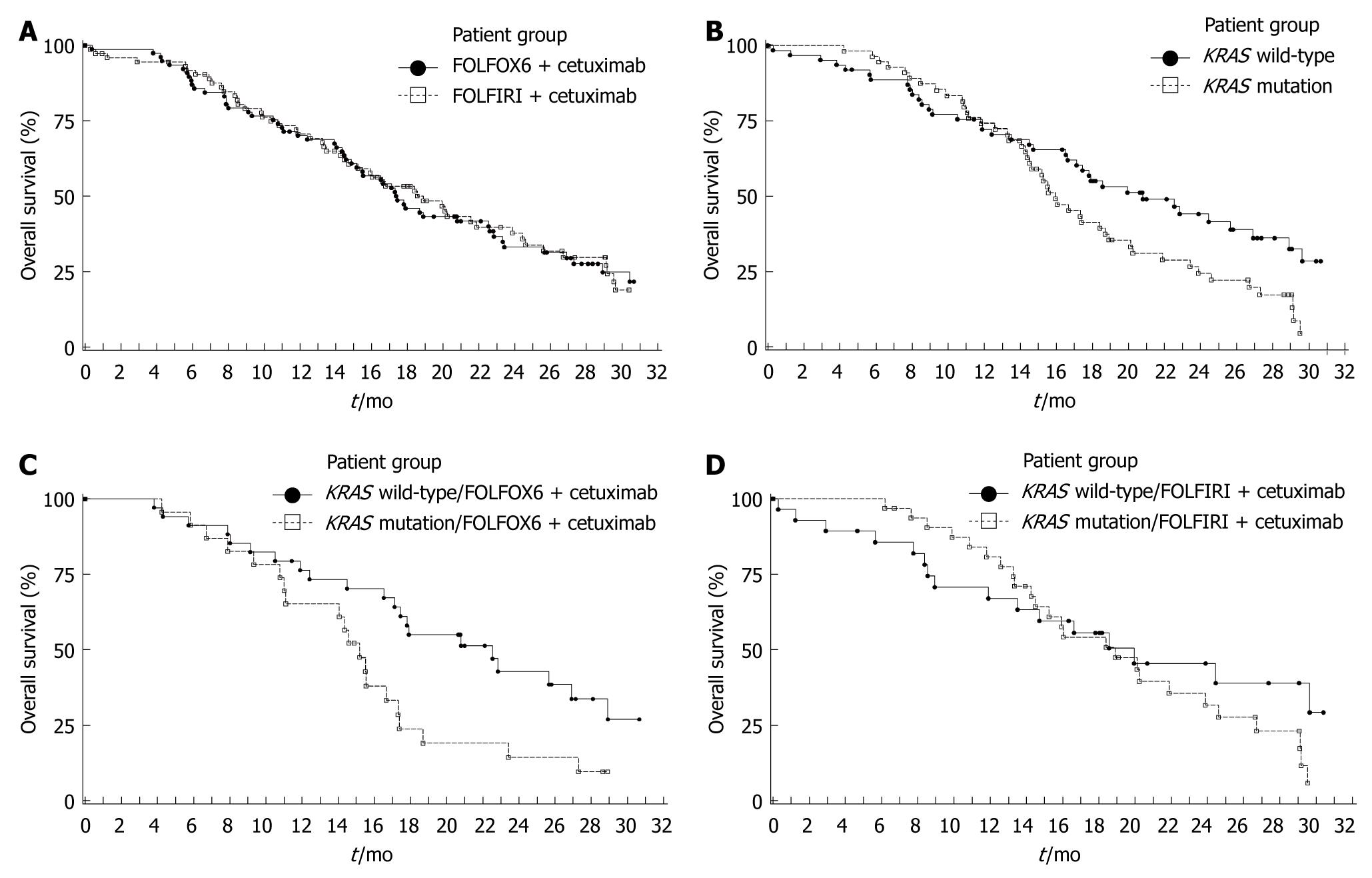
Efficacy data for the ITT population are summarized in Table 3. The 9-mo PFS rate was 11% higher in arm A than arm B (45% vs 34%); however, the 95% CI for the difference was -6% to 28%, indicating no significant difference. The risk of disease progression (Figure 2A), death (Figure 3A) and the ORR were also similar between treatment arms.
| Characteristic | FOLFOX6 plus cetuximab (arm A, n = 77) | FOLFIRI plus cetuximab (arm B, n = 74) |
| PFS | ||
| Events, n (%) | 61 (79) | 59 (80) |
| Median1, mo (95% CI) | 8.6 (6.3-9.7) | 8.3 (7.4-8.7) |
| Log rank P-value | 0.7375 | |
| Hazard ratio (95% CI) | 1.06 (0.74-1.52) | |
| PFS rate1, % (95% CI) | ||
| 3 mo | 92 (85-98) | 78 (68-88) |
| 6 mo | 69 (58-80) | 69 (58-80) |
| 9 mo | 45 (33-58) | 34 (23-46) |
| 12 mo | 18 (8-27) | 18 (8-27) |
| Overall survival | ||
| Events, n (%) | 54 (70) | 50 (68) |
| Median1, mo (95% CI) | 17.4 (14.9-22.6) | 18.9 (14.7-23.9) |
| Logrank P-value | 0.9230 | |
| Hazard ratio2 (95% CI) | 0.98 (0.67-1.44) | |
| Survival rate1, % (95% CI) | ||
| 9 mo | 79 (70-88) | 79 (70-89) |
| 12 mo | 70 (60-80) | 71 (60-81) |
| 18 mo | 46 (35-57) | 53 (42-65) |
| 24 mo | 33 (22-44) | 38 (26-50) |
| Best overall response, n (%) | ||
| CR | 2 (3) | 6 (8) |
| PR | 31 (40) | 27 (36) |
| SD | 31 (40) | 24 (32) |
| PD | 6 (8) | 9 (12) |
| NE | 7 (9) | 8 (11) |
| Objective response rate, n (%) | 33 (43) | 33 (45) |
| 95% CI | 32-55 | 33-57 |
| Odds ratio (95% CI) | 0.93 (0.49-1.77) | |
The influence of tumor KRAS mutation status on clinical outcome is summarized in Table 4. The 9-mo PFS rate was higher and the risk of disease progression was significantly reduced in patients with KRAS wild-type tumors compared with those with KRAS mutations (Figure 2B). A significant improvement in survival (Figure 3B) and an increase in ORR were also demonstrated in patients with KRAS wild-type tumors compared with KRAS mutated tumors. In multivariate analyses of the KRAS evaluable population using Cox proportional hazard models (baseline characteristics and acne-like rash), only KRAS tumor mutation status (wild-type vs mutant) was identified as a significant prognostic indicator for prolonged PFS (HR = 0.55, P = 0.006), while KRAS mutation status (wild type vs mutant, HR = 0.51, P = 0.003), prior adjuvant/neo-adjuvant therapy (no vs yes, HR = 0.32, P < 0.001) and acne-like rash during the first 6 wk (grade 2-3 vs grade 0-1, HR = 0.47, P = 0.004) were significant independent prognostic indicators for prolonged overall survival.
| Parameter | KRAS population | FOLFOX6 plus cetuximab (arm A) | FOLFIRI plus cetuximab (arm B) | |||
| KRAS wild-type (n = 62) | KRAS mutation (n = 55) | KRAS wild-type (n = 34) | KRAS mutation (n = 23) | KRAS wild-type (n = 28) | KRAS mutation (n = 32) | |
| PFS | ||||||
| Events, n (%) | 46 (74) | 47 (85) | 26 (76) | 20 (87) | 20 (71) | 27 (84) |
| Median1, mo (95% CI) | 8.9 (7.3-11.1) | 7.8 (6.4-8.4) | 9.1 (8.3-11.1) | 7.2 (5.5-9.7) | 8.4 (3.2-11.3) | 8.1 (7.3-8.5) |
| Logrank P-value | 0.0051 | 0.0196 | 0.1737 | |||
| HR2 (95% CI) | 0.55 (0.36-0.84) | 0.49 (0.27-0.91) | 0.66 (0.36-1.21) | |||
| PFS rate1, % (95% CI) | ||||||
| 3 mo | 81 (70-91) | 88 (80-97) | 90 (80-100) | 91 (79-100) | 69 (51-87) | 87 (75-99) |
| 6 mo | 70 (58-82) | 70 (57-83) | 77 (62-92) | 62 (41-83) | 61 (42-80) | 76 (60-91) |
| 9 mo | 49 (35-62) | 26 (14-39) | 53 (35-71) | 31 (11-52) | 43 (24-63) | 23 (7-39) |
| 12 mo | 29 (17-41) | 11 (2-20) | 28 (12-45) | 10 (0-24) | 30 (12-49) | 11 (0-24) |
| Overall survival | ||||||
| Events, n (%) | 37 (60) | 45 (82) | 21 (62) | 20 (87) | 16 (57) | 25 (78) |
| Median1, mo (95% CI) | 20.8 (16.6-26.9) | 15.9 (14.4-18.9) | 22.5 (17.1-28.9) | 15.2 (11.1-17.3) | 19.9 (11.9-na) | 18.9 (14.5-23.9) |
| Logrank P-value | 0.0296 | 0.0201 | 0.3608 | |||
| HR2 (95% CI) | 0.62 (0.40-0.96) | 0.48 (0.26-0.90) | 0.74 (0.39-1.40) | |||
| Survival rate1 (95% CI) | ||||||
| 9 mo | 79 (69-89) | 87 (78-96) | 85 (73-97) | 83 (67-98) | 71 (54-88) | 90 (80-100) |
| 12 mo | 72 (61-83) | 74 (63-86) | 76 (62-91) | 65 (46-85) | 67 (49-85) | 81 (67-95) |
| 18 mo | 55 (42-68) | 41 (28-55) | 55 (38-72) | 24 (6-42) | 56 (37-74) | 54 (36-72) |
| 24 mo | 44 (31-57) | 24 (12-36) | 43 (25-61) | 14 (0-29) | 45 (26-65) | 32 (14-49) |
| Best overall response, n (%) | ||||||
| CR | 6 (10) | 1 (2) | 2 (6) | - | 4 (14) | 1 (3) |
| PR | 27 (44) | 19 (35) | 17 (50) | 7 (30) | 10 (36) | 12 (38) |
| SD | 14 (23) | 26 (47) | 9 (26) | 12 (52) | 5 (18) | 14 (44) |
| PD | 8 (13) | 6 (11) | 3 (9) | 3 (13) | 5 (18) | 3 (9) |
| NE | 7 (11) | 3 (5) | 3 (9) | 1 (4) | 4 (14) | 2 (6) |
| ORR, n (%) | 33 (53) | 20 (36) | 19 (56) | 7 (30) | 14 (50) | 13 (41) |
| 95% CI | 40-66 | 24-50 | 38-73 | 13-53 | 31-69 | 24-59 |
| Odds ratio (95% CI) | 1.99 (0.95-4.18) | 2.90 (0.95-8.84) | 1.46 (0.53-4.07) | |||
In treatment arm A, the 9-mo PFS rate in patients with KRAS wild-type tumors was higher and the PFS time was significantly longer compared with patients with KRAS mutated tumors (Table 4, Figure 2C). In arm B, the 9-mo PFS rate was also higher in KRAS wild-type patients, although the PFS time was not significantly different compared with patients with KRAS mutated tumors (Figure 2D, Table 4). Similarly, in treatment arm A, survival time was significantly higher in patients with KRAS wild-type tumors compared with tumors with KRAS mutations (Figure 3C, Table 4). In arm B survival time was not significantly different according to tumor KRAS mutation status (Figure 3D, Table 4). In both treatment arms the ORR was higher in patients with KRAS wild-type tumors compared with KRAS mutated tumors (Table 4).
In treatment arm A vs B; median PFS was 8.2 vs 8.4 mo in patients with EGFR-detectable tumors (n = 43 vs n = 46), and 11.0 mo vs 8.1 mo in patients with EGFR-undetectable tumors (n = 17 vs n = 12). Median OS was also comparable by EGFR tumor status between the treatment groups. In arm A vs arm B; median OS was 15.5 mo vs 21.6 mo in patients with EGFR-detectable tumors and 23.3 mo vs 17.6 mo in patients with EGFR-undetectable tumors.
The number of patients experiencing serious AEs was balanced between the treatment groups (27% in arm A vs 28% in arm B). The frequencies of the most common treatment emergent AEs (TEAE) in arm A vs arm B were: neutropenia (47% vs 36%); nausea (40% vs 26%); diarrhea (44% vs 58%); rash (36% vs 34%); vomiting, (26% vs 23%); stomatitis (22% vs 18%); dermatitis acneiform (21% vs 23%); anorexia (22% vs 20%), pyrexia (22% vs 20%). Peripheral neuropathy was reported only in arm A (13%).
Grade 3/4 TEAEs related to study treatment (Table 5) were slightly higher in arm A than in arm B. Grade 4 neutropenia occurred more frequently in patients in arm A than in patients in arm B. The incidence of the special AEs, acne-like rash and infusion-related reactions (composite categories), was not significantly different between the treatment groups (Table 5).
| Adverse event | FOLFOX6 plus cetuximab (arm A) | FOLFIRI plus cetuximab (arm B) | ||
| Grade 3/4a | Grade 4 | Grade 3/4a | Grade 4 | |
| Safety populationb | ||||
| Any related AE | 48 (62) | 12 (16) | 37 (50) | 6 (8) |
| Neutropenia | 22 (29) | 9 (12) | 15 (20) | 4 (5) |
| Diarrhea | 7 (9) | - | 9 (12) | - |
| Rash | 5 (6) | - | 3 (4) | - |
| Dermatitis acneiform | 4 (5) | - | 2 (3) | - |
| Special AE categories | ||||
| Skin reactionsc | 11 (14) | - | 6 (8) | - |
| Acne-like rashd | 10 (13) | - | 6 (8) | - |
| Infusion-related reactionse | 5 (6) | 2 (3) | 1 (1) | 1 (1) |
| Allergy/anaphylaxis | 5 (6) | 2 (3) | 1 (1) | 1 (1) |
| KRAS wild-type populationf | ||||
| Any related AE | 24 (71) | 5 (15) | 10 (36) | 1 (4) |
| Neutropenia | 12 (35) | 3 (9) | 3 (11) | 1 (4) |
| Diarrhea | 3 (9) | - | 2 (7) | - |
| Dermatitis acneiform | 3 (9) | - | - | - |
| Mucosal inflammation | 3 (9) | - | - | - |
| Rash | 2 (6) | - | - | - |
| Neuropathy peripheral | 2 (6) | - | - | - |
| Hypersensitivity | 2 (6) | 1 (3) | - | - |
| Special AE categories | ||||
| Skin reactionsc | 6 (18) | - | 1 (4) | - |
| Acne-like skin rashd | 5 (15) | - | 1 (4) | - |
| Infusion-related reactionse | 2 (6) | 1 (3) | - | - |
| Allergy/anaphylaxis | 2 (6) | 1 (3) | - | - |
| KRAS mutation populationg | ||||
| Any related AE | 14 (61) | 4 (17) | 18 (56) | 4 (13) |
| Neutropenia | 6 (26) | 3 (13) | 9 (28) | 2 (6) |
| Diarrhea | 3 (13) | - | 4 (13) | - |
| Thrombocytopenia | 2 (9) | - | - | - |
| Rash | 1 (4) | - | 2 (6) | - |
| Mucosal inflammation | - | - | 2 (6) | - |
| Dehydration | - | - | 2 (6) | |
| Special AE categories | ||||
| Skin reactionsc | 2 (9) | - | 3 (9) | - |
| Acne-like rashd | 2 (9) | - | 3 (9) | - |
| Infusion-related reactionse | 2 (9) | 1 (4) | 1 (3) | 1 (3) |
| Allergy/anaphylaxis | 2 (9) | 1 (4) | 1 (3) | 1 (3) |
Fifty-four deaths (70%) were reported for patients in arm A and 50 deaths (68%) in arm B. Ten deaths (13%) occurred on-treatment or within 60 d after the last dose in arm A and six (8%) in arm B. None of these deaths were assessed as being due primarily to treatment. Progressive disease and death related to disease complications were the most common reasons for death in both treatment groups.
The frequencies of serious AEs by KRAS tumor mutation status and treatment group were similar across the 4 groups (29%-31%). The only noteworthy finding was the relatively low incidence of related grade 3/4 AEs in KRAS wild-type patients treated with FOLFIRI plus cetuximab (36%) in comparison to the other 3 groups (56%-71%), mainly due to a lower incidence of neutropenia (Table 5). However, the low sample size in the subgroup analysis should be taken into account, when considering this finding.
No significant differences in efficacy were found for cetuximab combined with FOLFOX6 or FOLFIRI in the first-line treatment of mCRC. Efficacy data in the current study are comparable with those reported from the corresponding arms of the CRYSTAL (median PFS of 8.9 mo, median overall survival of 19.9 mo and an ORR of 47%)[13] and OPUS studies (median PFS value of 7.2 mo and an ORR of 46%)[14].
The KRAS evaluable population was representative of the ITT population. The KRAS tumor mutation frequency (47%) was similar to that previously reported for mCRC[13,14,27]. Across the treatment groups, PFS and overall survival were significantly improved and there was an increased chance of a tumor response in patients with KRAS wild-type tumors compared with KRAS mutant tumors; differences in PFS and survival appeared to increase over time. Multivariate analysis also confirmed that tumor KRAS mutation status is a prognostic marker for PFS and overall survival after adjustment by other independent predictors such as acne-like rash in the first 6 wk.
Patients with KRAS wild-type tumors receiving cetuximab plus FOLFOX6 demonstrated significantly improved PFS and overall survival and an increased chance of tumor response compared with patients with KRAS mutated tumors. Similar findings were reported in the OPUS study in patients receiving cetuximab plus FOLFOX4, where patients with KRAS wild-type tumors had a reduced risk of disease progression (HR 0.45, P = 0.0009) and a higher response rate (61% vs 37%) compared with those with tumor mutations[14]. However in the present study for patients receiving FOLFIRI plus cetuximab, no significant benefit was apparent with regard to PFS, survival or ORR according to KRAS tumor mutation status. This contrasts somewhat with the CRYSTAL study, where a significant clinical benefit was associated with the addition of cetuximab to FOLFIRI in patients with KRAS wild-type tumors, but not in patients with KRAS mutant tumors[13]. This non-significance may be due to the comparatively small sample size in the KRAS subgroup analysis in the present study compared with the CRYSTAL study[13]. It should also be noted that the difference in the predictive power of KRAS tumor mutation status in patients receiving FOLFOX with cetuximab compared with those receiving FOLFIRI with cetuximab described here is consistent with the KRAS analysis from CRYSTAL and OPUS studies[13,14,26]. Furthermore, FOLFIRI and cetuximab were given until disease progression in the CRYSTAL study, whereas FOLFIRI was given for 6 mo and cetuximab until progression in the present study. Within this context it is noteworthy that the PFS curves cross after 6 mo in the FOLFIRI subgroup.
In the absence of chemotherapy-alone control arms, the present study was not able to accurately assess the influence of KRAS mutation status on clinical outcome for cetuximab or chemotherapy-alone as individual treatment components. The influence of KRAS tumor mutation status on patients treated with 5-FU-based chemotherapy remains controversial. The MRC FOCUS study of mCRC patients randomized to receive first-line 5-FU, 5-FU plus irinotecan or 5-FU plus oxaliplatin, reported that patients whose tumors harbored KRAS tumor mutations displayed significantly worse survival than those with KRAS wild-type tumors (HR = 1.24, P = 0.08)[28]. In contrast, in patients treated in the FOLFIRI-alone arm in the CRYSTAL trail, KRAS mutation status was not associated with clinical outcome. Furthermore, in the large PETACC-3 trial of stage II and III colon cancer patients treated with adjuvant chemotherapy, KRAS tumor mutation status was found not to be of prognostic value[29]. The data from the present study lends support to the findings from retrospective analyses of randomized trials that demonstrated a lack of efficacy of cetuximab (either in combination with chemotherapy or best supportive care) in the treatment of mCRC patients with KRAS mutant tumors[13,14,19], adding to the view that KRAS tumor mutation status is predictive of resistance to EGFR-targeted antibodies.
No marked difference in efficacy between the treatment groups for patients with EGFR-undetectable and EGFR-detectable tumors was found. Whilst this result should be treated with caution given the low numbers of EGFR-undetectable patients, the efficacy of cetuximab in combination with chemotherapy in EGFR-undetectable tumors has been reported previously[30,31].
The combination of FOLFIRI with cetuximab was generally better tolerated than FOLFOX6 plus cetuximab with regard to grade 3/4 related AEs, and the frequency of study withdrawal being slightly higher in the latter group. The observed chemotherapy toxicity profiles are similar to those previously reported[32]. AEs associated with cetuximab were typically acne-like skin rash, which was observed with both chemotherapy combinations. KRAS tumor mutation status did not appear to markedly influence the toxicity profiles of either treatment regimen as would be expected and as previously reported[26].
In summary, this CECOG study shows that combinations of cetuximab with either FOLFOX6 or FOLFIRI have similar efficacy and acceptable toxicity profiles, in the first-line treatment of patients with unresectable mCRC. Analyses of tumor KRAS mutational status demonstrated cetuximab in combination with chemotherapy to have an increased treatment effect on tumor response, overall survival and PFS in patients with KRAS-wild-type tumors compared with those with KRAS mutated tumors. Whether there is a stronger predictive effect of KRAS mutation status in patients treated with cetuximab plus FOLFOX6 compared with cetuximab plus FOLFIRI requires further investigation.
The standard first-line treatment for patients with metastatic colorectal cancer (mCRC) is a combination of 5-fluorouracil (5-FU) and folinic acid (FA) with either irinotecan or oxaliplatin. The addition of cetuximab, one of a new class of drug known as biological therapeutics, to both these regimens has led to an increase in efficacy in some studies. This is even more pronounced in patients who do not carry a mutation in the KRAS gene (some 60% of the CRC population). No direct comparison has been made between the efficacy of these two regimens and the current study was undertaken to address this.
Cetuximab is one of a number of biological therapies which have the potential to improve the outcome of patients with mCRC. However, as with all treatments, some patients respond well to this therapy while others do not. The current research hotspot is to identify key biomarkers which will predict the treatment to which patients are more likely to respond. The KRAS gene is one such biomarker and many others are under investigation.
A number of studies have produced encouraging results for combinations of cetuximab with various regimens containing 5-FU, FA and irinotecan or 5-FU, FA and oxaliplatin for the first-line treatment of mCRC. The current CECOG study was important in that it directly compared cetuximab combined with 5-FU FA and irinotecan (FOLFIRI) and 5-FU FA and oxaliplatin (FOLFOX) and showed that there was no statistical difference in efficacy between the two regimens in this setting. Efficacy data in the current study are also comparable with those reported from the corresponding arms of the CRYSTAL (cetuximab plus FOLFIRI) and OPUS studies (cetuximab plus FOLFOX). Of further interest is the retrospective analysis of KRAS data, which demonstrated that the 9-mo PFS rate was higher and the risk of disease progression was significantly reduced in patients receiving cetuximab in combination with chemotherapy who had KRAS wild-type tumors compared with those with KRAS mutations.
The results of this study suggest that cetuximab plus FOLFIRI and cetuximab plus FOLFOX are equally effective in treating patients with mCRC. The data consolidate the view that cetuximab in combination with standard chemotherapy should be tailored to patients with KRAS wild-type mCRC. Whether there is a stronger predictive effect of KRAS mutation status in patients treated with cetuximab plus FOLFOX6 compared with cetuximab plus FOLFIRI requires further investigation.
This is a well written manuscript which describes the effect of cetuximab combined with FOLFOX6 or FOLFIRI. The authors report that in general, in patients with KRAS wild-type tumors the treatment was more effective compared with patients with KRAS mutated tumors.
Peer reviewers: Pascal Gervaz, PhD, Department of Surgery, University Hospital Geneva, 4, Rue Gabrielle Perret Gentile, Geneva, 1211, Switzerland; Luca Stocchi, MD, Desk A 30, Department of Colorectal Surgery, Digestive Disease Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, United States; Dr. Abdel-Majid Khatib, PhD, INSERM, UMRS 940, Equipe Avenir, Cibles Thérapeutiques, IGM 27 rue Juliette Dodu, 75010 Paris, France
S- Editor Wang JL L- Editor Logan S E- Editor Lin YP
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 2. | Thirion P, Michiels S, Pignon JP, Buyse M, Braud AC, Carlson RW, O'Connell M, Sargent P, Piedbois P. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766-3775. |
| 3. | Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004;101:3-27. |
| 4. | Borner MM, Castiglione M, Bacchi M, Weber W, Herrmann R, Fey MF, Pagani O, Leyvraz S, Morant R, Pestalozzi B. The impact of adding low-dose leucovorin to monthly 5-fluorouracil in advanced colorectal carcinoma: results of a phase III trial. Swiss Group for Clinical Cancer Research (SAKK). Ann Oncol. 1998;9:535-541. |
| 5. | Poon MA, O'Connell MJ, Moertel CG, Wieand HS, Cullinan SA, Everson LK, Krook JE, Mailliard JA, Laurie JA, Tschetter LK. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7:1407-1418. |
| 6. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. |
| 7. | Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136-147. |
| 8. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. |
| 9. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. |
| 10. | Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311-1318. |
| 11. | Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301-311. |
| 12. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. |
| 13. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. |
| 14. | Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-671. |
| 15. | Lièvre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouché O, Landi B, Louvet C. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374-379. |
| 16. | De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508-515. |
| 17. | Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, Bastit L, Killian A, Sesboüé R, Tuech JJ. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166-1169. |
| 18. | Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230-3237. |
| 19. | Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757-1765. |
| 20. | Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459-465. |
| 22. | Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-1634. |
| 23. | Thelwell N, Millington S, Solinas A, Booth J, Brown T. Mode of action and application of Scorpion primers to mutation detection. Nucleic Acids Res. 2000;28:3752-3761. |
| 24. | Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. |
| 25. | Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley & Sons 1980; . |
| 26. | Van Cutsem E, Lang I, D'haens G, Moiseyenko V, Zaluski J, Folprecht G, Tejpar S, Kisker O, Stroh C, Rougier P. KRAS status and efficacy in the firstline treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLF1R1 with or without cetuximab: The CRYSTAL experience. J Clin Oncol. 2008;26:suppl; abstr 2. |
| 27. | McLellan EA, Owen RA, Stepniewska KA, Sheffield JP, Lemoine NR. High frequency of K-ras mutations in sporadic colorectal adenomas. Gut. 1993;34:392-396. |
| 28. | Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, Taylor G, Barrett JH, Quirke P. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931-5937. |
| 29. | Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466-474. |
| 30. | Lenz HJ, Van Cutsem E, Khambata-Ford S, Mayer RJ, Gold P, Stella P, Mirtsching B, Cohn AL, Pippas AW, Azarnia N. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol. 2006;24:4914-4921. |
| 31. | Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803-1810. |
| 32. | Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237. |