Published online Jun 28, 2010. doi: 10.3748/wjg.v16.i24.3078
Revised: April 23, 2010
Accepted: April 30, 2010
Published online: June 28, 2010
AIM: To generate recombinant adenoviral vector containing calreticulin (CRT)-hepatitis B surface antigen (HBsAg) fusion gene for developing a safe, effective and HBsAg-specific therapeutic vaccine.
METHODS: CRT and HBsAg gene were fused using polymerase chain reaction (PCR), endonuclease digestion and ligation methods. The fusion gene was cloned into pENTR/D-TOPO transfer vector after the base pairs of DNA (CACC) sequence was added to the 5′ end. Adenoviral expression vector containing CRT-HBsAg fusion gene was constructed by homologous recombinantion. The human embryo kidney (HEK) 293A cells were transfected with linearized DNA plasmid of the recombinant adenoviral vector to package and amplify recombinant adenovirus. The recombinant adenovirus titer was characterized using the end-dilution assay. The expression of the CRT/HBsAg fusion protein in Ad-CRT/HBsAg infected 293A cells was detected by Western blotting.
RESULTS: The CRT-HBsAg fusion gene was characterized by PCR and sequencing and its length and sequence were confirmed to be accurate. The CRT-HBsAg fusion gene recombinant pENTR/D-TOPO transfer vector was constructed. The recombinant adenoviral vector, Ad-CRT/HBsAg, was generated successfully. The titer of Ad-CRT/HBsAg was characterized as 3.9 × 1011 pfu/mL. The CRT-HBsAg fusion protein was expressed by HEK 293A cells correctly.
CONCLUSION: CRT/HBsAg fusion gene recombinant replication-defective adenovirus expression vector is constructed successfully and this study has provided an experimental basis for further studies of Hepatitis B virus gene therapy.
- Citation: Ma CL, Wang GB, Gu RG, Wang F. Construction and characterization of calreticulin-HBsAg fusion gene recombinant adenovirus expression vector. World J Gastroenterol 2010; 16(24): 3078-3082
- URL: https://www.wjgnet.com/1007-9327/full/v16/i24/3078.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i24.3078
Development of a safe, effective and therapeutic vaccine represents the best hope for treatment of hepatitis B virus (HBV) infection. Recent data have indicated that immunotherapeutic strategies stimulating both cellular and humoral immune responses to HBV antigens are essential to cure chronic HBV infection[1]. In this regard, DNA-based vaccination appears to be a particularly pertinent approach for chronic hepatitis B treatment, since it has been well documented to elicit durable humoral and cell mediated immunity including cytotoxic T lymphocytes and cytokines in normal mice[2,3], ducks[4] and chimpanzees[5]. It is a good candidate for immunization of non-responders to recombinant hepatitis B surface antigen (HBsAg) vaccines and for therapeutic vaccination[2]. However, even at higher doses of DNA, human clinical trials of HBV DNA vaccine have yielded a much lower level of immune responses than that observed in small animals[6,7]. Therefore, the potency of DNA vaccines must be enhanced for successful human application. The use of calreticulin (CRT) represents an innovative and feasible approach for enhancing immune responses and generating an antiangiogenic effect[8]. CRT is an abundant 46 kDa Ca2+-binding protein located in the endoplasmic reticulum (ER)[9]. CRT is considered to be related to the family of heat shock proteins[10,11]. The protein has been shown to associate with peptides delivered into the ER by transporters associated with antigen processing (TAP-1 and TAP-2)[12] and with major histocompatibility complex (MHC) class I-β2 microglobulin molecules to aid in antigen presentation[13].
It is well known that available recombinant HBsAg protein vaccine is effective for preventing HBV infection for healthy persons, but for the infected patients there have been no effective and therapeutic vaccines until now. In this study, CRT-HBsAg fusion gene recombinant adenovirus expression vector was generated to establish the basis for curing chronic HBV infection and HBsAg-positive liver cancer.
ViraPower Adenoviral Expression System including pENTR/D-TOPO Cloning Vector, Adenoviral Expression Vector, HEK293A cells, TOP10 competent cells of Escherichia coli (E. coli) were all purchased from Invitrogen Corporation (USA). The pJW4303, plasmid was provided by professor Lu Shan (Umass, USA).
Taq DNA polymerase, Pfu DNA polymerase, antibodies marked by HRP, PacI, plasmid DNA purification kit and Gel purification kit were purchased from Tianwei Shidai Corporation (China), New England Corporation (UK), Takara Corporation (China), and Promega Corporation (USA), respectively. Human anti-HBsAg antibody positive serum was collected by our laboratory. Primers were synthesized in Shanghai Invitrogen Corporation (China).
For the generation of pJW43033-CRT, CRT was amplified with polymerase chain reaction (PCR) using rabbit CRT cDNA as the template[14] and a set of primers, 5′-CCGGAGACTCATGCTGCTCCCTGTGCCGCT-3′ and 5′-CCGGGAATTCCAGCTCGTCCTTGGCCTGGC-3′. There is more than 90% homology between rabbit, human, mouse, and rat CRT[14]. The amplified product was then cloned into the SacI/EcoRI sites of pJW4303 vector. For the generation of pJW4303-CRT/HBsAg, HBsAg gene was amplified with a set of primers, 5′-GTGGGGAATTCATGGAGAACACAACATCAGG-3′ and 5′-GGGGTGGATCCAATTTACATATGGGTTTCTGT-3′, and then cloned into the EcoRI/BamHI sites of pJW4303-CRT to generate pJW4303-CRT/HBsAg. The accuracy of these constructs was confirmed by endonuclease digestion and DNA sequencing. The sequencing was finished by Shanghai Invitrogen Biological Engineering Corp (China).
CRT/HBsAg fusion gene was amplified using pJW4303-CRT/HBsAg plasmid as the template and a set of primers, 5′-CACCATGCTGCTCCCTGTGCCGCT-3′ and 5′-AATTTACATATGGGTTTCTGT-3′. In order to construct directional TOPO cloning transfer vector in the desired direction, the CACC sequence was incorporated into the PCR upstream primer at its 5’ end. The final optimized conditions of PCR consisted of 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 1 min. After PCR, the PCR products of fusion gene were purified for generating the pENTR/D-TOPO transfer vector. The ligated products were transformed into TOP10 chemically competent E. coli and incubated overnight at 37°C. Subsequently, extracted and purified plasmids containing CRT/HBsAg fusion gene were characterized by PCR and sequencing, respectively. The sequencing was finished by Shanghai Invitrogen Biological Engineering Corp.
Using LR Clonase Mix™ (Invitrogen, US) as catalysis, the purified CRT/HBsAg fusion gene recombinant pENTR/D-TOPO transfer vector was mixed with adenoviral expression vector DNA plasmid to generate the recombinant adenoviral expression vector. The recombinant products were transformed into TOP10 chemically competent E. coli and incubated on Luria-Bertani (LB) plates containing 100 μg/mL ampicillin at 37°C overnight. Subsequently, we selected eight putative positive clones which were ampicillin-resistant to amplify, extract and purify for PCR amplification and electrophoresis detection, respectively. The reason why using LB plates containing 30 μg/mL chloramphenicol to select positive clones is because true expression clones would be ampicillin-resistant and chloramphenicol-sensitive.
Purified Ad-CRT/HBsAg DNA plasmid was digested with PacI. The day before transfection, the 293A cells were trysinized, counted, and plated at 5 × 105 cells per well in a 6-well plate. Two milliliter of normally growing Dulbecco’s modified Eagle’s medium (D-MEM) containing 10% fetal bovine serum (FBS) was plated into each well. Those 293A cells were transfected using Lipofectamine 2000™ (Invitrogen, USA) and Pac I-digested expression plasmid DNA complexes. Culture medium was replaced with refresh complete culture medium every 2-3 d until visible regions of cytopathic effect (CPE) were observed. When approximately 80% of CPE was observed, adenovirus-containing cells and media were harvested.
LacZ gene recombinant adenoviral expression vector plasmid (Ad-LacZ) provided by the manufacturer (Invitrogen, USA) was used as control.
The titer of Ad-CRT/HBsAg was characterized using the End-Point Dilution Assay. When the 293A cells prepared 24 h before incubated at 37°C in a CO2 incubator were at more than 80% confluency in 96-well plates, the recombinant adenovirus stock solution harvested above was diluted to a concentration of 10-1-10-12 and 100 μL was added to each well. After cultured for 10 d, the number of wells with CPE was calculated according to the formula of titre (pfu/mL) = 10(x + 0.8).
After being cultured for 72 h, the Ad-CRT/HBsAg infected and normal control 293A cells were harvested and frozen to -80°C. Some of them were prepared for Western blotting analysis. Protein samples were fractionated on 5%-12% SDS-PAGE and transferred to PVDF membrane using protein transfer apparatus (Bio-Rad, USA). The membrane was blocked for 1 h with 5% skimmed milk in TBS buffer (50 mmol/L Tris base, 150 mmol/L NaCl, pH 7.5) containing 0.5% Triton X-100. The membrane was probed with human anti-HBsAg antibody positive serum. After washing the membrane, goat anti-human HRP-conjugated antibody was added and incubated. The results were finally revealed using the sensitive substrate of DAB kit (Wuhan Boster, China) for Western blotting detection.
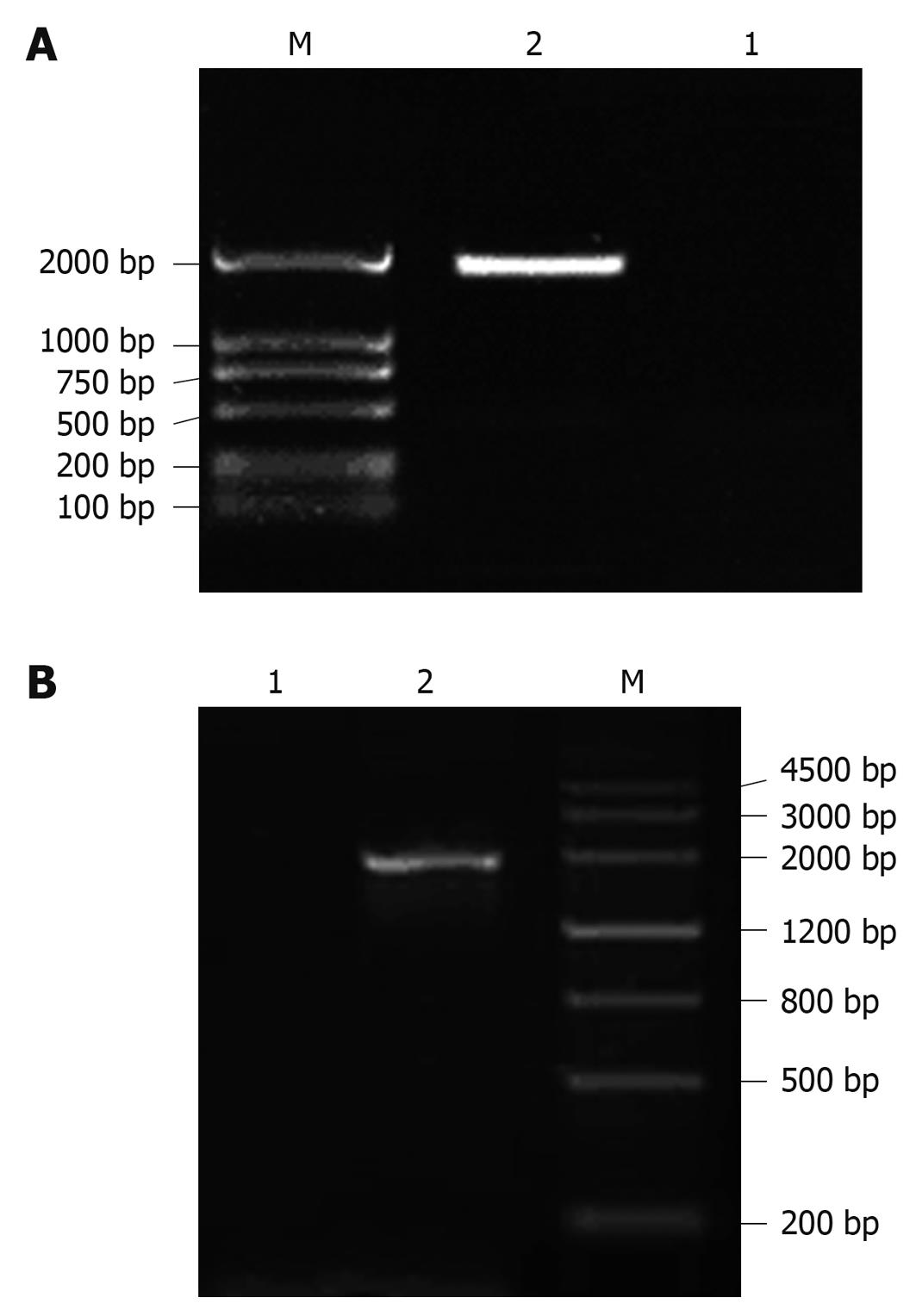
The CRT/HBsAg fusion gene recombinant plasmid pJW4303-CRT/HBsAg was digested by SacI and BamHI endonucleases. An approximately 2000 bp (1944 bp = 1263 bp + 681 bp) long DNA band was observed using 1.5% agarose gel electrophoresis (Figure 1A).
Kanamycin-resistant putative positive clones were prepared as template to amplify CRT/HBsAg fusion gene by specific PCR. Approximately 2000 bp long DNA bands were observed in those positive clones using 1.5% agarose gel electrophoresis (Figure 1B). The sequencing results of CRT and HBsAg gene were the same as CRT sequence of NM004343.3 and HBsAg sequence of AF013631 in GenBank.
All of the selected clones were sensitive for culturing in LB plates containing 30 μg/mL chloramphenicol because those true expression clones would be chloramphenicol-sensitive. After PCR amplification, a nearly 2000 bp long DNA band was observed using 1.5% agarose gel electrophoresis.
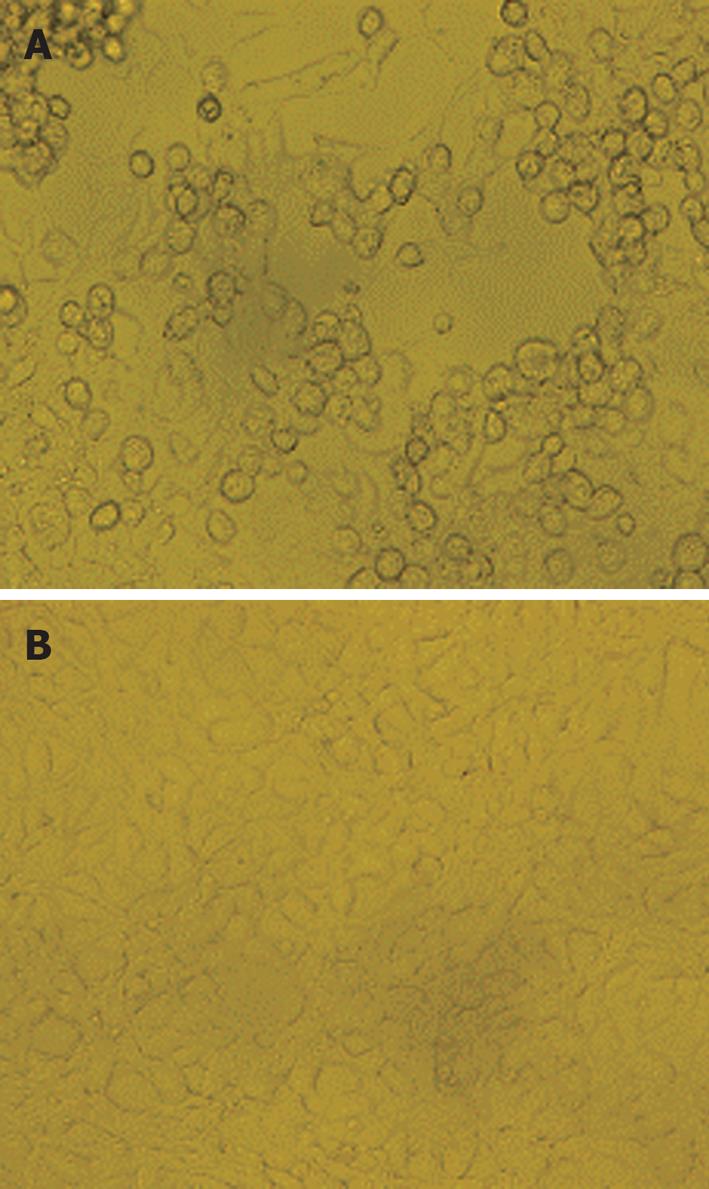
PacI-digested Ad-CRT/HBsAg DNA plasmid was transfected into 293A cells by Lipofectamine 2000™. About 10 d after transfection, 80% of the cells rounded up and were floating or lightly attached to the tissue culture dish. This indicated that cells were loaded with adenovirus particles (Figure 2A). The shape of normal 293A cells are shown in Figure 2B. The adenovirus-containing cells were harvested and characterized by PCR amplification. A DNA band, which was approximately 2000 bp long, was found with 1.5% agarose gel electrophoresis.
After having been amplified, the recombinant adenoviral stock was characterized by the End-Point Dilution Assay. Its titer was calculated as 3.9 × 1011 pfu/mL.
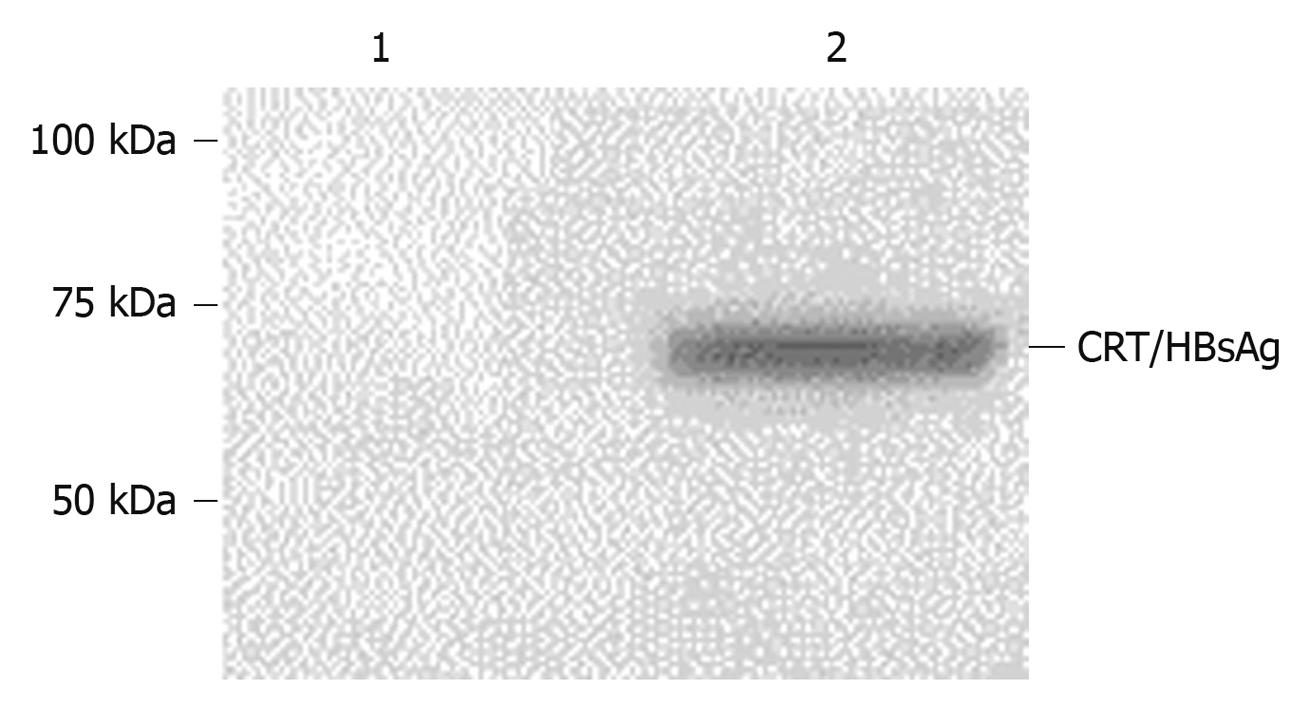
The expression of CRT/HBsAg fusion protein in Ad-CRT/HBsAg infected 293A cells was detected by Western blotting. Figure 3 shows the 71 kDa CRT/HBsAg fusion protein expression.
There are approximately four hundred million chronic hepatitis B patients in the world, and effective therapeutic vaccines are needed urgently. The purpose of this study is to facilitate the development of a new type of therapeutic HBV vaccine. The research hotspot in this field is how to develop an effective therapeutic vaccine by modifying the HBV antigens such as HBsAg, pres1Ag, pres2Ag and HBcAg to induce a high level of humoral and cellular immune responses for clearing the HBV infection. The HBV therapeutic vaccines include protein vaccine, DNA vaccine and virus vector vaccine, but none of them has been developed successfully.
Recently, investigators have performed a head-to-head comparison of linkage of antigen to CRT in a DNA vaccine, and achieved the greatest enhancement of the humoral and T-cell-mediated immune responses in vaccinated mice[15]. Previous studies have shown that adenoviral vectors expressing the fusion of CRT and E7 can effectively be used as a prophylactic and therapeutic vaccine against E7-expressing tumors[16]. In this study, the HBV surface gene was fused with CRT, which can improve immunogenicity by enhancing the MHC class I presentation of linked peptide/protein. CRT-HBsAg fusion gene recombinant adenovirus vector vaccine will be an ideal candidate for HBV therapeutic vaccine, because it can induce HBsAg specific high-level immune responses.
Members of adenovirus family infect a great variety of post-mitotic cells, even those associated with highly differentiated tissues such as skeletal muscle, lung, brain and heart. This characteristic, together with being relatively easy for preparation and purification, has led to their extensive use as gene vectors. Since they deliver their genome to the nucleus and can replicate with high efficiency, they are prime candidates for the expression and delivery of therapeutic genes[17]. The adenovirus vectors used in this study are to be utilized for delivering genes of CRT fused HBsAg to liver tissues to suppress and eliminate chronic inflammation and tumors caused by HBV infection. This is for the first time to construct the CRT-HBsAg gene recombinant adenovirus as a therapeutic vaccine. This study suggested that CRT-HBsAg fusion gene recombinant adenovirus vector may be a potent tool for treating chronic HBV carriers and liver cancer patients.
The hepatitis B virus (HBV) infection is one of the most widespread viral infections of human and causes acute and chronic hepatitis and hepatocellular carcinoma. Problem of HBV infection has necessitated the development of an effective therapeutic vaccine. Calreticulin (CRT) is a ubiquitously expressed Ca2+ binding protein in endoplasmic reticulum of eukaryotic cells. Previous studies have shown that CRT enhances the major histocompatibility complex (MHC) class I presentation of linked peptide/protein and may serve as an effective vaccination strategy for antigen-specific cancer treatment. In this study, CRT-hepatitis B surface antigen (HBsAg) fusion gene recombinant adenovirus expression vector was generated to establish the basis for curing acute and chronic HBV infection and HBsAg-positive hepatocellular carcinoma.
There are approximately four hundred million chronic hepatitis B patients in the world, and effective therapeutic vaccines are needed urgently. The research hotspot is how to develop an effective therapeutic vaccine by modifying the HBV antigens such as HBsAg, pres1Ag, pres2Ag and HBcAg to induce a high level of humoral and cellular immune responses for clearing the HBV infection. HBV therapeutic vaccines include protein vaccine, DNA vaccine and virus vector vaccine, but none of them has been developed successfully.
Most of the previous researches about HBV therapeutic vaccines focus on modifying HBsAg to enhance its immunogenicity. In this study, the HBV surface gene was fused with CRT, which can improve immunogenicity by enhancing the MHC class I presentation of linked peptide/protein. CRT-HBsAg fusion gene recombinant adenovirus vector vaccine will be an ideal candidate for HBV therapeutic vaccine, because it can induce HBsAg specific high-level immune responses. Adenovirus can infect a great variety of cell types and tissues in both dividing and non-dividing cells. This characteristic, together with being relatively easy for preparation and purification, has led to their extensive use as gene vectors. The adenovirus vectors used in this study are to be utilized for delivering genes of CRT fused HBsAg to liver tissues to suppress and eliminate tumors and inflammation caused by HBV infection. This is for the first time to construct the CRT-HBsAg gene recombinant adenovirus as a therapeutic vaccine.
CRT linked HBsAg gene recombinant adenovirus vectors were constructed in this study to develop a new sort of HBV therapeutic vaccine for the purpose of curing chronic hepatitis and hepatocellular carcinoma.
It is a nice work to construct CRT/HBsAg fusion gene recombinant replication-defective adenovirus expression vector and it has provided a therapeutic vaccine and an experimental basis for further research of HBV gene therapy.
Peer reviewer: Seong Gyu Hwang, Professor, MD, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, #351, Yatap-Dong, Bundang-Gu, Seongnam, Gyeonggi-Do, 463-712, South Korea
S- Editor Wang JL L- Editor Ma JY E- Editor Lin YP
| 1. | Trepo C, Maynard M, Zoulim F. Perspectives on therapy of hepatitis B. J Hepatol. 2003;39 Suppl 1:S220-S223. |
| 2. | Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci USA. 1998;95:15553-15558. |
| 3. | Oka Y, Akbar SM, Horiike N, Joko K, Onji M. Mechanism and therapeutic potential of DNA-based immunization against the envelope proteins of hepatitis B virus in normal and transgenic mice. Immunology. 2001;103:90-97. |
| 4. | Le Guerhier F, Thermet A, Guerret S, Chevallier M, Jamard C, Gibbs CS, Trépo C, Cova L, Zoulim F. Antiviral effect of adefovir in combination with a DNA vaccine in the duck hepatitis B virus infection model. J Hepatol. 2003;38:328-334. |
| 5. | Davis HL, McCluskie MJ, Gerin JL, Purcell RH. DNA vaccine for hepatitis B: evidence for immunogenicity in chimpanzees and comparison with other vaccines. Proc Natl Acad Sci USA. 1996;93:7213-7218. |
| 6. | Tacket CO, Roy MJ, Widera G, Swain WF, Broome S, Edelman R. Phase 1 safety and immune response studies of a DNA vaccine encoding hepatitis B surface antigen delivered by a gene delivery device. Vaccine. 1999;17:2826-2829. |
| 7. | Boyer JD, Cohen AD, Vogt S, Schumann K, Nath B, Ahn L, Lacy K, Bagarazzi ML, Higgins TJ, Baine Y. Vaccination of seronegative volunteers with a human immunodeficiency virus type 1 env/rev DNA vaccine induces antigen-specific proliferation and lymphocyte production of beta-chemokines. J Infect Dis. 2000;181:476-483. |
| 8. | Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, Wu TC. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669-678. |
| 9. | Nash PD, Opas M, Michalak M. Calreticulin: not just another calcium-binding protein. Mol Cell Biochem. 1994;135:71-78. |
| 10. | Conway EM, Liu L, Nowakowski B, Steiner-Mosonyi M, Ribeiro SP, Michalak M. Heat shock-sensitive expression of calreticulin. In vitro and in vivo up-regulation. J Biol Chem. 1995;270:17011-17016. |
| 11. | Basu S, Srivastava PK. Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J Exp Med. 1999;189:797-802. |
| 12. | Spee P, Neefjes J. TAP-translocated peptides specifically bind proteins in the endoplasmic reticulum, including gp96, protein disulfide isomerase and calreticulin. Eur J Immunol. 1997;27:2441-2449. |
| 13. | Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103-114. |
| 14. | Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochem J. 1999;344 Pt 2:281-292. |
| 15. | Kim TW, Hung CF, Ling M, Juang J, He L, Hardwick JM, Kumar S, Wu TC. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003;112:109-117. |
| 16. | Kim TW, Lee JH, Hung CF, Peng S, Roden R, Wang MC, Viscidi R, Tsai YC, He L, Chen PJ. Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J Virol. 2004;78:4638-4645. |
| 17. | Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000;81:2573-2604. |