Published online Jun 28, 2010. doi: 10.3748/wjg.v16.i24.3056
Revised: March 12, 2010
Accepted: March 19, 2010
Published online: June 28, 2010
AIM: To analyze the combined treatment of resection and intraoperative radiofrequency ablation (RFA) for multifocal hepatocellular carcinoma in terms of prognosis and surgical outcomes.
METHODS: This study was a retrospective case comparison study using prospectively collected data. The study covered the period from April 2001 to December 2006. The data of 200 patients with histologically confirmed hepatocellular carcinoma were reviewed. Nineteen patients (17 men and 2 women) having received resection in combination with RFA were chosen as subjects of the study (the combination group). Fifty-four patients (43 men and 11 women) having received resection alone were selected for comparison (the resection group). The two groups matched tumor number and tumor size, and all the patients in the two groups displayed no tumor rupture, major vascular involvement and distant metastasis. Their demographics, preoperative assessment, disease recurrence patterns, overall survival and disease-free survival were compared.
RESULTS: In the combination group, the median age was 65 years (range, 34-77 years), the median tumor number was 3 (range, 2-9), and the median tumor size was 6 cm (range, 1.2-14 cm). In the resection group, the median age was 51.5 years (range, 27-80 years, P = 0.003), the median tumor number was 3 (range, 2-9, P = 0.574), and the median tumor size was 6 cm (range, 1-14 cm, P = 0.782). The two groups were similar in characteristics of tumors and comorbidities, and had comparable results in preoperative liver function tests. All patients had Child-Pugh class A status. Bilobar involvement occurred in 14 patients (73.6%) in the combination group and 3 patients (5.5%) in the resection group (P = 0.04). Six patients (32%) in the combination group and 35 patients (65%) in the resection group underwent major hepatectomy. Thirteen patients (68%) in the combination group and 19 patients (35%) in the resection group underwent minor hepatectomy (P = 0.012). The combination group had fewer major resections (32% vs 65%, P = 0.012), less blood loss (400 vs 657 mL, P = 0.007), shorter operation time (270 vs 400 min, P = 0.001), and shorter hospital stay (7 vs 8.5 d, P = 0.042). The two groups displayed no major differences in surgical complications (15.8% vs 31.5%, P = 0.24), disease recurrence (63.2% vs 50%, P = 0.673), hospital mortality (5.3% vs 5.6%, P = 1), and overall survival (53 vs 44.5 mo, P = 0.496).
CONCLUSION: Safe and effective for selected patients with multifocal hepatocellular carcinoma, the combination of resection and intraoperative RFA widens the applicability of surgical intervention for the disease.
- Citation: Cheung TT, Ng KK, Chok KS, Chan SC, Poon RT, Lo CM, Fan ST. Combined resection and radiofrequency ablation for multifocal hepatocellular carcinoma: Prognosis and outcomes. World J Gastroenterol 2010; 16(24): 3056-3062
- URL: https://www.wjgnet.com/1007-9327/full/v16/i24/3056.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i24.3056
Management of hepatocellular carcinoma (HCC) in patients with cirrhosis is always a great challenge for clinicians. Prognosis of most HCC patients is poor due to the low rate of tumor resectability (20%-37%)[1,2]. Although resection is regarded as the gold-standard treatment for HCC, many patients are not suitable for it because of unfavorable anatomical location, major vessel involvement, multifocal involvement, distant metastasis, or poor liver function[3-7]. In the hope of improving the survival of patients having unresectable HCC, locoregional treatment aiming at local tumor control has been developed.
Radiofrequency ablation (RFA) is a locoregional treatment which has gained recognition in the management of liver diseases including HCC. During RFA treatment, heat energy generated by high-frequency alternating currents (460-480 kHz) targeted at the living tissues causes protein denaturation at a temperature of 60°C through ionic vibration; coagulative necrosis of the target lesion then follows. RFA outperforms other locoregional treatments by its effective tumor ablation, minimal liver damage, low morbidity, and low mortality[3,8-12].
Lately, there have been detailed studies on RFA treating small HCC as well as isolated reports on the combined employment of resection and RFA for the treatment of liver metastasis of HCC[13-15]. Curley et al[16] reported a series of RFA treatment on 123 patients bearing unresectable primary (n = 48) or metastatic (n = 75) liver tumors. A total of 169 tumors were ablated with a clearance rate of 100%. There was no operative mortality. However, the efficacy of RFA on large tumors is still questionable. Although several reports showed that RFA with multiple processes and overlap of ablation zones was feasible for treating large liver tumors, the high recurrence rates did not favor it as a primary treatment tool for them[17-22]. Resection is still considered the first treatment option for large HCC.
Patients with multiple HCCs can opt for multiple resections, but multiple resections entail a higher risk of liver failure for patients with cirrhosis. Hence, in the management of multifocal HCC, combined employment of resection (for large tumors) and RFA (for small tumors) sounds like a reasonable measure for the achievement of complete tumor clearance.
Both resection and RFA are good treatment options for HCC while at the same time they both have their own limitations. Resection is limited by the patient’s liver function and the location of tumor, and RFA is limited to small tumors only. In general, around one-fourth of patients with HCC are amenable to surgical intervention, and among them, around one-fourth develop multiple tumors on presentation. The combination of resection and RFA can subject more patients to potentially curative surgical intervention.
This study compared the combined treatment of resection and RFA with conventional hepatectomy alone in terms of prognosis and surgical outcomes in treating multifocal HCC.
This study was a retrospective case comparison study using prospectively collected data. In our hospital, the data of all patients of liver surgery are input into a detailed computerized database by the surgeon-in-charge after patient discharge. Data collected include demographics, preoperative investigation results, operative details, and postoperative investigation results. This study covered the period from April 2001 to December 2006. The data of 200 patients with histologically confirmed HCC were reviewed. Nineteen patients having received resection in combination with RFA were chosen as subjects of the study (the combination group). Fifty-four patients having received resection alone were selected for comparison (the resection group). The two groups matched in terms of tumor number (range, 2-9) and tumor size (within 14 cm), and all patients in the two groups displayed no tumor rupture, major vascular involvement and distant metastasis. Their demographics, preoperative assessment, disease recurrence patterns, overall survival and disease-free survival were compared.
All the 73 patients had their medical history reviewed and were fully assessed with physical examination. They received chest X-ray (CXR) and computed tomography (CT) scan of the abdomen. They also received serum laboratory tests which yielded information on complete blood count, platelet count, coagulation profile, hepatitis B and hepatitis C serology, total serum bilirubin, serum albumin, serum alanine aminotransferase, serum aspartate aminotransferase, serum gamma-glutamyl transferase, serum alkaline phosphatase, serum α-fetoprotein (AFP), electrolytes, renal function, and indocyanine green (ICG) clearance rate. Resectability of tumor was confirmed upon three criteria: first, there was no distant metastasis according to the results of CXR and CT scan; second, the ICG rate was less than 14.4% at 15 min; and third, the estimated mass volume of the residual liver after resection was more than 30% of the original liver.
Each patient in the combination group underwent resection and RFA in one single operation intended to cure. The operation was performed with the open approach, starting with an initial exploration of the abdomen and pelvis to confirm the absence of extrahepatic lesion. Intraoperative ultrasound was used to identify tumor location and number as well as the relation between the tumors and the vasculature of the liver. Anatomical resection was performed for the largest tumors or the largest groups of tumors which were deemed surgically resectable with clear margins. The extent of resection was determined by lesion location, the relation between the lesion and the surrounding vasculature, and biliary involvement. Parenchymal transection was performed with a Cavitron ultrasonic surgical aspirator. Segmental pedicles and other major vasculatures were divided with vascular staplers or ligatures. Pringle’s maneuver was not applied.
Lesions not treated with resection were then subjected to RFA with standard treatment algorithm. Intraoperative ultrasound was used to guide the placement of the RFA needle at the target lesion. Single needle or needle cluster was used according to the size of the target tumor. For tumors larger than 3 cm, cluster probe was used. A margin of 1 cm was included in ablation. The Cool-tip™ system which consisted of a generator that supplied power up to 200 W was used. The electrode was optimally positioned to achieve complete tumor destruction and a normal parenchymal ablation zone of at least 1 cm.
All the patients were originally enlisted for resection only, but due to various reasons, the operative surgeon adopted RFA as an additional treatment modality with curative intention for complete control of tumors. The reasons for the adoption of a combination therapy are listed in Table 1.
| Combination group (n = 19) | |
| Bilobar disease | 14 (73.6) |
| Proximity to major vessel or bile duct | 5 (26.3) |
| Dense adhesion | 3 (15.8) |
| Large resection required for small tumors | 5 (26.3) |
| ICG rate at 15 min > 14.4% | 5 (26.3) |
| Low platelet count (< 100 × 109/L) | 3 (15.8) |
| Severe cirrhosis | 9 (47.4) |
Monitoring of complete blood picture, coagulation profile, blood gas, liver function and renal function was carried out on day 1 and day 7 as part of the standard protocol. ICG clearance test was done on day 7 if possible. CXR, CT scan of the abdomen and serum AFP investigation were performed at one month, then quarterly in the first two years, and half-yearly afterwards.
All clinical data were analyzed with SPSS version 11.5 under the Window 98 operating system. Categorical variables were compared by a χ2 test or Fisher’s exact test. Continuous variables were compared by the Mann-Whitney U test. Survival rates were calculated by the Kaplan-Meier method, and differences in survival were analyzed by the log rank test. P value < 0.05 was considered statistically significant. The primary outcome measurement was overall survival and the secondary outcome measurement was disease-free survival. Immediate postoperative performance was also analyzed.
In the combination group, 17 men and 2 women, the median age was 65 years (range, 34-77 years). The median tumor number was 3 (range, 2-9) and the median tumor size was 6 cm (range, 1.2-14 cm). In the resection group, 43 men and 11 women, the median age was 51.5 years (range, 27-80 years, P = 0.003). The median tumor number was 3 (range, 2-9, P = 0.574) and the median tumor size was 6 cm (range, 1-14 cm, P = 0.782). The two groups were similar in characteristics of tumors and comorbidities, and had comparable results in preoperative liver function tests on serum bilirubin, serum albumin, prothrombin time, platelet count and ICG clearance. All patients in the two groups had Child-Pugh class A status (Table 2). Bilobar involvement occurred in 14 patients (73.6%) in the combination group and 3 patients (5.5%) in the resection group (P = 0.04).
| Combination group (n = 19) | Resection group (n = 54) | P value | |
| Age (yr) | 65 (34-77) | 52 (27-80) | 0.003 |
| Gender (M:F) | 17:2 | 43:11 | 0.492 |
| Comorbidities | 10 (53%) | 20 (37%) | 0.235 |
| Renal impairment | 0 (0%) | 2 (3.7%) | 1.000 |
| Diabetes mellitus | 5 (26.3%) | 10 (18.5%) | 0.469 |
| Chest infection | 1 (5.3%) | 3 (5.6%) | 1.000 |
| Coronary complications | 6 (31.6%) | 14 (25.9%) | 0.635 |
| Hepatitis B infection | 16 (84.2%) | 42 (77.8%) | 0.745 |
| Hepatitis C infection | 2 (10.5%) | 2 (3.7%) | 0.478 |
| Child-Pugh class A | 19 | 54 | 1.000 |
| Platelet count (× 109/L) | 165 (91-64.5) | 177 (86-458) | 0.396 |
| ICG (% at 15 min) | 11.8 (3-25.7) | 9 (3.7-18.2) | 0.083 |
| AFP level (ng/mL) | 248 (6-38 040) | 133 (2-530 600) | 0.915 |
| Tumor size (cm) | 6 (1.2-14) | 6 (1-12.5) | 0.782 |
| Tumor number | 3 (2-9) | 3 (2-9) | 0.574 |
Six patients (32%) in the combination group and 35 patients (65%) in the resection group received major hepatectomy. Thirteen patients (68%) in the combination group and 19 patients (35%) in the resection group received minor hepatectomy (P = 0.012). The types of resection performed are listed in Table 3. In the combination group, 5 patients had small tumor but received a large volume of resection for clearance, and 5 patients had tumors located close to major vessel, bile duct, or junction of hepatic veins. A total of 31 tumors were ablated, and the median size of the tumors was 1 cm (range, 1-3.8 cm). Ten patients had 1 tumor ablated, 6 patients 2, and 3 patients 3. Biopsies of the ablation sites were taken and all proven to be positive for HCC. The ablated sites were monitored with CT scan with contrast at one month, and no incomplete ablation was noted.
| Combination group (n = 19) | Resection group (n = 54) | |
| Right hepatectomy | 1 (5.3) | 17 (31.5) |
| Extended right hepatectomy | 0 (0) | 6 (11.1) |
| Right trisectionectomy | 0 (0) | 2 (3.7) |
| Left hepatectomy | 3 (15.8) | 3 (5.6) |
| Extended left hepatectomy | 2 (10.5) | 4 (7.4) |
| Left trisectionectomy | 0 (0) | 3 (5.6) |
| Left lateral sectionectomy | 3 (15.8) | 1 (1.9) |
| Segmentectomy | 1 (5.3) | 11 (20.4) |
| Wedge resection of liver | 9 (47.4) | 7 (13) |
The combination group demonstrated certain advantages over the resection group in terms of surgical outcomes: the former had median blood loss of 400 mL (range, 20-1650 mL) whereas the latter had 657 mL (range, 50-3750 mL) (P = 0.007), the former had median operation time of 270 min (range, 150-465 min) whereas the latter had 400 min (range, 165-773 min) (P = 0.001), and the former had median hospital stay of 7 d (range, 1-20 d) and the latter had 8.5 d (range, 4-69 d) (P = 0.042). There was no difference in overall complications (15.8% vs 31.5%, P = 0.241) and hospital mortality (5.3% vs 5.6%, P = 1) between the two groups. Only one patient from the resection group needed blood transfusion. The median serum bilirubin level at day 1 was 21 μmol/L (range, 10-37 μmol/L) in the combination group and 25 μmol/L (range, 9-110 μmol/L) in the resection group (P = 0.012). The median serum albumin level at day 1 was 31.5 g/L (range, 10-37 g/L) in the combination group and 32 g/L (range, 17-39 g/L) in the resection group (P = 0.215). The median serum bilirubin level at day 7 was 18 μmol/L (range, 11-27 μmol/L) in the combination group and 21 μmol/L (range, 3-190 μmol/L) in the resection group (P = 0.075). The median serum albumin level at day 7 was 30 g/L (range, 24-39 g/L) in the combination group and 32 g/L (range, 26-41 g/L) in the resection group (P = 0.036).
With regard to the pathology of tumors, the combination group and the resection group shared similar characteristics. Margin involvement happened on none of the patients in the former group while on 4 patients (7.4%) in the latter (P = 0.567). Vascular permeation happened on 7 patients (36.8%) in the former group and 31 patients (57.4%) in the latter (P = 0.123). Microsatellite lesions appeared in 3 patients (15.8%) of the former group and 14 patients (25.9%) of the latter (P = 0.53). Poorly differentiated cell types by Edmondson classification appeared in 2 patients (10.5%) of the former group and 8 patients (14.8%) of the latter (P = 0.857). There was no statistical difference in histopathology between the two groups (Table 4).
| Combination group (n = 19) | Resection group (n = 54) | P value | |
| Margin involvement | 0 (0) | 4 (7.4) | 0.567 |
| Vascular permeation | 7 (36.8) | 31 (57.4) | 0.123 |
| Microsatellite lesion | 3 (15.8) | 14 (25.9) | 0.530 |
| Poorly differentiated cell type | 2 (10.5) | 8 (14.8) | 0.857 |
| Complete ablation | 19 (100) | - | |
| Histological proof of HCC at ablation sites | 19 (100) | - |
Twelve patients (63.2%) in the combination group were found developing recurrence at the end of the study. Intrahepatic recurrence occurred in 8 patients (42.1%), and among them 4 patients (20.5%) had ablation site local recurrence, which happened at a median time of 3.6 mo (range, 3-24 mo). Extrahepatic recurrence occurred in 1 patient (5.3%) and intrahepatic and extrahepatic recurrence in 3 (15.8%). In the resection group, 27 patients (50%) were found developing recurrence. Among them, 15 patients (27.8%) had intrahepatic recurrence, 4 patients (7.4%) extrahepatic recurrence, and 8 patients (14.8%) intrahepatic and extrahepatic recurrence (P = 0.673).
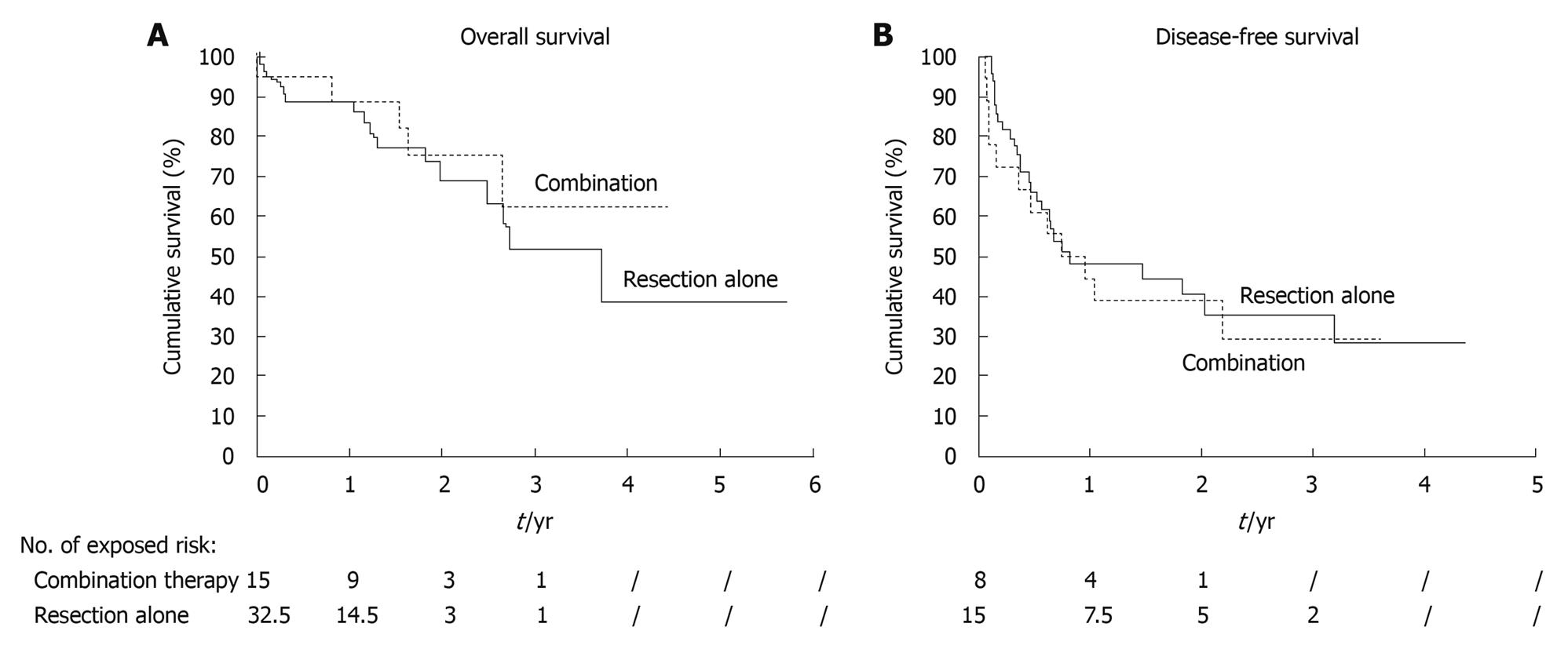
The median follow-up period was 18.4 mo for the entire study period, 21 mo (range, 0.03-53 mo) for the combination group and 15.1 mo (range, 0.37-68.4 mo) for the resection group. No patient was lost to follow-up. The rates of overall survival at 1 year and 3 years were 88.8% and 62.6%, respectively for the former group (median 53 mo), and 88.9% and 51.8%, respectively for the latter (median 44.5 mo) (P = 0.496) (Figure 1A). The median disease-free survival of the former was 8.8 mo and that of the latter was 9.8 mo (P = 0.64) (Figure 1B). The two groups showed no statistically significant differences in survival.
A variety of treatment options are available for HCC. Liver transplantation offers good hope of cure to HCC patients, even those with poor liver function. However, donated livers are scarce, and patients having tumors exceeding the size limit for liver transplant or having low score in Model for End-stage Liver Disease are not suitable for transplantation. So, the first-line option is still, if technically feasible, hepatectomy[1,4,23,24]. Hepatectomy with clear margin can provide long postoperative survival to patients. Unfortunately, poor liver function poses extra challenge to hepatectomy because hepatectomy will diminish the already limited liver function reserve, thus risking liver failure. In Southeast Asia including Hong Kong, HCC patients usually have poor liver function because their HCC often coexists with hepatitis B cirrhosis. Striking a balance between achieving adequate resection and preventing liver failure is not easy at all. In patients with multifocal HCC, equilibrium between complete tumor clearance and minimal hepatectomy volume is even more difficult to grasp. Multiple partial resections, on the one hand, can achieve complete tumor clearance but, on the other hand, carry a higher risk of liver failure with doubtful long-term oncological outcomes.
RFA is another treatment option for HCC. Several clinical trials showed that RFA was as effective as hepatectomy for HCC smaller than 5 cm with a morbidity rate of less than 15%[22,25,26]. Hence, RFA has been gaining recognition in treating liver cancer including advanced HCC. Nonetheless, the efficacy of RFA on larger HCC is still questionable. In a study using a porcine model, RFA of 30%-35% of liver and hepatectomy of the same volume were compared, and it was found that the former resulted in significantly adverse systemic inflammatory response syndrome[27-29]. Hoshida et al[30] showed in another study that large parenchymal RFA volume was an independent poor prognostic factor for HCC patients. With recent advances in the design of radiofrequency electrodes and refinements of ablation techniques, favorable outcomes of RFA treatment on large tumors have been reported. However, extra caution should always be taken in treating large tumors with RFA since ablation of large tumors is associated with a high incidence of local recurrence, and the large amount of necrotic tissue left behind can cause serious problems for the patients. To ensure better outcome of RFA, careful patient selection, meticulous techniques and close monitoring of hepatic and renal functions after the procedure are essential.
Combined employment of hepatectomy and RFA for metastatic liver cancer has been documented. This combination of treatments can improve the tumor resectability rate for patients having multiple tumors. In this comparatively novel treatment, large tumors are resected and smaller ones at difficult locations are ablated. Currently, data on its performance in the management of HCC are very limited[14-16,22,25,26,31]. In our present study, the surgical outcomes of the combined treatment of hepatectomy and RFA on patients with multifocal HCC were investigated, and it was shown that patients having bilobar HCC could be treated safely with it. In the study, considerably less major hepatectomies were performed for the combination group, yet complete tumor clearance was still achieved. This is of crucial importance as hepatectomy in a lesser extent means better preservation of liver reserve, less liver failure, and quicker recovery[32,33].
For managing multifocal HCC, one may argue that multiple anatomical resections can achieve similar results as the combination approach. But in our study, we found that there are situations in which the combination approach is easier, if not better, than resection alone. First of all, if a tumor is small but requires a large resection volume for tumor clearance, RFA comes in as a safer option in terms of liver function preservation. Secondly, if a tumor is close to an important anatomical structure such as major vessels or bile ducts, RFA together with bile duct cooling can offer complete tumor clearance, obviating major hepatectomy. Thirdly, in the case of reoperation at a site where there is dense adhesion, RFA is a less traumatic treatment option, as mobilization for resection may lead to bleeding problem, especially in patients with thrombocytopenia and coagulopathy.
In this study, the median age of the combination group was 65 years, which was 13 years older than the 52-year median of the resection group. Choice of treatment was up to individual surgeons. It turned out that more elderly patients received the combined treatment. This was probably because the combined treatment would require less operation time and induce seemingly less surgical trauma. The resultant shorter operation time, less blood loss and shorter hospital stay have vindicated the postulation.
An ablation rate of 100% was achieved as confirmed by CT scan of the abdomen performed one month after RFA treatment. The two groups displayed similar recurrence patterns. A margin of 1 cm was sought in all resections and ablations since a 1-cm margin is usually effective in preventing microscopic involvement by the major tumor. However, a clear margin alone is not enough for preventing tumor recurrence, as apart from margin involvement, there are other factors affecting recurrence such as poorly differentiated cell type, lymphovascular permeation, and microsatellite lesion, among which the latter two are the most significant independent risk factors. Unfortunately, we cannot alter these factors even by providing generous margins[24].
The combination group had more bilobar cases than the resection group (73.6% vs 7.4%). Theoretically, patients with stage IVa HCC would fare worse[34,35]. The predominance of bilobar disease in the combination group also explains why the group had more, though not significantly, intrahepatic and extrahepatic recurrences. A more advanced stage of HCC probably entails a poorer prognosis in general. Sub-group analysis of overall survival of these patients in the two groups was performed, and the median survival was 53 mo in the combination group and 44.5 mo in the resection group (P = 0.496). No significant difference was noted because of the small sample number in the resection group (n = 3). In our hospital, the median survival of patients having stage IVa HCC is 10 mo if they undergo no treatment. In the past, bilobar liver disease was considered a contraindication to hepatectomy, but with new tools and techniques, the whole concept of resectability is changing. Liu et al[36] pointed out that resection offered better survival outcomes than non-resectional treatments did to HCC patients with stage IVa bilobar disease. For this category of patients, resection in combination with RFA is an attractive choice of treatment. As to survival in our present study, the combination group and the resection group displayed comparable rates of overall survival and disease-free survival.
This study has clearly shown that when complete resection by major hepatectomy is dangerous because of marginal liver function or difficult tumor location, selective use of RFA is helpful. The integration of RFA into resectional surgery contributes to complete removal of tumors with adequate margin, diminishes the extent of parenchymal resection, and improves the resectability rate for patients with stage IVa liver disease. Safe and quick, it is especially handy when unexpected tumor is discovered during laparotomy, allowing complete tumor clearance in an unplanned situation during surgical operation without altering the original plan of treatment procedure too much; this is particularly important to patients having marginal hepatic function.
Fresh hope emerges as tumors previously considered unresectable due to multifocal involvement or poor liver function reserve can now be removed by the combined treatment, a new option in treating HCC. At present, a randomized controlled trial comparing it with surgical resection alone in patients with multifocal HCC is much desirable.
Management of hepatocellular carcinoma (HCC) in patients with cirrhosis is always a great challenge for clinicians. Prognosis of these patients is poor because many of their tumors are unresectable (with a resectability rate of 20%-37% only). Although resection is regarded as the gold-standard treatment for HCC, it is not applicable to cases with unfavorable anatomical location, major vessel involvement, multifocal involvement, distant metastasis, or poor liver function. On the other hand, radiofrequency ablation (RFA) is a treatment which has been gaining more and more recognition in the management of liver diseases including HCC. Both resection and RFA are good treatment options for HCC. However, they have their own limitations. Resection is hampered by patient’s liver function as well as the location of tumor, whilst RFA is limited to smaller tumors only. In reality, only around one-fourth of HCC patients are amenable to surgical intervention, and in this sub-group, around one-fourth develop multiple tumors on presentation. There is no doubt that the combination of resection and RFA can subject more patients to potentially curative surgical intervention. The present study compared the combined treatment of resection and RFA with the treatment of sole resection and investigated the surgical outcomes of the combined treatment in patients with multifocal HCC.
There is no detailed report on the combined use of resection and RFA in the management of HCC. However, this is an important topic as treating HCC with RFA is getting popular, and combining RFA with resection can definitely extend the operability of surgical intervention to patients with multifocal HCC.
In the management of multifocal HCC, one may argue that multiple resections can achieve what the combined treatment achieves, and thus there is no need to call in RFA. However, in the present study, it has been found that in some situations the combined treatment is easier than resection alone. First of all, since RFA is less traumatic and hence better for liver function preservation, it is preferable to resection when the tumor is small but still requires a large volume of resection for tumor clearance. Secondly, when the tumor is close to an important anatomical structure such as major vessel or bile duct, RFA together with bile duct cooling can achieve complete tumor clearance with lower risk. Thirdly, if dense adhesion is encountered, which is not uncommon in re-operation, RFA is safer as mobilization of the liver for resection may lead to bleeding problem especially in patients with thrombocytopenia and coagulopathy.
This study has clearly shown that when complete resection by major hepatectomy is dangerous because of marginal liver function or difficult tumor location, selective use of RFA provides the benefit of increased tumor resectability. The integration of RFA into resectional surgery contributes to complete removal of tumors with adequate margin, diminishes the extent of parenchymal resection, and improves the resectability rate for patients with stage IVa liver disease. Being safe and quick, it is especially handy when unexpected tumor is encountered during laparotomy, allowing achievement of complete tumor clearance in an unplanned situation during surgical operation without altering the original plan of treatment procedure too much; this is particularly important to patients with marginal liver function.
RFA is a locoregional treatment. RFA treats tumors by inducing coagulative necrosis of the target lesion. During RFA, heat energy generated by high-frequency alternating currents (460-480 kHz) targeted at the lesion through ionic vibration causes protein denature at a temperature of 60°C, which causes coagulative necrosis of tissues.
The Hong Kong group describes their experience with surgical treatment of HCC by performing a retrospective analysis of resected-only patients versus resected + RFA patients with HCC. This is important data to present and very clinically relevant as centers all over the world are taking care of rising numbers of patients with HCC.
Peer reviewers: Koert P de Jong, MD, PhD, Department of Hepato-Pancreato-Biliary surgery and Liver Transplantation, University Medical Center Groningen, PO Box 30 001, 9700 RB Groningen, The Netherlands; Michelle Lai, MD, MPH, Instructor in Medicine, Harvard University, Department of Medicine, Division of Gastroenterology/Hepatology, Beth Israel Deaconess Medical Center, 110 Francis Street, Suite 4A, Boston, MA 02215, United States
S- Editor Wang YR L- Editor Ma JY E- Editor Lin YP
| 1. | Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322-330. |
| 2. | Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790-799; discussion 799-800. |
| 3. | Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466-486. |
| 4. | Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225-235. |
| 5. | Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381-388. |
| 6. | Llovet JM, Sala M, Bruix J. Nonsurgical treatment of hepatocellular carcinoma. Liver Transpl. 2000;6:S11-S15. |
| 7. | Little SA, Fong Y. Hepatocellular carcinoma: current surgical management. Semin Oncol. 2001;28:474-486. |
| 8. | Ng KK, Lam CM, Poon RT, Ai V, Tso WK, Fan ST. Thermal ablative therapy for malignant liver tumors: a critical appraisal. J Gastroenterol Hepatol. 2003;18:616-629. |
| 9. | Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol. 2004;11:281-289. |
| 10. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655-661. |
| 11. | Livraghi T, Lazzaroni S, Meloni F. Radiofrequency thermal ablation of hepatocellular carcinoma. Eur J Ultrasound. 2001;13:159-166. |
| 12. | Ng KK, Poon RT. Radiofrequency ablation for malignant liver tumor. Surg Oncol. 2005;14:41-52. |
| 13. | Curley SA, Izzo F. Radiofrequency ablation of primary and metastatic liver tumors. Surg Technol Int. 2002;10:99-106. |
| 14. | Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10:1059-1069. |
| 15. | Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-825; discussion 825-827. |
| 16. | Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1-8. |
| 17. | Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10:338-347. |
| 18. | Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129. |
| 19. | Lam CM, Ng KK, Poon RT, Ai V, Yuen J, Fan ST. Impact of radiofrequency ablation on the management of patients with hepatocellular carcinoma in a specialized centre. Br J Surg. 2004;91:334-338. |
| 20. | Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450-458. |
| 21. | Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST, Wong J. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441-449. |
| 22. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. |
| 23. | Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70. |
| 24. | Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann Surg. 2000;231:544-551. |
| 25. | Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, Dai Y. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology. 2004;232:260-271. |
| 26. | Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Effectiveness of radiofrequency ablation for hepatocellular carcinomas larger than 3 cm in diameter. Arch Surg. 2004;139:281-287. |
| 27. | Ng KK, Lam CM, Poon RT, Shek TW, To JY, Wo YH, Ho DW, Fan ST. Comparison of systemic responses of radiofrequency ablation, cryotherapy, and surgical resection in a porcine liver model. Ann Surg Oncol. 2004;11:650-657. |
| 28. | Ng KK, Lam CM, Poon RT, Shek TW, Ho DW, Fan ST. Safety limit of large-volume hepatic radiofrequency ablation in a rat model. Arch Surg. 2006;141:252-258. |
| 29. | Ng KK, Lam CM, Poon RT, Shek TW, Yu WC, To JY, Wo YH, Lau CP, Tang TC, Ho DW. Porcine liver: morphologic characteristics and cell viability at experimental radiofrequency ablation with internally cooled electrodes. Radiology. 2005;235:478-486. |
| 30. | Hoshida Y, Shiratori Y, Koike Y, Obi S, Hamamura K, Teratani T, Shiina S, Omata M. Hepatic volumetry to predict adverse events in percutaneous ablation of hepatocellular carcinoma. Hepatogastroenterology. 2002;49:451-455. |
| 31. | Curley SA, Izzo F. Radiofrequency ablation of primary and metastatic hepatic malignancies. Int J Clin Oncol. 2002;7:72-81. |
| 32. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602-611. |
| 33. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373-382. |
| 34. | Poon RT, Fan ST, Ng IO, Wong J. Prognosis after hepatic resection for stage IVA hepatocellular carcinoma: a need for reclassification. Ann Surg. 2003;237:376-383. |
| 35. | Kawarada Y, Yamagiwa K. [Up to date of multidisciplinary treatments centering around hepatectomy for advanced liver cancer in stage IV-A]. Gan To Kagaku Ryoho. 2000;27:1489-1495. |