Published online Jun 28, 2010. doi: 10.3748/wjg.v16.i24.3011
Revised: March 30, 2010
Accepted: April 6, 2010
Published online: June 28, 2010
AIM: To communicate our findings on successful treatment of recto-vaginal fistulas (RVFs) after prosthetic reinforcement surgery of pelvic organ prolapse (POP).
METHODS: A retrospective single center study between 1998 and 2008 was performed. A total of 80 patients with RVF were identified, of which five patients (6%), with a mean age of 65 years (range: 52-73), had undergone previous surgery for POP with prosthetic reinforcement.
RESULTS: All patients complained about ongoing vaginal infections and febrile episodes. These symptoms were reported after a mean period of 18 mo after POP repair. As a first intervention, three patients underwent ablation of the prosthetic material (PM). As a second intervention, open proctectomy with a primary anastomosis, an omental patch, and a protective ileostomy were performed in two patients. One patient required a terminal colostomy due to complete destruction of the anal sphincters. In two other patients, ablation of the PM and proctectomy was performed as a one-step procedure. The postoperative course in all patients was uneventful, with a mean length of hospitalization of 20 d (range: 15-30). Closure of the ileostomy was achieved in all four patients within four months. After a mean period of 35 mo (range: 4-60) of follow-up, no recurrence was observed with normal continence in four patients.
CONCLUSION: In our experience, the definitive treatment of high RVFs after PM repair for POP necessitates ablation of the PM, proctectomy with a primary colo-rectal anastomosis, an omental patch interposition, and a temporary ileostomy.
- Citation: Ouaïssi M, Cresti S, Giger U, Sielezneff I, Pirrò N, Berthet B, Grandval P, Consentino B, Sastre B. Management of recto-vaginal fistulas after prosthetic reinforcement treatment for pelvic organ prolapse. World J Gastroenterol 2010; 16(24): 3011-3015
- URL: https://www.wjgnet.com/1007-9327/full/v16/i24/3011.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i24.3011
Pelvic organ prolapse (POP) is a significant health issue in women worldwide. Approximately 250 000 procedures are performed annually in the United States alone[1,2]. The search for a permanent cure for POP has been ongoing for more than a century and remains a challenge today. A number of different approaches have been described for POP repair, including abdominal (open, laparoscopic, and robotic) and vaginal techniques. Recently, the use of prosthetic material (PM) in pelvic floor surgery has become increasingly popular due to the high incidence of recurrence with primary repairs and no surrogate material. Nevertheless, our knowledge about the possible benefit of PM reinforcement for POP repair remains limited. Currently, randomized trials support the superiority of techniques using PM for cystocele repair, whereas the results for colpo- and rectocele repair remain unclear or controversial[3,4].
With the use of PM, new and specific complications occur; these include PM infection, PM erosion or shrinkage, and visceral extrusion. In a systematic review of 2653 patients who underwent surgery for apical vaginal prolapse, Feiner et al[5] reported mesh erosion as the single most frequent complication in 4.6% to 10.7%, depending on the different PMs used. In a retrospective study of monofilament mesh placement in the anterior and posterior compartment for POP repair, Dwyer et al[6] reported a patient that had developed an recto-vaginal fistula (RVF). Two years later, Hilger et al[7] published a case study of RVF formation three months after a posterior intravaginal slingplasty and mesh augmented rectocele repair was performed, which ended in a permanent colostomy after two unsuccessful attempts to correct the fistula. Our knowledge on the incidence of RVF formation after prosthetic material repair for POP is still limited. In addition, uncertainty over the best management of this complication remains. This is in part due to the small number of case reports present in the medical literature. Here, we report our experience with POP management; to the best of our knowledge, this is the largest case series in the literature of RVFs after previous reinforcement surgery for POP.
This was a retrospective single center study between 1998 and 2008. During that period, a total of 80 patients with RVF were treated at the Department of Visceral- and Oncological Surgery, Hôpital Timone, Marseille, France. Five female patients, with a mean age of 65 years (range: 52-73), were identified that had undergone previous surgery for POP with PM reinforcement. All patients had POP surgery done elsewhere. Details on previous POP repair were as follows: one Prolift® procedure (Ethicon Women’s Health and Urology, Somerville, NJ) for urinary stress incontinence, one vaginal wall sling procedure (Bologna technique) and a laparoscopic repair with two multifilamentous polypropylene meshes implanted in both cases for a colpocystocele. Two other patients had open transabdominal implantation of a multifilamentous polypropylene mesh for a colpocysto- and a colporectocele, respectively, with the mesh fixed to the sacrospinous ligaments. Three patients were referred to our department after the PM had been removed transperinealy or transabdominaly, with creation of a temporary colostomy in one case.
At our department, all patients underwent a Gadolinium enhanced magnetic resonance imaging (MRI) of the pelvis using T1 and T2 weighted sequences, such as ano-rectoscopy or endorectal ultrasound (EUS), to evaluate the anal sphincter and a full gynecological work-up. Finally, in all cases, a complete colonoscopy to search for other underlying pathologies that could provoke RVF was routinely performed.
All surgical interventions were performed under general anesthesia and antibiotic cover. The definitive surgical therapy at our department consisted of an open proctectomy, colo-rectal anastomosis, an omental patch, and a protective ileostomy. One patient underwent a Hartmann procedure. A closed suction pelvic drainage was used in all patients.
For postoperative follow-up, all patients in our department underwent an initial clinical control (including Wexner score[8]), ano-rectoscopy, gynecological examination, and an MRI of the pelvis six months after final surgery had been performed.
Patient characteristics, details of surgery and follow-up are summarized in Table 1. The mean time interval between POP repair and the onset of ailments was 18 mo (range: 2-48). All five patients complained about vaginal discharge, dyspareunia, repetitive vaginal infections, and febrile episodes. However, in one patient (No. 3) the leading clinical symptom was complete stool incontinence. Patients No. 1, 2, and 3 underwent ablation of the PM before they had been referred to our department. Patient No. 1 had undergone open transabdominal ablation of the PM with creation of a temporary colostomy. Ablation of the PM in patients No. 2 and 3 was done via a transperineal or laparoscopic approach, respectively.
| Patients No. | Age (yr) | Primary pathology POP surgery/type of PM | Onset of symptoms (mo) | Primary surgery | Delay to secondary surgery (mo) | Secondary surgery | Follow-up (mo)/Wexner score |
| 1 | 73 | Colpo-cystocele laparoscopic approach/two polypropylene meshes | 24 | Temporary colostomy + ablation of PM | 6 | Open proctectomy, omental patch + PI | 36/3 |
| 2 | 65 | Colpo-cystocele Bologna procedure/two polypropylene meshes | 12 | Laparoscopic ablation of PM | 12 | Open proctectomy, omental patch + PI | 36/2 |
| 3 | 60 | SUI Prolift® procedure/two polypropylene meshes | 6 | Transperineal ablation of PM | 6 | Hartmann | 36/NA |
| 4 | 52 | Colpo-rectocele transabdominal approach/one polypropylene mesh | 2 | - | - | Open proctectomy, omental patch + PI | 60/0 |
| 5 | 73 | Cysto-colpocele transabdominal approach/one polypropylene mesh | 48 | - | - | Open proctectomy, omental patch + PI | 6/0 |
Pelvic MRI at our department provided evidence of a high RVF in all five patients. In patient No. 3 a complex low RVF with a destruction of the internal and external anal sphincter complex was additionally diagnosed (Figure 1).
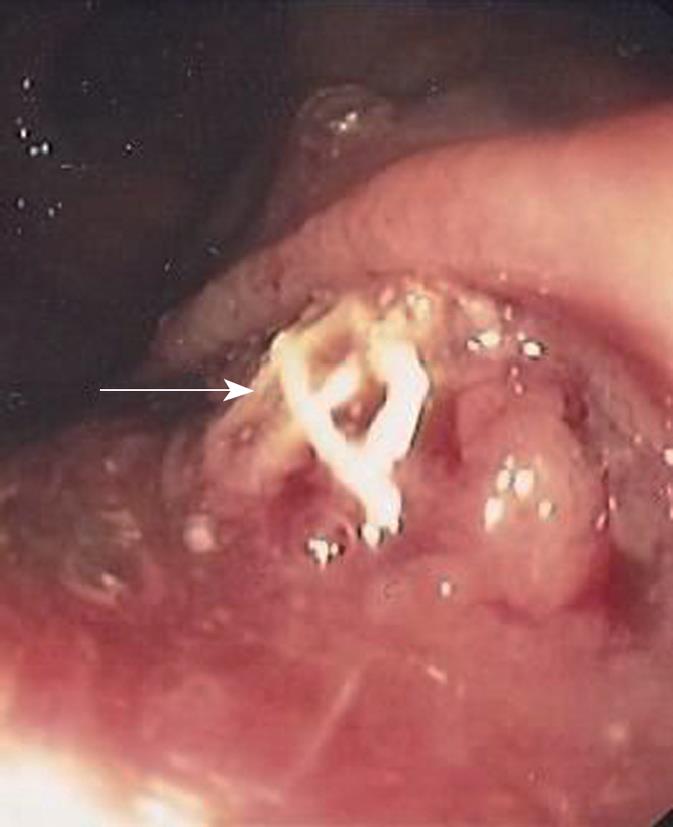
EUS examination was normal in three patients; however, in patients No. 1 and 3, the anal sphincters were found with an inflammatory inhibition or destruction at a circumference of 20° and 180°, respectively. Nevertheless, in all patients, except for one (No. 3, Wexner score 19[8]), the preoperative Wexner score was normal. Diagnostic colonoscopy (including routine biopsies) was normal except for patient No. 5, where the mesh had completely eroded and migrated into the rectum (Figure 2).
The intra- and postoperative course was uneventful in all patients. Ileostomy closure was achieved in all four patients within four months after final surgery. The mean hospitalization (including closure of the ileostomy) was 20 d (range: 15-40).
After a mean postoperative follow-up period of 34 mo (range: 6-60), no fistula recurrences were observed. In all patients where colo-rectal continuity could have been preserved, only patients No. 1 and 2 suffered from mild postoperative stool incontinence, with Wexner scores of 3 and 2, respectively. Stool continence in patients No. 4 and 5 was found to be completely normal (Wexner 0).
POP is a significant health problem in women worldwide, with an estimated 250 000 annual procedures performed in the United States alone[1,2]. These figures are predicted to rise by 45% over the next 30 years due to the increased life expectancy of women in the Western world, and an increasing prevalence of pelvic floor dysfunction with age[9].
Hundreds of techniques have been reported in POP surgery, whether vaginal, abdominal, or laparoscopic, but there is little or no real consensus on which procedure is most appropriate. This is in part due to the lack of high-quality, randomized controlled trials assessing the long-term anatomical and functional outcomes using validated tools and procedures[10].
As traditional surgical techniques for POP utilizing the patient’s own tissue do not restore normal anatomy and have a very high failure rate, the use of PM has recently become more frequent. As far as the treatment for cystocele is concerned, better results are reported compared to the traditional surgical approach without use of PM[3,4].
However, with the more liberal use of different types of PM, new adverse effects and complications in patients who underwent POP surgery have been observed. In a public health notification published in October 2008 by the U.S Food and Drug Administration (FDA) it is stated, that over a period of three years, nine different mesh manufacturers reported about 1000 complications associated with transvaginal placement of surgical mesh in repair of POP and SUI. Furthermore, it stated that specific characteristics of patients at increased risk for complications have not been determined. However, contributing factors might include the overall health of the patient, the mesh material, the size and shape of the mesh, the surgical technique used, concomitant procedures undertaken (e.g. hysterectomy), and possibly estrogen status[11].
In our current series of five patients with high RVF after mesh repair of POP, all patients were in good overall health at the time of POP surgery. None of them had previous POP surgery or were under a concomitant medication or medical treatment that could have promoted RVF formation.
The type of mesh implanted has been found to be critical (reported to be pivotal). Mesh erosion through the vaginal epithelium is the most frequently observed mesh-related complication. However, this complication differs between the different types of meshes used. A high rate of mesh erosion has been reported for non-resorbable synthetic meshes such as Gore-Tex®, Marlex®, and Mersilene®[12].
In our case series, RVF formation was related to multifilamentous polypropylene mesh implantation in all five cases, although the use of polypropylene mesh is currently promoted due to good biocompatability characteristics with a somewhat lower incidence of mesh related complications compared to other mesh types reported in the literature[12,13].
Information concerning RVF formation after mesh surgery for POP is rare in the medical literature. In a retrospective study of monofilament mesh placement in the anterior and posterior compartment for POP repair in 97 patients, Dwyer et al[6] observed one patient (1%) who had developed an RVF. In a case report, Hilger et al[7] reported an RVF that developed three months after a posterior intravaginal slingplasty and mesh augmented rectocele repair was performed. In another retrospective multicenter study of 687 patients who underwent a vaginal cure with the Prolift® procedure, one patient with an RVF (0.15%) in the postoperative follow-up period was found. However, in the same series, an intraoperative rectal erosion (0.15%), a rectal lesion (0.15%), and a recto-vesical fistula (0.15%) were also reported[14]. The exact incidence of this serious complication remains unknown, and is currently between 0.15% and 1%, as reported in the literature. However, the true incidence of RVF is probably somewhat higher, as the formation of RVFs is very likely the sequel of mesh infection and/or mesh extrusion, which are quite common.
Mesh extrusion after POP is generally observed between four and sixteen months postoperatively[15]. In our study, patients started to complain of vaginal discharge, repetitive vaginal infections, and febrile episodes after a mean period of 18 mo (range: 2-48) after POP repair. Therefore, the symptoms outlined above, even several years after POP surgery, should prompt clinicians to search for an RVF. In all five of our patients, MRI of the pelvis revealed a high RVF and furthermore helped to estimate the extent of pelvic inflammation and tissue destruction. Three patients underwent ablation of the PM before they were referred to our department. In one patient, a temporary colostomy had also been performed at the time of PM ablation. In cases of mesh infection, ablation of the mesh by a minimal invasive procedure, if possible, is currently recommended as a first treatment option[16]. This manoeuvre is generally facilitated by a bacterial biofilm, which isolates the PM from the surrounding tissue[13,17]. The definitive surgical treatment should then be performed after a delay of three to six months after pelvic inflammation has minimized[15]. In cases of a simple RVF, it is thought that once the infected PM has been removed, a spontaneous cicatrisation of the initially inflamed tissue will lead to spontaneous RVF closure[18].
However, in the three patients who underwent ablation of the PM in a primary surgical intervention outside our department, pelvic inflammation did not substantially minimize within 6 to 12 mo. Even protective colostomy did not resolve pelvic inflammation and closure of the RVF. This might be explained, in some part, by the finding that in all three patients some parts of PM were still found in the pelvis during final surgery, which might have promoted the ongoing pelvic inflammatory process. Therefore, it is unlikely that simple ablation of the PM, with or without the creation of a temporary colostomy, will completely resolve pelvic inflammation and lead to a permanent cure. In those two patients who were directly transferred to our department, ablation of the PM, proctectomy, colo-rectal anastomosis, an omental patch, and temporary ileostomy was performed as a one-step procedure. In cases of severe sphincter destruction, a definitive colostomy is the procedure of choice.
RVF after mesh surgery for POP is a rare, but severe, complication. The exact incidence of this complication depends on the type of PM implanted and the type of previous surgeries performed, and is currently about 0.15% to 1%, as reported in the medical literature. However, it is likely that the true incidence is somewhat higher. Ablation of the PM in a first surgical procedure is unlikely to remove all PM and there is a risk that parts of the infected mesh are left in place, which probably promotes an ongoing inflammation of the pelvis. If the general condition of the patient allows, we recommend a radical one-step surgical procedure with open PM ablation, proctectomy, an omental patch, colo-rectal anastomosis, and creation of a temporary ileostomy.
An estimated 11% of women will require surgery for pelvic floor dysfunction, with 29% requiring at least a second surgery. To improve upon the results obtained with the use of native tissue, many surgeons have turned to different graft materials, especially synthetic mesh. Polypropylene mesh has been widely used and studied in such procedures, as abdominal sacral colpopexies and numerous mid-urethral slings. Over the last several years, the use of polypropylene mesh has been extended to augment vaginal repair of the anterior and/or posterior vaginal wall. With the use of prosthetic material (PM), new and specific complications have occurred. These include PM infection, PM erosion or shrinkage, and visceral extrusion. In a systematic review of 2 653 patients that underwent surgery for apical vaginal prolapse, Feiner et al reported mesh erosion as the single most frequent complication in 4.6% to 10.7%, depending on the different PMs used.
Knowledge on the incidence of rectovaginal fistula (RVF) formation after prosthetic material repair for pelvic organ prolapse (POP) is still limited. In addition, uncertainly on the best management of this complication remains. This is in part due to the small number of case reports present in the medical literature. Here, the authors report on their experience with POP management, and to the best of their knowledge this is the largest case series the literature of RVFs after previous reinforcement surgery for POP.
In case of a simple RVF, it is thought that once the infected PM has been removed, a spontaneous cicatrisation of the initially inflamed tissue will lead to spontaneous RVF closure. However, in three patients who underwent ablation of the PM in a primary surgical intervention outside our department, pelvic inflammation did not substantially minimize within 6 to 12 mo. Even protective colostomy did not resolve pelvic inflammation and closure of the RVF. This might be explained, in some part, by the finding that, in all three patients, some parts of the PM were still found in the pelvis during final surgery, which might have promoted the ongoing pelvic inflammatory process. Therefore, it is unlikely that simple ablation of the PM, with or without the creation of a temporary colostomy, will completely resolve pelvic inflammation and lead to a permanent cure.
RVF after mesh surgery for POP is a rare, but severe and complication. The exact incidence of this complication depends on the type of PM implanted and the type of previous surgeries performed, and is currently about 0.15% to 1%, as reported in the medical literature. However, it is likely that the true incidence is somewhat higher. Ablation of the PM in a first surgical procedure is unlikely to remove all PM and there is a risk that parts of the infected mesh are left in place, which probably promotes an ongoing inflammation of the pelvis. If the general condition of the patient allows, the authors recommend a radical one-step surgical procedure with open PM ablation, proctectomy, an omental patch, colo-rectal anastomosis, and creation of a temporary ileostomy.
This is a good report of uncommon problem with clear-cut guidelines for this problem.
Peer reviewer: Dr. Rajesh Gupta, Professor, Surgical Gastroenterology Division, in General Surgery, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh, 160012, India
S- Editor Wang YR L- Editor Stewart GJ E- Editor Lin YP
| 1. | Beer M, Kuhn A. Surgical techniques for vault prolapse: a review of the literature. Eur J Obstet Gynecol Reprod Biol. 2005;119:144-155. |
| 2. | Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506. |
| 3. | Savary D, Fatton B, Velemir L, Amblard J, Jacquetin B. [What about transvaginal mesh repair of pelvic organ prolapse? Review of the literature since the HAS (French Health Authorities) report]. J Gynecol Obstet Biol Reprod (Paris). 2009;38:11-41. |
| 4. | Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2007;CD004014. |
| 5. | Feiner B, Jelovsek JE, Maher C. Efficacy and safety of transvaginal mesh kits in the treatment of prolapse of the vaginal apex: a systematic review. BJOG. 2009;116:15-24. |
| 6. | Dwyer PL, O'Reilly BA. Transvaginal repair of anterior and posterior compartment prolapse with Atrium polypropylene mesh. BJOG. 2004;111:831-836. |
| 7. | Hilger WS, Cornella JL. Rectovaginal fistula after Posterior Intravaginal Slingplasty and polypropylene mesh augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:89-92. |
| 8. | Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77-97. |
| 9. | Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol. 2001;184:1496-1501; discussion 1501-1503. |
| 10. | Novara G, Galfano A, Mancini M, Ficarra V, Artibani W. Critical assessment of pelvic floor surgical reconstruction outcome. EEUS. 2006;4:202–213. |
| 11. | FDA Public Health Notification: Serious Complications Associated with Transvaginal Placement of Surgical Mesh in Repair of Pelvic Organ Prolapse and Stress Urinary Incontinence. October 20, 2008. Available from: http://www.fda.gov/MedicalDevices/Safety. |
| 12. | Iglesia CB, Fenner DE, Brubaker L. The use of mesh in gynecologic surgery. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:105-115. |
| 13. | Bader G, Fauconnier A, Guyot B, Ville Y. [Use of prosthetic materials in reconstructive pelvic floor surgery. An evidence-based analysis]. Gynecol Obstet Fertil. 2006;34:292-297. |
| 14. | Cosson M, Caquant F, Collinet P, Rosenthal C, Clave H, Debodinance P, Garbin O, Berrocal J, Villet R, Jacquetin B. Prolift mesh (Gynecare) for pelvic organ prolapse surgical treatment using the TVM group: a retrospective study of 687 patients. Montreal: Communication in the ICS meeting, August 21 2005; . |
| 15. | Visco AG, Weidner AC, Barber MD, Myers ER, Cundiff GW, Bump RC, Addison WA. Vaginal mesh erosion after abdominal sacral colpopexy. Am J Obstet Gynecol. 2001;184:297-302. |
| 16. | Kodner IJ, Mazor A, Shemesh EI, Fry RD, Fleshman JW, Birnbaum EH. Endorectal advancement flap repair of rectovaginal and other complicated anorectal fistulas. Surgery. 1993;114:682-689; discussion 689-690. |
| 17. | Cosson M. [Risk of infection and prostheses: time out or a red flag?]. J Gynecol Obstet Biol Reprod (Paris). 2004;33:559-560. |
| 18. | Brizzolara S, Pillai-Allen A. Risk of mesh erosion with sacral colpopexy and concurrent hysterectomy. Obstet Gynecol. 2003;102:306-310. |