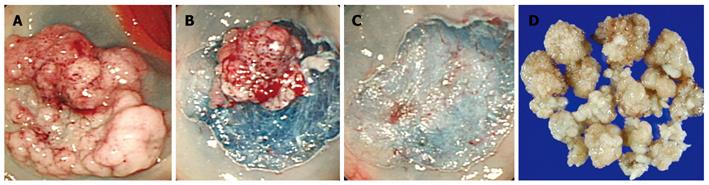
Published online Jun 14, 2010. doi: 10.3748/wjg.v16.i22.2806
Revised: March 10, 2010
Accepted: March 17, 2010
Published online: June 14, 2010
AIM: To evaluate the safety and outcomes of endoscopic piecemeal mucosal resection (EPMR) for large sessile colorectal polyps.
METHODS: The patients enrolled in this study were 47 patients with 50 large sessile polyps (diameter, 2 cm or greater) who underwent EPMR using a submucosal saline injection technique between December 2002 and October 2005. All medical records, including characteristics of the patients and polyps, complications, and recurrences, were retrospectively reviewed. The first follow-up endoscopic examination was performed at 3-6 mo after initial endoscopic resection, and the second at 12 mo post-EPMR. Subsequent surveillance colonoscopic examinations were individualized, taking risk factors into account.
RESULTS: The patients were 23 men and 24 women, with a mean age of 60 years. Mean polyp size was 30.1 mm. Of 50 polyps identified, 34 (68%) were benign and 16 (32%) were malignant. There were 6 (12%) cases with EPMR-related bleeding: 5 intra-procedural and 1 early post-procedural bleeding. All bleeding episodes were managed by endoscopic clipping or argon beam coagulation. There were no perforations. Recurrence was identified in 5 cases (12.2%): 4 local recurrences detected at 3 mo post-EPMR and 1 local recurrence detected at 14 mo post-EPMR. The recurrence rate after EPMR was 3.1% for benign polyps and 33.3% for malignant polyps (P < 0.05). Median follow-up time was 37 mo.
CONCLUSION: EPMR is safe, but should be applied carefully in malignant polyps. Close follow-up endoscopic examinations are necessary for early detection of recurrence.
- Citation: Seo GJ, Sohn DK, Han KS, Hong CW, Kim BC, Park JW, Choi HS, Chang HJ, Oh JH. Recurrence after endoscopic piecemeal mucosal resection for large sessile colorectal polyps. World J Gastroenterol 2010; 16(22): 2806-2811
- URL: https://www.wjgnet.com/1007-9327/full/v16/i22/2806.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i22.2806
Endoscopic resection of large sessile colorectal polyps remains challenging because of its technical difficulty and high complication rate[1]; even so, the endoscopic removal of colonic adenomatous polyps is a commonly used technique that reduces the incidence of colorectal cancer. Some investigators have reported that endoscopic piecemeal mucosal resection (EPMR) is a safe and effective procedure for large sessile colorectal polyps[2-4]; however, this approach remains controversial because of the high possibility of coexisting malignancy and a high recurrence rate associated with large sessile polyps. Endoscopic submucosal dissection (ESD) has recently been attempted by expert endoscopists for en bloc resection of large sessile polyps[5,6]; however, this procedure has a long procedure time and high complication rate, and is not currently widely used due to its technical difficulty[7].
Several studies have shown that the incomplete removal of large sessile colorectal polyps, particularly by piecemeal resection, can contribute to a higher subsequent incidence of colorectal cancers[8,9]. Thus, recent guidelines recommend that when large sessile polyps are removed by piecemeal resection, a repeat examination should be performed at a short interval (2-6 mo) to verify complete removal[10]. However, few studies report the recurrence rate after EPMR, and the long-term outcomes of EPMR have yet to be established. This study was designed to evaluate the safety, efficacy, and long-term outcomes of EPMR of large sessile colorectal polyps.
We retrospectively reviewed and identified 77 patients with 80 large sessile colorectal polyps (2 cm or greater) detected by colonoscopy at the National Cancer Center, Korea, between December 2002 and October 2005. Among these patients, 30 were excluded for the following reasons: co-existence of synchronous advanced colorectal cancer (n = 15), non-lifting tumor (n = 4), encircling lesion > 70% (n = 4), transfer to other institution (n = 4), suspicion of muscle invasion by endoscopic ultrasound (n = 1), and recurrent tumor (n = 2). A final total of 47 patients with 50 large sessile polyps who underwent EPMR using submucosal saline injection technique were enrolled in this study. We reviewed medical records, including patient demographics, endoscopic findings, histopathological reports, and follow-up data. The study was performed in accordance with the Declaration of Helsinki and informed consent was obtained from all patients.
Patients were prepared with mechanical bowel preparation. The patients received either two 45 mL doses of sodium phosphate (Fleet®; C.B. Fleet Co. Inc., Lynchburg, VA, USA) or 4 L of polyethylene glycol solution (Colyte-F®; Taejoon Pharm, Seoul, Korea) and underwent colonoscopy under conscious sedation with midazolam. Colonoscopy was performed to the cecum or terminal ileum with white light colonoscopic examination. Polyps suspicious of invasive cancer (the presence of ulceration, induration, friable mucosa, or non-lifting sign[11]) were referred for surgical resection. Polyp size (measured in comparison with open biopsy forceps) and morphology were generally estimated and recorded by the endoscopist.
Endoscopic resection was performed using a snare piecemeal method with submucosal saline injection technique (Figure 1) according to the strip biopsy method described by Karita et al[12] Colonoscopy was performed with a standard video colonoscope (CF Q260L; Olympus Optical Co., Ltd., Tokyo, Japan). Briefly, the injection catheter was passed through the channel, and saline solution mixed with diluted epinephrine (1:100 000) was injected into the submucosal layer near the sessile polyp until the entire polyp was elevated. If the polyp was not elevated after one injection, additional injections were made around the polyp. The injection catheter was removed and a snare device was inserted through the channel. The surrounding normal mucosa, along with the lesion, was encircled by the snare. After the snare device was positioned, resection was performed using electrosurgical coagulation current, a combination of coagulation and blended currents in sequence. If necessary, argon plasma coagulation (APC) was used to treat the polyp base in an attempt to destroy any residual polyp.
EPMR-induced bleeding was defined as intraprocedural (occurring during EMR), early (within 24 h after EPMR), or delayed (≥ 24 h after EPMR), as described previously[13]. The diagnosis of early or delayed bleeding was based on the passage of blood per rectum. Bleeding was controlled by endoscopic clip (HX-600-135, Olympus) placement and/or APC. Perforation was defined as the presence of free air on plain abdominal film or computed tomography (CT).
All resected material was retrieved for histopathologic evaluation. Specimens were collected using a basket or by aspiration into the suction channel. For entire retrieval of sessile polyps located in the right colon, the colonoscope was withdrawn and reinserted as many times as necessary. A single pathologist assessed all histopathologic specimens and was not blinded to the endoscopic findings. Malignant polyps with unfavorable histology, such as poor differentiation, angiolymphatic invasion, and deep submucosal invasion (≥ 1000 μm), or having a positive deep resection margin, were referred for surgical treatment after polypectomy.
Patients who had undergone endoscopic treatment alone were followed up with colonoscopy to evaluate whether endoscopic resection of the sessile polyps had been complete. Endoscopic examinations were scheduled as follows: the first follow-up endoscopic examination was performed at 3-6 mo after the initial endoscopic resection, and the second was performed at 12 mo post-EPMR. Subsequent surveillance colonoscopic examinations were individualized with consideration of risk factors. If a polyp was detected on follow-up examinations, it was resected if possible. Residual polypoid tissue with the appearance of granulation tissue was biopsied, but not counted as residual adenoma. Recurrence or residual polyp was defined as the presence of any amount of adenomatous or carcinomatous tissue on follow-up, even as small as 1 mm, confirmed by histology at the site of prior piecemeal polypectomy[14]. All recurrences were demonstrated by pathology to contain dysplastic (adenoma) or carcinomatous tissue. The difference in recurrence between EPMR for benign polyps and EPMR for malignant polyps was determined by the log-rank test using SPSS 14.0 for Windows (SPSS Inc., Chicago, IL, USA). P values of < 0.05 were considered statistically significant.
The clinicopathologic characteristics of the 47 patients with 50 polyps are listed in Table 1. Among the patients, 5 had a history of non-steroidal anti-inflammatory drugs (NSAIDs) administration that was stopped at least 7 d before endoscopy. The mean size of resected polyps was 30.1 ± 10.9 mm (range, 20-60 mm). Locations of the large sessile polyps were as follows: cecum in 2 polyps, ascending in 7, hepatic flexure in 6, transverse in 4, splenic flexure in 1, descending in 5, sigmoid in 6, and rectum in 19. Of the 50 polyps, 34 (68%) were benign and 16 (32%) were malignant. Histological examination revealed tubular adenoma in 20 polyps, tubulovillous adenoma in 7, villous adenoma in 1, serrated adenoma in 4, hyperplastic polyp in 2, and carcinoma in 16 (Tis, 11; T1, 4; T2, 1).
| Variable | n |
| Male/female | 23/24 |
| Mean age (range), yr | 60 (27-78) |
| Mean polyp size (range), mm | 30.1 (20-60) |
| Location | |
| Cecum | 2 |
| Ascending | 7 |
| Hepatic flexure | 6 |
| Transverse | 4 |
| Splenic flexure | 1 |
| Descending | 5 |
| Sigmoid | 6 |
| Rectum | 19 |
| Histology | |
| Benign (n = 34) | |
| Tubular adenoma | 20 |
| Tubulovillous adenoma | 7 |
| Villous adenoma | 1 |
| Serrated adenoma | 4 |
| Hyperplastic | 2 |
| Malignant (n = 16) | |
| Tis | 11 |
| T1 | 4 |
| T2 | 1 |
The outcomes of EPMR for large sessile polyps are listed in Table 2. After EPMR, there were 6 (12%) cases of procedural bleeding: 5 intraprocedural and 1 early after initial endoscopic resection, which were managed by endoscopic means with the application of hemoclips alone in 4 cases, APC alone in 1 case, and hemoclips with APC plus fibrin glue in 1 case. There was no significant bleeding requiring blood transfusion or surgical intervention, and no delayed bleeding following polypectomy. No patient suffered colonic perforation as a result of endoscopic resection. Bleeding prophylaxis was performed in 6 cases (12%) by applying a hemoclip. The median hospital stay (from procedure to discharge) was 3 d (range, 1-5 d), with the exception of 4 patients: 3 who were not admitted and 1 who underwent consecutive colonic resection.
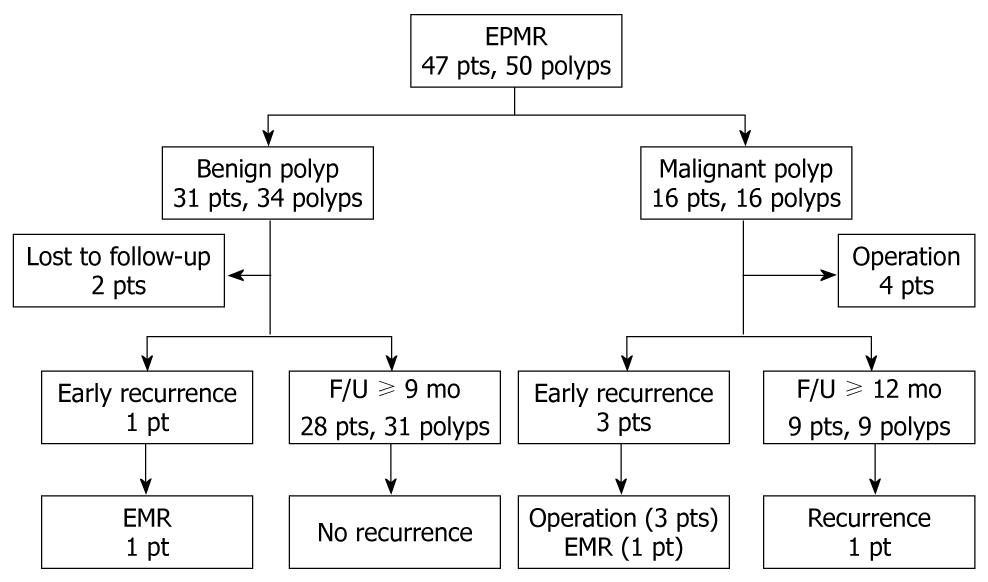
Of the 47 patients, follow-up data were available for 45 patients (95.7%); the exceptions were 2 without follow-up endoscopy. The median follow-up time was 37 mo (range, 3-72 mo). Recurrence was identified in 5 patients (12.2%): including 4 cases of local recurrence detected at 3 mo post-EPMR and 1 local recurrence detected at 14 mo post-EPMR (Table 3).
| No. | Age/sex | Location | Size (mm) | Primary histology | Time to recurrence (mo) | Recurrent histology | Method of treatment | Follow-up (mo) |
| 1 | 72/M | Cecum | 40 | Adenoma | 3 | Adenoma | EMR | 25 |
| 2 | 65/M | Rectum | 40 | Carcinoma (Tis) | 3 | Carcinoma (T2) | LAR | 16 |
| 3 | 78/F | Rectum | 40 | Carcinoma (T1) | 3 | Adenoma | EMR | 51 |
| 4 | 64/F | Rectum | 60 | Carcinoma (T1) | 14 | Carcinoma (Tis) | LAR | 42 |
| 5 | 49/M | Sigmoid | 40 | Carcinoma (T1) | 3 | HGD adenoma | AR | 40 |
Of the 32 patients with benign polyps, there was 1 (3.1%) case of recurrence: a patient with benign recurrent adenoma who was successfully treated by endoscopic resection. Other follow-up endoscopy showed no recurrence. Of the 16 patients with malignant polyps, 4 polyps with unfavorable histology underwent surgery. Among the remaining 12 patients with malignant polyps, 4 (33.3%) had recurrence: 1 patient with benign recurrent adenoma was treated by endoscopic resection and 3 patients underwent surgery because their recurrent lesions could not be removed by endoscopic resection (Table 3).
The difference in recurrence between EPMR for benign polyps and that for malignant polyps was statistically significant (3.1% vs 33.3%, P < 0.05).
The follow-up results of the 50 large sessile polyps are shown in Figure 2.
Endoscopic resection of large sessile colorectal polyps (2 cm or greater) is increasingly used as an alternative to surgery, but remains challenging because of its technical difficulty, the high risk of complications such as bleeding or perforation, and the possibility of coexisting malignancy. Recent advances in endoscopic technique and equipment have enabled the development of techniques such as EPMR and ESD to remove large sessile colorectal polyps[1-6]. Comparing these two techniques, the mean procedure times for EPMR[4,6] and ESD[6,15] ranged from 29 to 55 min and from 70.5 to 108 min, respectively. Regarding complications, the perforation rates for EPMR[6,8,14] and ESD[4,6,15] ranged from 0.8% to 1.3% and 6.2% to 10.0%, respectively. Because EPMR has a shorter procedure time and lower perforation rate than ESD, EPMR appears to be an easy and safe procedure; however, Saito et al[6] demonstrated that EPMR resulted in a higher recurrence rate compared with ESD (14% vs 2%). The rate of recurrence at the polypectomy site after EPMR was reported to be 20%-55% in several other studies[8,14,16]. The recurrence rate after EPMR of 12.2% in the present study (11.36% in 44 polyps) is relatively low for the 44 large sessile polyps that had follow-up endoscopy. Several investigators have reported that additional techniques (e.g. APC) may further improve the success of polypectomy, and hence lower the recurrence rate[3,14,17]. In the present study, 91% of cases were treated with APC. Our low recurrence rate was probably influenced by the high application rate of APC to the tumor bed following EPMR. With regard to histopathology of the polyps, the recurrence rate was 3.1% for benign polyps and 33.3% for malignant polyps; in other words, EPMR of malignant polyps resulted in a higher recurrence rate than that of benign polyps. In contrast, Conio et al[13] reported similar recurrence rates for benign and malignant polyps. The recurrence rate of malignant colorectal polyps after EPMR varies among studies; however, it is difficult to compare the results of different series because there are wide variations in polyp size and the length of follow-up[13]. In addition, it is difficult to explain the reason why the incidence of recurrence after EPMR in malignant lesions is higher than that in benign lesions. However, we should try to remove all cancer cells completely because microscopic residual cancer cells after EPMR can cause recurrences. Further studies are needed to confirm the usefulness of EPMR for malignant colorectal polyps.
The most common complication after polypectomy is bleeding; the risk of post-polypectomy bleeding ranges from 0.3% to 6.1%[18,19]. The risk factors for bleeding include large polyp size and location in the proximal colon[19,20]. In large sessile polyps (2 cm or greater), the incidence of bleeding during and after polypectomy has been reported to be as high as 13.5%[2,3,8,14,16]. In the present study, bleeding occurred in 5 cases (12.2%), and all patients with bleeding were treated by endoscopic management, without surgery or blood transfusion. Thus, we can consider these cases as having “minor complications,” as described in a previous report[3]. In the present study, none of the patients with bleeding were taking NSAIDs (aspirin) or had known coagulopathy at the time of the EPMR procedure. In our endoscopic database, any procedural bleeding requiring additional endoscopic treatment was described, and we classified EPMR-induced bleeding as intraprocedural, early, or delayed. Doniec et al[21] suggested that it is doubtful whether hemorrhage should be classified as a complication during endoscopic treatment when it can be managed endoscopically; no surgeon would regard bleeding as a complication during an operation such as mucosectomy. We used submucosal injection of epinephrine-saline mixture 1:100 000 in all EPMR cases, not only for submucosal elevation but also to prevent procedure-related bleeding. Iishi et al[2] injected only 0.9% saline solution alone to elevate sessile polyps, and reported bleeding in 7% of cases; however, no study has definitely proved the superiority of submucosal solution. In the present study, there was no delayed bleeding or perforations. Taking our experience into consideration, it is clear that the risk of perforation increases with increased width of the polypectomized colonic wall during snaring, rather than with increasing polyp size.
Rectal polyps are considered easy to remove due to the relatively low rate of perforation. In our study, 19 (40.4%) out of 47 polyps were located in the rectum. However, the recurrence rate after EPMR was not different between rectal polyps and colonic polyps (17.6% vs 7.4%, P = 0.359).
Previous studies have reported the risk of malignancy in large sessile colorectal polyps (2 cm or larger) as being up to 29%[22,23]. In the present study, 16 polyps (32%) were found to be adenomas containing an area of carcinoma; of these, 5 (10%) were invasive cancer. We evaluated the lifting sign of the tumor using saline injection to the submucosal layer before EPMR in all cases. This technique may have caused the relatively low incidence of invasive cancer in the large sessile colorectal polyps in the present study.
It is well known that initial colonoscopy has a significant miss rate of 24% for all types of adenomas[24]. Yamaji et al[25] reported recurrence rates for small adenomas and advanced lesions of 19.3% and 22.9%, respectively. In the present study, 80% of recurrences were identified at 3 mo post-EPMR, and the other recurrences were detected at 14 mo post-EPMR. Missed or metachronous adenomas detected at 3-6 mo, 1-3 years, and 3 years post-EPMR were detected in 32%, 46%, and 32% of cases, respectively. The higher rate of metachronous adenomas may have been influenced by the fact that our patients had more advanced lesions (i.e. all had large sessile polyps), including invasive cancers. The present results support current guidelines which recommend that patients who undergo piecemeal resection of large sessile adenomas should have an initial follow-up colonoscopy within 3-6 mo, followed by an additional colonoscopy 1 year later[10].
In conclusion, EPMR is a safe procedure for large sessile colorectal polyps, but should be applied carefully in malignant polyps because of high recurrence rate. Close follow-up endoscopic examinations are necessary for early detection of recurrence.
Endoscopic removal of colonic adenomatous polyps is a commonly used technique that reduces the incidence of colorectal cancer. However, endoscopic resection of large sessile colorectal polyps is still challenging because of its technical difficulty.
Endoscopic piecemeal mucosal resection (EPMR) can be used for large sessile colorectal polyps. However, this approach remains controversial because of the high possibility of coexisting malignancy and the high recurrence rate associated with large sessile polyps. Few studies report the recurrence rate after EPMR, and the long-term outcomes of EPMR have yet to be established.
Several studies have shown that the incomplete removal of large sessile colorectal polyps, particularly by piecemeal resection, can contribute to a higher subsequent incidence of colorectal cancers. This study shows that EPMR is safe for benign colorectal polyps, but should be applied carefully in malignant polyps due to high recurrence rate.
By identifying the recurrence rate and long term follow-up results after EPMR, this study may contribute to the development of a therapeutic guideline for patients with large sessile colorectal polyps.
EPMR, which is used to increase safety, is a technique for removing large sessile polyps. During EPMR, the polyp can be broken down into multiple pieces. However, this makes it difficult to evaluate the resection margin pathologically.
This paper is devoted to obtaining knowledge on the outcome of piecemeal mucosal and large sessile polyps. The authors have concluded that EPMR should be carefully applied in malignant polyps.
Peer reviewers: Josep M Bordas, MD, Department of Gastroenterology IMD, Hospital Clinic, Llusanes 11-13 at, Barcelona 08022, Spain; Dr. Shinji Tanaka, Director, Department of Endoscopy, Hiroshima University Hospital, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8551, Japan
S- Editor Wang YR L- Editor Webster JR E- Editor Ma WH
| 1. | Seitz U, Bohnacker S, Seewald S, Thonke F, Soehendra N. Long-term results of endoscopic removal of large colorectal adenomas. Endoscopy. 2003;35:S41-S44. |
| 2. | Iishi H, Tatsuta M, Iseki K, Narahara H, Uedo N, Sakai N, Ishikawa H, Otani T, Ishiguro S. Endoscopic piecemeal resection with submucosal saline injection of large sessile colorectal polyps. Gastrointest Endosc. 2000;51:697-700. |
| 3. | Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Añaños FE, Domènech E, Ojanguren I, Gassull MA. Endoscopic removal of large sessile colorectal adenomas: is it safe and effective? Dig Dis Sci. 2007;52:840-844. |
| 4. | Salama M, Ormonde D, Quach T, Ee H, Yusoff I. Outcomes of endoscopic resection of large colorectal neoplasms: an Australian experience. J Gastroenterol Hepatol. 2010;25:84-89. |
| 5. | Fujishiro M. Perspective on the practical indications of endoscopic submucosal dissection of gastrointestinal neoplasms. World J Gastroenterol. 2008;14:4289-4295. |
| 6. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. |
| 7. | Tanaka S, Oka S, Chayama K. Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol. 2008;43:641-651. |
| 8. | Walsh RM, Ackroyd FW, Shellito PC. Endoscopic resection of large sessile colorectal polyps. Gastrointest Endosc. 1992;38:303-309. |
| 9. | Robertson DJ, Greenberg ER, Beach M, Sandler RS, Ahnen D, Haile RW, Burke CA, Snover DC, Bresalier RS, McKeown-Eyssen G. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129:34-41. |
| 10. | Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O'brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56:143-159; quiz 184-185. |
| 11. | Han KS, Sohn DK. Biopsy and nonlifting sign in endoscopically resectable colorectal cancers. Gastrointest Endosc. 2008;68:615. |
| 12. | Karita M, Tada M, Okita K, Kodama T. Endoscopic therapy for early colon cancer: the strip biopsy resection technique. Gastrointest Endosc. 1991;37:128-132. |
| 13. | Conio M, Repici A, Demarquay JF, Blanchi S, Dumas R, Filiberti R. EMR of large sessile colorectal polyps. Gastrointest Endosc. 2004;60:234-241. |
| 14. | Zlatanic J, Waye JD, Kim PS, Baiocco PJ, Gleim GW. Large sessile colonic adenomas: use of argon plasma coagulator to supplement piecemeal snare polypectomy. Gastrointest Endosc. 1999;49:731-735. |
| 15. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. |
| 16. | Brooker JC, Saunders BP, Shah SG, Williams CB. Endoscopic resection of large sessile colonic polyps by specialist and non-specialist endoscopists. Br J Surg. 2002;89:1020-1024. |
| 17. | Regula J, Wronska E, Polkowski M, Nasierowska-Guttmejer A, Pachlewski J, Rupinski M, Butruk E. Argon plasma coagulation after piecemeal polypectomy of sessile colorectal adenomas: long-term follow-up study. Endoscopy. 2003;35:212-218. |
| 18. | Repici A, Tricerri R. Endoscopic polypectomy: techniques, complications and follow-up. Tech Coloproctol. 2004;8 Suppl 2:s283-s290. |
| 19. | Consolo P, Luigiano C, Strangio G, Scaffidi MG, Giacobbe G, Di Giuseppe G, Zirilli A, Familiari L. Efficacy, risk factors and complications of endoscopic polypectomy: ten year experience at a single center. World J Gastroenterol. 2008;14:2364-2369. |
| 20. | Tolliver KA, Rex DK. Colonoscopic polypectomy. Gastroenterol Clin North Am. 2008;37:229-251, ix. |
| 21. | Doniec JM, Löhnert MS, Schniewind B, Bokelmann F, Kremer B, Grimm H. Endoscopic removal of large colorectal polyps: prevention of unnecessary surgery? Dis Colon Rectum. 2003;46:340-348. |
| 22. | Nivatvongs S, Snover DC, Fang DT. Piecemeal snare excision of large sessile colon and rectal polyps: is it adequate? Gastrointest Endosc. 1984;30:18-20. |
| 23. | Christie JP. Colonoscopic excision of large sessile polyps. Am J Gastroenterol. 1977;67:430-438. |
| 24. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. |