Published online Jun 14, 2010. doi: 10.3748/wjg.v16.i22.2764
Revised: January 15, 2010
Accepted: January 22, 2010
Published online: June 14, 2010
AIM: To investigate a relationship between the clinicopathological features and mucin phenotypes in advanced gastric adenocarcinoma (AGA).
METHODS: Immunohistochemical staining was performed to determine the mucin phenotypes in 38 patients with differentiated adenocarcinomas (DACs), 9 with signet-ring cell carcinomas (SIGs), and 48 with other diffuse-type adenocarcinomas (non-SIGs) of AGA. The mucin phenotypes were classified into 4 types: gastric (G), gastrointestinal (GI), intestinal, and unclassified.
RESULTS: The G-related mucin phenotypes were highly expressed in all the histological subtypes of AGA. The expression of the GI phenotype in SIG patients was lower than that in DAC patients (P = 0.02), and this phenotype was observed in 56% of the non-SIG patients in the intramucosal layer. Among non-SIG cases, the expression of the GI phenotype was significantly higher in patients with extended adenocarcinomas and those with positive rates of lymph node metastasis. There was no difference between the expressions of the G and other GI phenotypes factors. Among DAC and non-SIG patients, there were no differences between the survival rates of the corresponding patient groups.
CONCLUSION: The GI phenotype might possess more invasive characteristics than the G phenotype in non-SIG. Neither of the phenotypes indicated a poor prognosis of DAC and non-SIG.
- Citation: Toki F, Takahashi A, Aihara R, Ogata K, Ando H, Ohno T, Mochiki E, Kuwano H. Relationship between clinicopathological features and mucin phenotypes of advanced gastric adenocarcinoma. World J Gastroenterol 2010; 16(22): 2764-2770
- URL: https://www.wjgnet.com/1007-9327/full/v16/i22/2764.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i22.2764
Recently, an immunohistochemical method for mucin staining has been developed, and the expression of the mucin phenotype in gastric adenocarcinoma has been reported[1-4]. Lauren[5] and Nakamura et al[6] histologically classified gastric adenocarcinomas into 2 main types: intestinal type of Lauren’s histology or differentiated type, and diffuse type or poorly differentiated type. The diffuse type adenocarcinomas, as classified using Lauren’s method[7] (non-solid type of poorly differentiated adenocarcinoma and the signet-ring cell carcinoma according to a Japanese classification[8]: PDC), which do not show clustering or glandular formation, can be further divided into 2 histological subtypes. In one of these subtypes, the adenocarcinomas are predominantly (> 50%) composed of isolated malignant cells containing intracellular mucin and the nucleus is located at the periphery of the cytoplasm (signet-ring cell carcinoma: SIG according to the Japanese classification). In the other subtype, the adenocarcinoma contains few (< 50%) signet-ring cancer cells (non-SIGs in PDC).
On the basis of the differences in immunohistochemical staining, the mucin phenotypes in gastric adenocarcinoma can generally be divided into 4 types: gastric (G), mixed or gastrointestinal (GI), intestinal (I), and unclassified (UC). Some studies on the intestinal or differentiated types of adenocarcinoma have described the relationship between the expression of mucin phenotypes and the occurrence, progression, and clinicopathological features of adenocarcinomas[9-14]. The expression of the mucin phenotype in diffuse or poorly differentiated adenocarcinomas has also been studied[15-22]. However, some of these studies have been restricted to SIG[15-22], and there have been few studies on advanced gastric adenocarcinoma (AGA)[23-27]. Therefore, there is little information on the effects of the mucin phenotypes on the clinicopathological and histological subtype-based features of AGA, particularly in the diffuse type as defined by Lauren’s method[7]. To clarify the role of the mucin phenotype in the clinicopathological features and prognosis of AGA, further studies should take into account the histological subtypes of AGA.
To this end, we examined the expression of mucin phenotypes in the above-mentioned histological subtypes of AGA. The mucin phenotype-based analysis was performed according to the classification proposed by Watanabe et al[28,29]. We also determined the relationship between the expression of the mucin phenotypes and the clinicopathological features, including the prognosis of patients, who were grouped according to the different histological subtypes of gastric adenocarcinoma.
We investigated 95 subjects with AGA who had undergone gastric resection at the Surgery I Department of Gunma University Hospital between 1994 and 2000. Among the 95 subjects, 38 had differentiated adenocarcinoma (DAC), 9 had SIG, and 48 had non-SIG of PDC. We defined AGA as an adenocarcinoma that invades deep into the muscularis propria. The definition of DAC includes papillary and tubular adenocarcinomas classified using the Japanese classification[8]. The definitions of SIG and PDC are described above. The definition of non-SIG is the adenocarcinoma of NSC excluding SIG.
We used a staining method which had been previously reported[25,26]. In brief, the samples obtained after gastric resection were fixed in 10% buffered neutral formalin, macroscopically examined, and photographed. Thereafter, the resected tumor, which included the tumor center, was cut into 3-4 mm wide slices. These slices were then embedded in paraffin and stained with hematoxylin-eosin (HE). The slices were then examined and color images were used for the histochemical mapping of the tissues and for measuring tumor size. For immunohistochemistry, we selected 1 or 2 HE-stained sections obtained from the tumor areas with the largest diameters and the deepest mucosal invasion. Paraffin blocks containing the selected HE-stained sections were cut into consecutive 3 μm sections for immunohistochemical staining.
The following protocol was employed for staining. Deparaffinized sections were treated with citrate buffer (pH 6.0), heated in a microwave oven for 20 min, and allowed to be cooled to room temperature. Endogenous peroxidase activity was blocked by incubating the sections for 20 min with 0.3% hydrogen peroxidase in absolute methanol, and the sections were then washed in tap water. Non-specific binding was blocked by using normal serum (Nichirei, Japan). The sections were incubated with a primary antibody overnight at 4°C and then incubated with a biotinylated secondary antibody for 30 min at room temperature (Nichirei, Japan). Immunohistochemical staining was performed using a streptavidin-biotin-peroxidase kit (Nichirei, Japan) according to the manufacturer’s instructions, with slight modifications. We used MUC5AC (diluted 1:100; antibody CLH2, Novocastra, UK) and human gastric mucin (HGM, diluted 1:50; antibody 45M1, Novocastra) antibodies as markers for the gastric foveolar phenotype; MUC6 (diluted 1:100; antibody CLH5, Novocastra) and M-GGMC-1 (diluted 1:50; antibody HIK1083, Kantou Chemicals, Japan) antibodies as markers for the pyloric gland phenotype; MUC2 (diluted 1:500; antibody Ccp58, Novocastra) as a marker for intestinal goblet cell mucin; and CD10 (diluted 1:200; antibody 56C6, Novocastra) as a marker for small intestinal enterocytes.
The markers HGM, M-GGMC-1, and CD10 exhibited both cytoplasmic and luminal membranous reactivity, whereas MUC5AC, MUC6, and MUC2 exhibited only cytoplasmic reactivity. The reactivity was estimated on the basis of the percentage of stained cells among the total number of tumor cells in each stained section. On the basis of the frequency of positive staining for the relevant marker, the adenocarcinoma phenotypes were classified into 4 groups: G, GI, I, and UC. The following criteria were used for the classification of the mucin phenotypes: (1) if more than 5% of the cells were positive for HGM, MUC5AC, MUC6, or M-GGMC-1, the phenotype was classified as G; (2) if more than 5% of the cells were positive for MUC2, the phenotype was classified as I; and (3) if even a single cell was positive for CD10, the phenotype was classified as I. If none of the above criteria were met, the phenotype was classified as UC. The mucin phenotype was determined by examining the intramucosal layer.
In addition to determining the mucin phenotype, we reviewed the patients’ profiles (age at operation and gender), the tumor site (upper segment of the stomach, U; middle segment of the stomach, M; and lower segment of the stomach, L), the tumor size, the histological findings (lymphatic and vessel invasion, metastasis to the lymph nodes and other sites), and patient survival. The relationships among these factors, particularly the comparison between the G and GI phenotypes, were determined.
The survival rates of the patients with G and GI phenotypes grouped according to the histological subtypes were examined by Kaplan-Meier analysis.
Statistical analysis was performed using the Pearson χ2 test [Fisher’s exact test (extended)] and the Student t-test. Two-tailed P-values < 0.05 were considered significant.
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association and all the participants gave their written informed consent.
The clinical profiles and tumor characteristics of the patients with DAC, SIG, and non-SIG are summarized in Table 1. Among women, the number with SIG was significantly higher than those with DAC (P = 0.05). There were no intergroup differences in the age at operation or size of tumor. For the site of tumor, the number in the lower segment in DAC was higher than that in SIG or DAC.
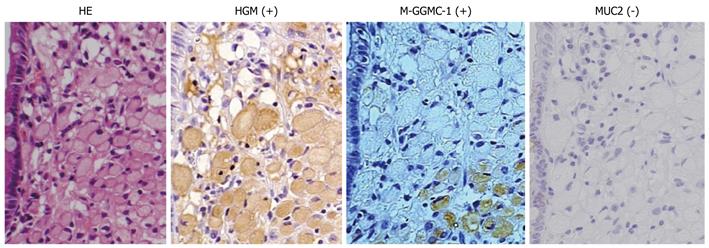
Typical photographs of a patient with SIG expressing the G phenotype are shown in Figure 1. Immunohistochemical staining revealed a large number of HGM- or M-GGMC-1-positive cells in the mucosal portion of the tumor. No MUC2-positive cells were detected in the tumor area. The cancer cells, most of which were signet-ring cells, were scattered without clustering or glandular formation.
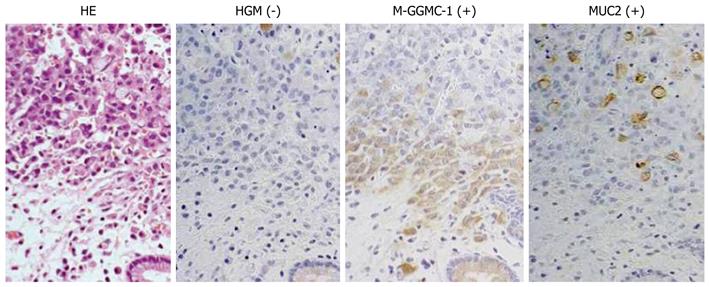
Figure 2 shows typical photographs of a patient with non-SIG expressing the GI phenotype. Immunohistochemical staining revealed a number of M-GGMC-1-positive cells in the mucosal portion of the tumor. We also observed sporadically distributed MUC2-positive cells. No HGM-positive cells were detected in the tumor area of the examined tissues. We observed scattered non-SIG cells similar to the SIG shown in Figure 1. No cluster and glandular formation of cancer cells was detected in any region of tumor.
The expression rates of various mucin phenotypes at the mucosal layer among the patients classified according to the histological subtype are shown in Table 2. The expression rates of HGM and MUC5AC were higher than those of MUC6 and M-GGMC-1 in all the histological subtypes, particularly in the cases of SIG (P = 0.02) and non-SIG (P < 0.01). The expression rate of M-GGMC-1 in the cases of DAC was higher than that in the cases of SIG (P < 0.01) and non-SIG (P < 0.01).
The MUC2-expression rates in the cases of DAC, SIG, and non-SIG were 76%, 33%, and 56%, respectively. The MUC2-expression rate was lowest in the cases of SIG, and the expression rate in the cases of DAC was higher than that in the cases of SIG (P = 0.02) and non-SIG (P = 0.06). No CD10 expression was detected in any of the cases.
The expressions of the G, GI, I, and UC phenotypes at the mucosal layer among the patients classified according to the different histological subtypes are shown in Table 3.
The expressions of the G, GI, I, and UC phenotypes in patients with DAC were 24%, 76%, 0%, and 0%, respectively; the expressions in patients with SIG were 67%, 33%, 0%, and 0%, respectively; and the expressions in patients with non-SIG were 40%, 56%, 0%, and 4%, respectively. For all the histological subtypes, the expressions of the I and UC phenotypes were extremely low and those of the G and GI phenotypes were extremely high. The expression of the GI phenotype in the cases of DAC was higher than that in the cases of SIG (P = 0.02) and non-SIG (P = 0.06).
Table 4 presents a comparison between the expressions of the G and GI phenotypes with respect to the clinical profiles, tumor site, tumor size, metastasis rate, and histological findings among the patients classified according to the histological subtypes. In SIG, all 6 female patients expressed the G phenotype and all 3 male patients expressed the GI phenotype (P = 0.01). Among the subjects with non-SIG, the number of GI phenotype cases with a tumor diameter greater than 5 cm was significantly higher than the corresponding number of G phenotype cases (P = 0.01). The positive rate of lymph node metastasis in the GI phenotype cases was also significantly higher than the corresponding value in the G phenotype cases (P = 0.01). There were no significant differences in other factors between the patients expressing the G and GI phenotype.
| Histological subtype | DAC | SIG | Non-SIG | |||
| Mucin phenotype | G (n = 9) | GI (n = 29) | G (n = 6) | GI (n = 3) | G (n = 19) | GI (n = 27) |
| Male:female | 6:3 | 12:17 | 0:6a | 3:0a | 9:10 | 10:17 |
| Mean age (yr) | 57.2 | 60.3 | 63.2 | 55.3 | 68.2 | 60.9 |
| Tumor site | ||||||
| U | 2 | 7 | 2 | 0 | 2 | 5 |
| M | 2 | 10 | 0 | 1 | 9 | 18 |
| L | 5 | 12 | 4 | 2 | 8 | 4 |
| Tumor size (cm) | ||||||
| < 5 | 3 | 17 | 3 | 0 | 9b | 3b |
| > 5 | 6 | 12 | 3 | 3 | 10b | 24b |
| Metastasis rate (expression rate, %) | ||||||
| Lymph node | 67 | 45 | 83 | 100 | 42c | 81c |
| Other site | 22 | 3 | 50 | 33 | 11 | 19 |
| Histological findings (expression rate, %) | ||||||
| Vessel invasion | 67 | 69 | 67 | 100 | 84 | 93 |
| Lymph invasion | 100 | 86 | 83 | 100 | 95 | 100 |
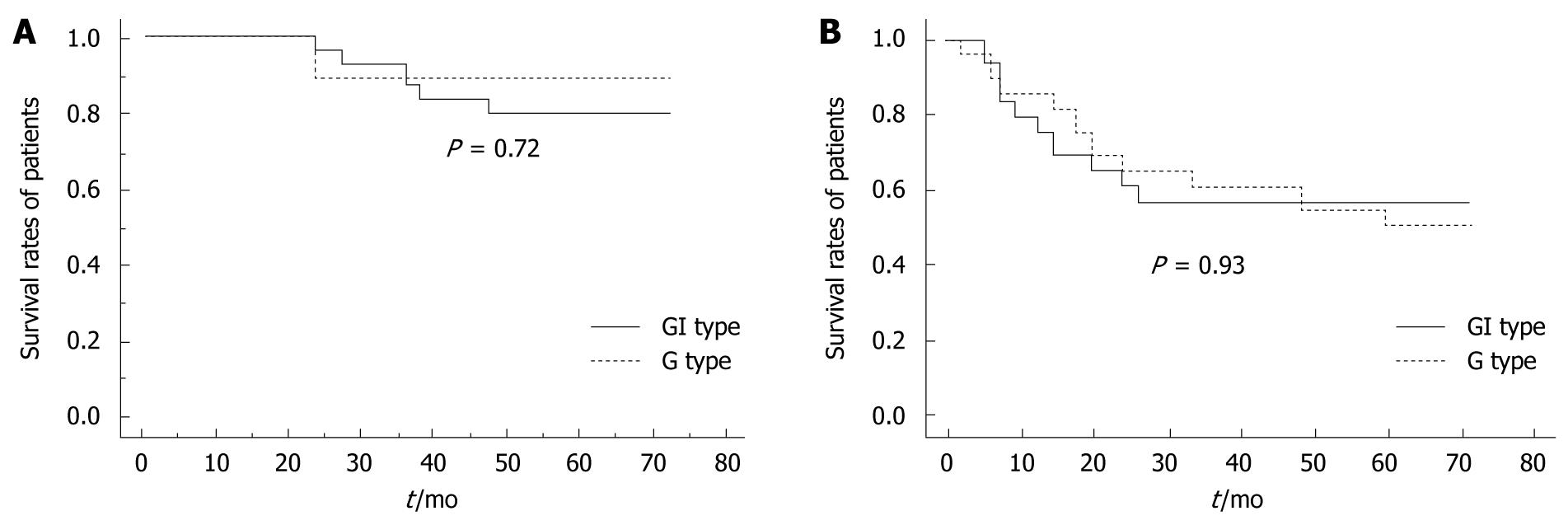
The survival rates of the G and GI phenotype cases classified according to the histological subtypes are shown in Figure 3. Among the patients with DAC, there was no significant difference between the survival rates of patients expressing the G phenotype and those expressing the GI phenotype (Figure 3A). The survival rates among the non-SIG patients expressing the G phenotype and those expressing the GI phenotype (Figure 3B) were also not significantly different (P = 0.93).
In the present study, HGM and MUC5AC were highly expressed in all histological subtypes. Among the SIG and non-SIG cases examined, the expression rates of MUC6 and M-GGMC-1 were lower than those of HGM and MUC5AC. The expression rates of MUC6 and M-GGMC-1 in the DAC were higher than those in the SIG and non-SIG cases. Pinto-de-Sousa et al[18] showed that the mucin phenotype is associated with the tumor site. In the SIG and non-SIG cases in the present study, the upper or middle segments of the stomach were the most common tumor sites. The differences between the rates of expression of the G-related phenotypes were believed to have been influenced by the tumor location. In addition, most cases of AGA showed the presence of gastric foveola phenotype. Further, we observed that the proportion of women in the SIG group was high and that none of the women with SIG expressed the GI phenotype. With regard to the clinical features of the mucin phenotype, the SIGs in women had specific clinical features.
On the basis of the examination of various mucin phenotype-expressing antigens, we classified almost all the AGAs (over 95%) as having either a G or GI phenotype. Furthermore, we failed to detect the expression of the pure I phenotype in any of the cases. The expression rate of the UC phenotype was also extremely low. Pinto-de-Sousa et al studied the mucin phenotypes of 23 diffuse-type adenocarcinomas classified according to Lauren’s method[7] and showed that the MUC5AC-expression rate in these adenocarcinomas was significantly higher than that in the unclassified and expansive adenocarcinomas[18]. Further, Reis et al[4] studied the expression of MUC5AC in early gastric adenocarcinomas and suggested that all gastric adenocarcinomas retain at least some G phenotype cells in the initial stages of neoplasm development. Therefore, we postulate that almost all the cases of advanced DAC, SIG, and non-SIG exhibit or retain the features of G-related phenotypes in the intramucosal layer.
In the present study, the I phenotype (GI phenotype) was expressed in 56% of the non-SIG cases. Barresi et al[21] reported that 8 out of every 10 cases of diffuse-type adenocarcinomas were MUC2-positive. Tajima et al[23] studied the expression rates of the GI and I phenotypes in the cases of undifferentiated type AGA and reported that the I phenotype was expressed in half of the cases. Yamachika et al[15] and Bamba et al[16] reported that the progression of SIG was associated with a phenotypic shift from the G-type to the I-type expression. Other studies have described the invasive features of the I phenotype in gastric adenocarcinoma[19,22,26]. The results of our study and the abovementioned studies suggest that more than 50% of the cases with non-SIG AGA exhibit the features of the I phenotype and that this phenotypic feature is acquired or transformed during the initial progression stage of these adenocarcinomas. In addition, our results indicate that the GI phenotype is associated with invasive features.
The expression rate of the I phenotype (GI phenotype) among the SIG cases was 33% and the number of SIG cases was low (9 cases). We assume that SIG cells with the G phenotype cannot progress to the deep layer. It is also suggested that the morphological features of the SIG cells change and are subsequently classified as non-SIG during tumor progression.
In the present study, we also examined the relationships between the expression of mucin phenotypes and the clinicopathological features and prognosis of patients with DAC, SIG, and non-SIG. Among the non-SIG cases, the expression rate of the GI phenotype in both the patients with extended tumors and those with lymph node metastasis was significantly high. The survival rates of the DAC and non-SIG patients who expressed the G or GI phenotypes were not significantly different. These results indicate that the acquisition of the I phenotype in patients with non-SIG AGA is related to tumor extension and lymph node metastasis and that the existence or acquisition of this phenotype does not affect the survival rate of these patients. Our results indicate that the GI phenotype is associated with invasive features as described above. In SIG, the relationship between the survival rates of G and GI phenotype patients is unknown due to the small number of patients with these phenotypes.
The high rate of lymph node metastasis and the invasive tendency of the non-SIG cells with the GI phenotype may influence the 5-year survival rate, and the patients with the GI phenotype may thus show poor prognosis. However, in the present study, there was no difference between the survival rates of G and GI phenotype patients showing these features. With regard to the features of the mucin phenotype in differentiated-type gastric adenocarcinoma, the G phenotype has been reported to have malignant features[12,13]. In contrast to our study, Tajima et al[23] studied the expression of the mucin phenotype in patients with AGA and reported that patients with the G phenotype adenocarcinomas had a poorer outcome than those with the I phenotype adenocarcinomas. Mizoshita et al[24] reported that AGA patients expressing the GI phenotype had a relatively good prognosis. Although the reason for the difference is unclear, it could be attributed to the difference in the histological subtypes of the examined adenocarcinomas; this is because almost all the studies reporting the malignant potential of the G phenotype have considered differentiated adenocarcinomas[12,13]. Further, we mainly studied the diffuse type of AGA, which was classified into the SIG and non-SIG types, namely the restricted diffuse type of Lauren’s classification. In addition, we assume that the prognosis of the patients with advanced non-SIG will also depend on postoperative treatments such as postoperative chemotherapy.
Therefore, we assume that the expression of the G or GI phenotypes in cases of the advanced non-SIG type of pure diffuse-type gastric adenocarcinoma does not indicate a poor prognosis during the 5-year postoperative period.
In conclusion, the GI phenotype showed a high expression rate (56%) in patients with advanced non-SIG, thereby indicating the acquisition of I phenotypic features during the progression of adenocarcinomas. In patients with advanced non-SIG, although the GI phenotype may be associated with greater invasiveness than the G phenotype, the survival rates of patients expressing either phenotype are similar, suggesting that neither the G nor the I phenotype indicates a poor prognosis in this type of adenocarcinoma. However, the presence of the I phenotype in patients with advanced SIG is unknown.
The relationship between the clinicopathological features and mucin phenotypes in advanced gastric adenocarcinoma (AGA) classified according to the histological subtype [differentiated adenocarcinoma (DAC), signet-ring cell carcinoma (SIG), and diffuse-type adenocarcinoma (non-SIG)] is unclear.
Recently, the expression of the mucin phenotype in gastric adenocarcinoma was reported. Lauren and Nakamura histologically classified gastric adenocarcinomas into 2 main types: intestinal type of Lauren’s histology or differentiated type, and diffuse type or poorly differentiated type. There is little information on the effects of the mucin phenotypes on the clinicopathological and histological subtype-based features of AGA, particularly in the diffuse type as defined by Lauren’s method.
This study is the first report that investigated the mucin phenotype in advanced differentiated, SIG and non-SIG gastric adenocarcinomas. The GI phenotype might possess more invasive characteristics than the G phenotype in non-SIG patients. However, neither of the phenotypes indicates a poor prognosis of DAC and non-SIG.
Mucin phenotypes of gastric adenocarcinoma are related to the biological features in these cancers. The study of mucin phenotype is useful to clarify the biological features in gastric adenocarcinoma.
Mucins of stomach are heavily glycosylated glycoproteins that are the major components of the mucous viscous gel covering gastric surface mucous cells, pyloric gland cells, intestinal goblet cells of the mature gastrointestinal tract, and the brush border of intestinal epithelial cells, etc.
The data presented are interesting and helpful for further understanding the possible clinical value of mucin phenotypes of gastric carcinomas. The conclusions extracted are reasonable, although most of them are disproving.
Peer reviewers: Tamara Vorobjova, Senior Researcher in Immunology, Department of Immunology, Institute of General and Molecular Pathology, University of Tartu, Ravila, 19, Tartu, 51014, Estonia; Qin Su, Professor, Department of Pathology, Cancer Hospital and Cancer Institute, Chinese Academy of Medical Sciences and Peking Medical College, PO Box 2258, Beijing 100021, China
S- Editor Tian L L- Editor Cant MR E- Editor Lin YP
| 1. | Tatematsu M, Furihata C, Katsuyama T, Miki K, Honda H, Konishi Y, Ito N. Gastric and intestinal phenotypic expressions of human signet ring cell carcinomas revealed by their biochemistry, mucin histochemistry, and ultrastructure. Cancer Res. 1986;46:4866-4872. |
| 2. | Saito K, Shimoda T. The histogenesis and early invasion of gastric cancer. Acta Pathol Jpn. 1986;36:1307-1318. |
| 3. | Tahara E. Genetic alterations in human gastrointestinal cancers. The application to molecular diagnosis. Cancer. 1995;75:1410-1417. |
| 4. | Reis CA, David L, Nielsen PA, Clausen H, Mirgorodskaya K, Roepstorff P, Sobrinho-Simões M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997;74:112-121. |
| 5. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. |
| 6. | Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann. 1968;59:251-258. |
| 7. | Hamilton SR, Aaltonen LA; Members of Working Group in World Health Organization Classification of Tumors. Tumor of the stomach. Pathology and Genetics: Tumors of the Digestive System. Lyon, France: IARC Press 2000; 37-52. |
| 8. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 13th ed. Tokyo: Kanahara Inc 1996; 26. |
| 9. | Endo Y, Tamura G, Mtoyama T, Ajioka Y, Watanabe H. Well-differentiated adenocarcinoma mimicking complete-type intestinal metaplasia in the stomach. Hum Pathol. 1999;30:826-832. |
| 10. | Yoshikawa A, Inada Ki K, Yamachika T, Shimizu N, Kaminishi M, Tatematsu M. Phenotypic shift in human differentiated gastric cancers from gastric to intestinal epithelial cell type during disease progression. Gastric Cancer. 1998;1:134-141. |
| 11. | Nakamura T, Yao T, Kabashima A, Nishiyama K, Maehara Y, Tsuneyoshi M. Loss of phenotypic expression is related to tumour progression in early gastric differentiated adenocarcinoma. Histopathology. 2005;47:357-367. |
| 12. | Shibata N, Watari J, Fujiya M, Tanno S, Saitoh Y, Kohgo Y. Cell kinetics and genetic instabilities in differentiated type early gastric cancers with different mucin phenotype. Hum Pathol. 2003;34:32-40. |
| 13. | Koseki K, Takizawa T, Koike M, Ito M, Nihei Z, Sugihara K. Distinction of differentiated type early gastric carcinoma with gastric type mucin expression. Cancer. 2000;89:724-732. |
| 14. | Yamazaki K, Tajima Y, Makino R, Nishino N, Aoki S, Kato M, Sakamoto M, Morohara K, Kaetsu T, Kusano M. Tumor differentiation phenotype in gastric differentiated-type tumors and its relation to tumor invasion and genetic alterations. World J Gastroenterol. 2006;12:3803-3809. |
| 15. | Yamachika T, Inada K, Fujimitsu Y, Nakamura S, Yamamura Y, Kitou T, Itzkowitz SH, Werther JL, Miki K, Tatematsu M. Intestinalization of gastric signet ring cell carcinomas with progression. Virchows Arch. 1997;431:103-110. |
| 16. | Bamba M, Sugihara H, Kushima R, Okada K, Tsukashita S, Horinouchi M, Hattori T. Time-dependent expression of intestinal phenotype in signet ring cell carcinomas of the human stomach. Virchows Arch. 2001;438:49-56. |
| 17. | Saito A, Shimoda T, Nakanishi Y, Ochiai A, Toda G. Histologic heterogeneity and mucin phenotypic expression in early gastric cancer. Pathol Int. 2001;51:165-171. |
| 18. | Pinto-de-Sousa J, David L, Reis CA, Gomes R, Silva L, Pimenta A. Mucins MUC1, MUC2, MUC5AC and MUC6 expression in the evaluation of differentiation and clinico-biological behaviour of gastric carcinoma. Virchows Arch. 2002;440:304-310. |
| 19. | Aihara R, Mochiki E, Nakabayashi T, Akazawa K, Asao T, Kuwano H. Clinical significance of mucin phenotype, beta-catenin and matrix metalloproteinase 7 in early undifferentiated gastric carcinoma. Br J Surg. 2005;92:454-462. |
| 20. | Kabashima A, Yao T, Maehara Y, Tsuneyoshi M. Relationship between biological behavior and phenotypic expression in undifferentiated-type gastric carcinomas. Gastric Cancer. 2005;8:220-227. |
| 21. | Barresi V, Vitarelli E, Grosso M, Tuccari G, Barresi G. Relationship between immunoexpression of mucin peptide cores MUC1 and MUC2 and Lauren's histologic subtypes of gastric carcinomas. Eur J Histochem. 2006;50:301-309. |
| 22. | Tian MM, Zhao AL, Li ZW, Li JY. Phenotypic classification of gastric signet ring cell carcinoma and its relationship with clinicopathologic parameters and prognosis. World J Gastroenterol. 2007;13:3189-3198. |
| 23. | Tajima Y, Shimoda T, Nakanishi Y, Yokoyama N, Tanaka T, Shimizu K, Saito T, Kawamura M, Kusano M, Kumagai K. Gastric and intestinal phenotypic marker expression in gastric carcinomas and its prognostic significance: immunohistochemical analysis of 136 lesions. Oncology. 2001;61:212-220. |
| 24. | Mizoshita T, Tsukamoto T, Nakanishi H, Inada K, Ogasawara N, Joh T, Itoh M, Yamamura Y, Tatematsu M. Expression of Cdx2 and the phenotype of advanced gastric cancers: relationship with prognosis. J Cancer Res Clin Oncol. 2003;129:727-734. |
| 25. | Tajima Y, Yamazaki K, Nishino N, Morohara K, Yamazaki T, Kaetsu T, Suzuki S, Kawamura M, Kumagai K, Kusano M. Gastric and intestinal phenotypic marker expression in gastric carcinomas and recurrence pattern after surgery-immunohistochemical analysis of 213 lesions. Br J Cancer. 2004;91:1342-1348. |
| 26. | Yamagishi M, Noda M, Tatsumi Y, Mukaisho K, Mitsufuji S, Sugihara H, Okanoue T, Hattori T. Correlation between cyclooxygenase-2, proliferative activity, and mucin phenotype in human advanced gastric cancer. J Gastroenterol. 2004;39:1143-1149. |
| 27. | Kim GH, Song GA, Park DY, Lee SH, Lee DH, Kim TO, Jo HJ, Heo J, Kang DH, Cho M. CDX2 expression is increased in gastric cancers with less invasiveness and intestinal mucin phenotype. Scan J Gastroenterol. 2006;41:880-886. |
| 28. | Shiroshita H, Watanabe H, Ajioka Y, Watanabe G, Nishikura K, Kitano S. Re-evaluation of mucin phenotypes of gastric minute well-differentiated-type adenocarcinomas using a series of HGM, MUC5AC, MUC6, M-GGMC, MUC2 and CD10 stains. Pathol Int. 2004;54:311-321. |
| 29. | Watanabe G, Watanabe H, Ajioka Y, Shiroshita H, Nishikura K. Well-differentiated type adenocarcinomas of gastric mucin phenotype transform into intestinal type carcinomas. Stomach and Intestine. 2003;38:693-700. |