Published online Jun 7, 2010. doi: 10.3748/wjg.v16.i21.2648
Revised: March 2, 2010
Accepted: March 9, 2010
Published online: June 7, 2010
AIM: To evaluate the prophylactic properties of integrin CD18-βA peptide in a murine model of abdominal polymicrobial peritonitis and sepsis.
METHODS: Bacterial sepsis was induced in Institute of Cancer Research (ICR) mice by cecal ligation and puncture (CLP) surgery. Inflicted mice were then injected with either sterile saline or CD18-βA peptide intraperitoneally at 2 h after surgery, and were sacrificed at 12 and 24 h after surgery. Blood samples were immediately collected, and analyzed for endotoxin activity and tumor necrosis factor (TNF)-α and interleukin (IL)-6. Lungs and liver were studied for CD45+ leukocyte and CD3 mRNA content. Pulmonary expression of intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM) and E-selectin was also determined.
RESULTS: Intraperitoneal injection of CD18-βA peptide significantly suppressed circulating endotoxin activity (P < 0.01) at 24 h, as well as serum levels of TNF-α (P < 0.05 at 12 and 24 h) and IL-6 (P < 0.01 at 12 h, P < 0.05 at 24 h) in CLP-inflicted mice. CD18-βA peptide also abrogated leukocyte infiltration into liver and lungs as unveiled by reduced CD45+ leukocyte and CD3 mRNA contents. Furthermore, the peptide significantly reduced pulmonary expression of VCAM (P < 0.01 at 12 h, P < 0.001 at 24 h), E-selectin (P < 0.01 at 12 and 24 h), and ICAM-1 (P < 0.01 at 12 h, P < 0.001 at 24 h). These actions of CD18-βA peptide collectively protected septic mice against lethality (P < 0.01).
CONCLUSION: CD18-βA peptide is a potent endotoxin antagonist that can protect surgical patients against sepsis-associated lethality.
- Citation: Wong KF, Wo J, Ho D, Poon RT, Casasnovas JM, Luk JM. Prophylactic uses of integrin CD18-βA peptide in a murine polymicrobial peritonitis model. World J Gastroenterol 2010; 16(21): 2648-2656
- URL: https://www.wjgnet.com/1007-9327/full/v16/i21/2648.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i21.2648
Endotoxin [or lipopolysaccharide (LPS)] was first identified by Richard Pfeiffer from the late log phase culture of Vibrio cholerae, of which the heat inactivated bacterial lysates could induce septic shock and death in experimental animals[1,2]. In clinical settings, circulating endotoxin acts as a pivotal initiator in the pathogenesis of bacterial sepsis and signals front-line immune host cells to trigger the “cytokine storm”, resulting in systemic inflammatory response syndrome (SIRS)[3]. Given that the deleterious SIRS goes unchecked, patients may develop and die from acute respiratory distress, shock, and multiple organ dysfunction. Severely ill patients in surgical wards and intensive care units are at risk from sepsis-associated death, despite the use of broad-spectrum antibiotics, corticosteroids, and other new drugs like drotrecogin alfa[4]. To resolve the stringent clinical situation, many experimental therapeutics like cultured bone marrow stromal cells[5], cholinergic agonists[6] and lysophosphatidylcholine[7] have emerged as potential therapeutics. Studies on their therapeutic efficacies however still remain in pre-clinical stages.
Our previous finding has identified that leukocyte integrin CD18 βA peptide is able to bind bacterial LPS and to alleviate the pro-inflammatory responses in LPS-stimulated Jurkat cells[8]. In the present study, we evaluated the potential prophylactic use of CD18-βA peptide in a murine model of cecal ligation and puncture (CLP) that resembles abdominal polymicrobial peritonitis and sepsis in humans[9].
We designed the CD18-βA peptide basing on the residues 266-318 of CD18 of leukocyte β2 integrins. We cloned the cDNA fragment into pET43.1B vector (Novagen, San Diego, CA, USA), expressed recombinant CD18-βA peptide in E. coli. BL21 (DE3) (Novagen), and subsequently purified the resulting protein using ion mobilized affinity chromatography following our published procedure[8,10]. Before we injected CD18-βA peptide into mice, we dialyzed the peptide against sterile phosphate buffered saline. We verified the purity using electrophoresis, and measured the concentration of CD18-βA peptide as described[11].
We obtained male Institute of Cancer Research (ICR) mice (30 g in weight) from the AAALAC-accredited Laboratory Animal Unit of the University of Hong Kong (Pokfulam, Hong Kong), and divided them randomly into three experimental groups of equal size. We then maintained all mice in a vivarium on a 12-h light/dark cycle at 21°C. Mice had ad libitum access to a sterilized animal chow and water. We performed all experimental procedures on mice in accordance with the ethical guidelines set forth by the University’s Committee on Using Live Animals for Teaching and Research.
We performed CLP as previously described[5] in ICR mice. We anaesthetized mice with an intraperitoneal injection of 50 mg/kg sodium pentobarbital (Nembutal, Rhone Merieux, Pinkenba, QLD, Australia), and opened the anterior abdomen by a 15 mm midline incision. Afterwards, we ligated the exposed cecum non-obstructively with surgical silk, punctured it twice with a 19-gauge needle, and then replaced it back to the peritoneal cavity. For sham-operated mice, we opened the abdomen, but the cecum was neither ligated nor punctured. Finally, we completed CLP surgery by closing the abdomen wall with nylon sutures, and gave no analgesics and antibiotics to mice. At 2 h after the completion of CLP surgery, we injected 0.1 mL sterile saline intraperitoneally into 12 mice, and 0.8 mg/kg CD18-βA peptide (this optimal dosage was prior determined in a pilot study ranging from 0.6-1.2 mg/kg) to another 12 mice. An additional injection of sterile saline or CD18-βA peptide, respectively, was given to mice at 14 h after the completion of CLP surgery. We gave no treatment to the sham-operated mice throughout the experiment. We killed six mice from each of the three experimental groups at 12 and 24 h after CLP. Therefore, mice that were sacrificed at 12 and 24 h received one and two injections of CD18-βA peptide, respectively. We collected blood from the interior vena cava, and harvested lung and liver. We obtained serum from the collected blood by centrifugation at 3000 rpm for 10 min at 4°C. For each harvested lung and liver, we minced it into two pieces that were formalin-fixed and snap-frozen separately.
We studied the survival of septic mice receiving different treatments after CLP in another group of ICR mice (n = 8 in each experimental group, in total 24 mice). We performed CLP as previously described, and injected mice intraperitoneally with either sterile saline or CD18-βA peptide at 2, 12 and 24 h after closure of abdomen. Sterile saline was used as a control according to our earlier studies and reported procedures[5,7,12]. We assessed survival after CLP continually for 48 h, and regarded mice that still remained alive beyond the observation period as survivors. Survival data were analyzed using the Kaplan-Meier module in GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA).
We determined the endotoxin activity in circulation using the Limulus amoebocyte lysate (LAL) pyrochrome kit (Associates of Cape Cod Inc, East Falmouth, MA, USA) as previously described[13,14]. All buffers for the LAL assay were prepared using endotoxin-free water according to manufacturer’s instructions. We measured the resulting signal spectrophotometrically at 540 nm using a microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA), and each sample was done in triplicate. We finally determined the endotoxin activity from the measured signals using a standard curve with a dynamic range from 0 to 0.623 Endotoxin Units (EU)/mL.
We measured the serum level of tumor necrosis factor (TNF)-α and interleukin (IL)-6 of mice using commercially available enzyme-linked immunosorbent assay kits that were purchased from Dakewe (Shenzhen, China) and eBioscience (San Diego, CA, USA), respectively, in accordance with instructions previously reported[15,16].
We followed our published procedures to perform all immunohistochemical staining[17,18]. To study the CD45+ leukocyte content in lungs and liver of mice, we stained tissue paraffin sections with 10 μg/mL monoclonal antibody against CD45 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as described[19]. We stained lung tissues with 20 μg/mL monoclonal antibody against VCAM (20 μg/mL; BD Biosciences, San Jose, CA, USA) and 20 μg/mL monoclonal antibody against E-selectin (BD Biosciences) to study pulmonary expression of the two adhesion molecules. We incubated tissue sections with respective antibodies at 4°C overnight. After washing, we added a horseradish peroxidase-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA). We visualized the resulting signals with a liquid DAB substrate kit (Invitrogen), and thereafter, we counterstained tissue sections with hematoxylin (Vector Laboratories, Burlingame, CA, USA).
We measured the level of CD3 mRNA in lung and liver of mice after CLP using real-time polymerase chain reaction (PCR). This method provides robust and quantitative measurement on tissue infiltration of inflammatory leukocytes[20]. To study the expression of ICAM-1, VCAM, and E-selectin in lungs, we measured their mRNA levels. We performed RNA extraction, first-strand cDNA synthesis, and real-time PCR in ABI PRISM 7700 sequence detector system (Applied Biosystems Inc., Forest Hill, CA, USA) as previously described[21-23].
We performed all statistical analysis using the GraphPad Prism (GraphPad Software Inc.); group means of the study parameters were compared by one-way ANOVA, and P values < 0.05 were considered to indicate statistical significance.
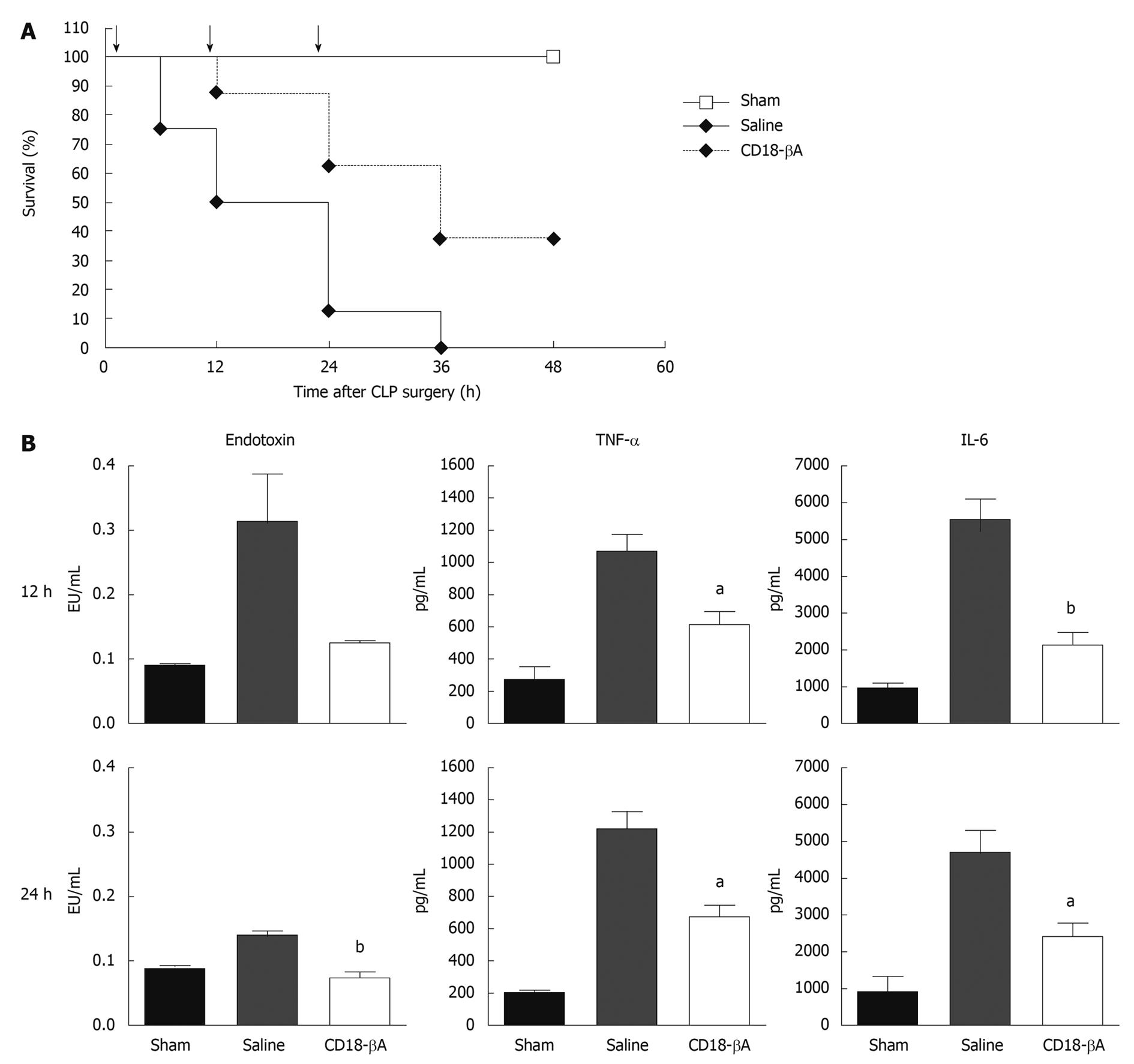
Diagnosis of bacterial sepsis in most clinical scenarios is always delayed, therefore in this study we injected mice intraperitoneally with CD18-βA peptide (0.8 mg/kg) (or sterile saline) at 12-h intervals beginning two hours after the CLP surgery. We first looked at the survival rates after CLP surgery in saline- and CD18-βA peptide-treated mice. A statistically significant (P < 0.01) improvement in the survival of mice injected CD18-βA peptide intraperitoneally was noted (Figure 1A); about 40% of mice survived over the 48 h post-operative observation period, and remained alive thereafter despite discontinuation of therapy. In contrast, mice that received saline all died within the 36 post-operative hour period.
We next investigated whether the beneficial effect of CD18-βA peptide on the survival of septic mice was associated with reductions in endotoxin activity in circulation. To this end, we measured both biological activity of endotoxin and serum levels of TNF-α and IL-6 after we sacrificed mice at 12 and 24 h after CLP surgery and collected blood samples at the interior vena cava. Mice inflicted with CLP surgery showed up-regulation of the circulating endotoxin activity and TNF-α and IL-6 levels in serum samples, which could be remarkably modulated by treatment with CD18-βA peptide but not with saline control (Figure 1B). Intraperitoneal injection of CD18-βA peptide achieved a statistically significant reduction in circulating endotoxin activity (P < 0.01) at 24 h, as well as serum levels of TNF-α (P < 0.05 at 12 and 24 h) and IL-6 (P < 0.01 at 12 h, P < 0.05 at 24 h).
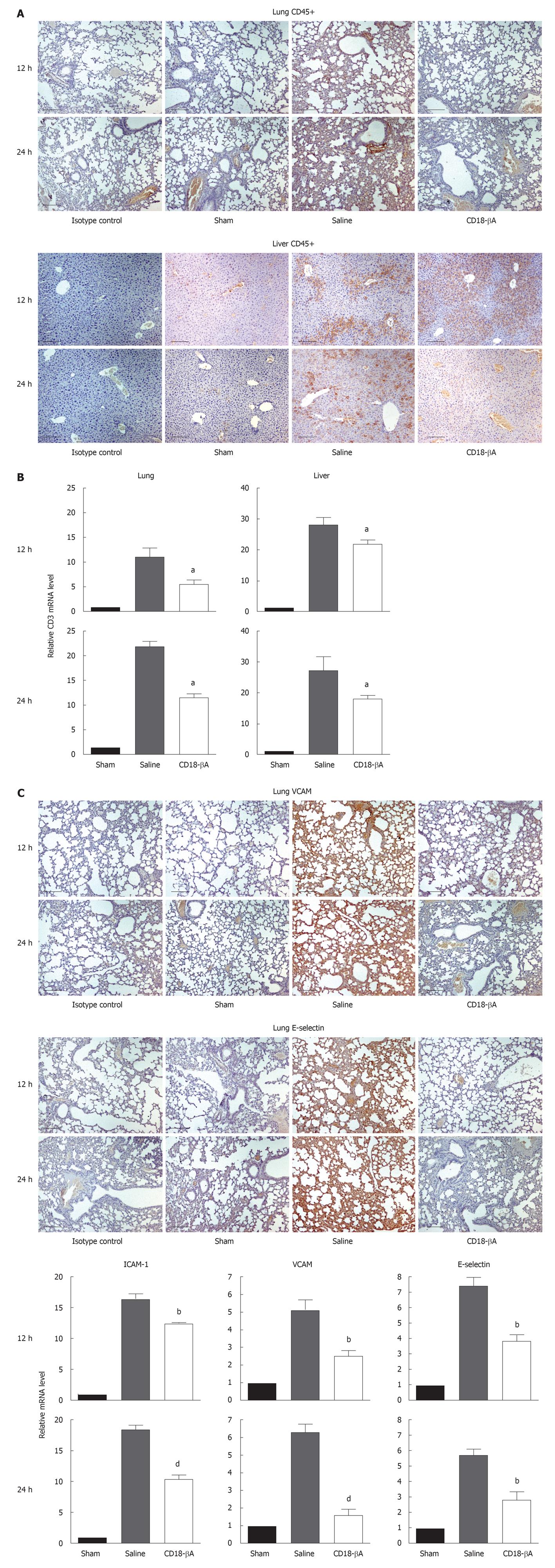
In view of the fact that infiltrating leukocytes usually injure tissues, we first studied histologically the effect of CD18-βA peptide on CD45+ leukocyte infiltration in lungs and liver of septic mice (Figure 2A). Intense CD45 reactivity was detected in the lungs and liver organs from the placebo group. Strikingly, a bolus of CD18-βA peptide injection drastically diminished CD45+ cells in liver, and almost completely eradicated the CD45+ signals in lungs. Further to confirm this observation, we measured the level of CD3 mRNA that is expressed by infiltrating leukocytes using real-time PCR (Figure 2B). Compared to the sham control, CD3 mRNA levels of lungs and liver were up-regulated by over 10-20 fold by CLP surgery, and these elevations were reduced significantly by treatment with the CD18-βA peptide (P < 0.05). Taken together, we suggest that CD18-βA peptide can be used to prevent leukocyte infiltration in bacterial sepsis.
Adhesion molecules facilitate leukocyte trafficking, and are implicated in mediating organ injuries[24]. To study the effect of CD18-βA peptide on adhesion molecule expression, we first determined the pulmonary expression of VCAM and E-selectin by immunohistochemistry (Figure 2C). Intense immunoreactivity toward VCAM and E-selectin was detected in lung paraffin sections prepared from saline-treated septic mice, whereas the corresponding immunoreactivities were markedly diminished from the CD18-βA peptide-treated mice. Expression of ICAM-1, VCAM, and E-selectin mRNA in lungs has also been determined by real-time PCR. Similarly, mRNA levels of the three adhesion molecules surged in lungs of saline-treated septic mice, whereas such elevations were significantly suppressed after CD18-βA peptide treatment: VCAM (P < 0.01 at 12 h, P < 0.001 at 24 h), E-selectin (P < 0.01 at 12 and 24 h), and ICAM-1 (P < 0.01 at 12 h, P < 0.001 at 24 h) (Figure 2C).
We illustrated in a stringent murine model of bacterial sepsis that CD18-βA peptide was able to neutralize circulating endotoxin, to reduce serum TNF-α and IL-6 levels, to inhibit leukocyte infiltrating into lungs and liver, and to suppress endotoxin-induced adhesion molecule expression. All these protective actions of CD18-βA peptide might contribute to survival benefits of the animals in the sepsis model.
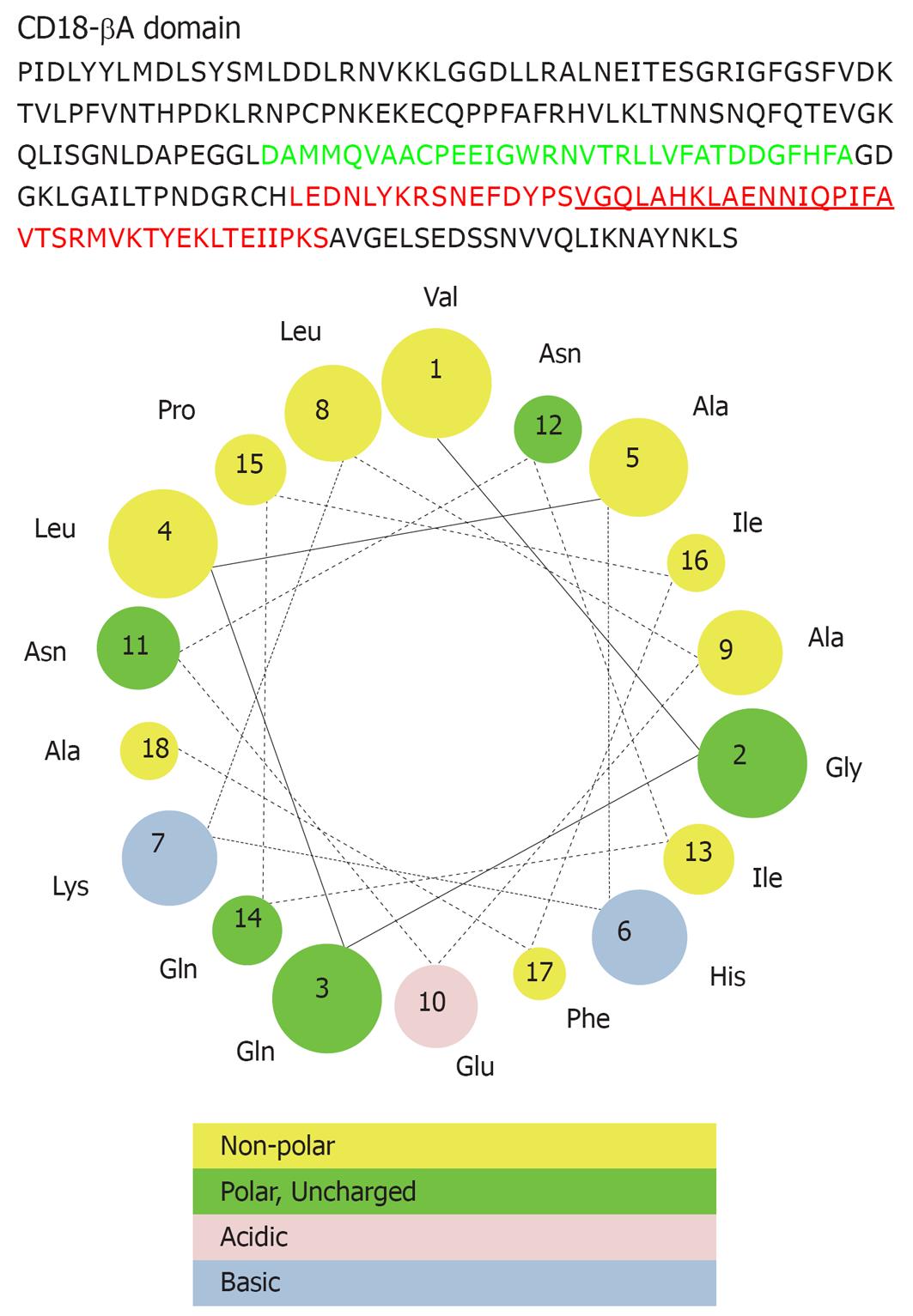
We found the protection of CD18-βA peptide on septic mice was in some ways impressive. Notably, CD18-βA peptide still afforded protection on septic mice even when it was administered after onset of bacterial sepsis. This is in contrast to many other therapeutic interventions that are incapable of halting the already-initiated super-inflammatory responses in surgical patients. In view of this, CD18-βA peptide can serve as an adjunctive therapeutic particularly for patients who are being challenged by high concentration of endotoxin, which usually results with a delayed diagnosis of bacterial sepsis. One of the possible underlying mechanisms for the effective endotoxin neutralization could be attributed to the presence of an amphipathic helix motif (VGQLAHKLAENNIQPIFA) at the C-terminal end (Figure 3). Such structural moiety favors endotoxin binding and neutralization of endotoxicity[25]. In addition, we have previously shown that the positively charged residues (e.g. arginine, lysine) along the CD18-βA peptide are contributing to the effective binding to LPS of various bacterial strains[8].
We showed CD18-βA peptide is an effective agent to suppress the “cytokine storm” in bacterial sepsis, given that the peptide significantly reduced TNF-α and IL-6 levels in the circulation of septic mice. This aspect of CD18-βA peptide is essential to sepsis treatment because pro-inflammatory cytokines can stimulate phagocytes to release reactive oxygen species, increasing vascular permeability and leading to organ injury[26]. In fact, therapeutic agents like anti-cytokine antibodies and receptor antagonists that are aiming to abolish cytokine functions have long been developed. Unfortunately, none of these agents proved efficacious in protecting sepsis patients[27,28], apparently because of the redundancy of cytokine functions. Unlike these therapeutic agents, use of CD18-βA peptide can circumvent this problem because in our recent study, we have shown CD18-βA peptide could down-modulate the NF-κB-mediated signaling that is central to the release of many pro-inflammatory cytokines.
The clinical utility of CD18-βA peptide as a sepsis therapeutic is further enhanced by its ability to inhibit leukocyte infiltration into lungs and liver, given that leukocyte-mediated injury on these two organs cause the high mortality rate of sepsis patients[29,30]. The inhibition is likely attributed to the suppression of adhesion molecules in lungs. In addition to this mechanism, it was apparent that CD18-βA peptide could block the integrin binding sites on adhesion molecules (e.g. ICAM-1) because CD18-βA peptide is homologous to the CD18 antigen of leukocyte β2 integrins. Indeed, this hypothesis is supported by our early study showing that a recombinant CD18 subunit could inhibit the binding of leukocytes to immobilized adhesion molecules (Luk JM, unpublished observation). We also hypothesized that CD18-βA peptide could antagonize direct activation of leukocytes by bacterial endotoxin as our recent study has illustrated that CD18-βA peptide could prevent the sensing of Jurkat cells by bacterial LPS in vitro[8].
Furthermore, a recent in vivo animal study of necrotizing enterocolitis has readily demonstrated the potential therapeutic efficacy of CD18-βA peptide against LPS-induced proinflammatory reactions[31]. In that study, rat intestine was distended and then challenged by bacterial LPS, culminating in profound TNF-α release from immune cells, intense leukocyte influx into the intestine, and severe intestinal tissue damage. CD18-βA peptide was able to neutralize the LPS endotoxicity and modulate the above-mentioned deleterious immune responses.
In summary, CD18-βA peptide is a promising therapeutic for intervention of bacterial sepsis. Unlike anti-LPS antibodies that failed to protect septic patients because of their inability to inhibit pro-inflammatory cytokine releases from immune cells[32], CD18-βA peptide can halt the deleterious “cytokine storm” in bacterial sepsis. To further assess the therapeutic efficacy of CD18-βA peptide, we are going to study the protective action in a porcine model of endotoxemia[33].
Patients severely infected with Gram-negative bacteria can die of endotoxemia and sepsis. Indeed, despite the emergence of broad-spectrum antibiotics, corticosteroids, and advanced surgical resuscitative supports, sepsis remains one of the leading causes of death in intensive care units. In this context, many experimental therapeutic agents have been developed over the last few decades. Disappointingly, only a few of these agents were shown to improve survival, and nearly none of them could halt the unchecked inflammatory response of sepsis.
CD18-βA peptide is a novel endotoxin antagonistic peptide derived from a lipopolysaccharide (LPS)-binding site of leukocyte integrins, and was found able to suppress the stimulation of LPS on leukocytes in vitro. The present work is the first published data concerning the use of CD18-βA peptide in the treatment of mice with polymicrobial peritonitis.
The key pathogenesis of sepsis is the super-inflammatory response (or “cytokines storm”) that can ultimately lead to multiple organ failure and inevitably death. To date, clinical uses of corticosteroids and antibiotics, and experimental uses of cytokine antibodies all fail to halt the deleterious immunological cascades. In this study, CD18-βA peptide was shown to antagonize endotoxicity in circulation, preventing the pro-inflammatory “cytokines storm” from initiation. This mitigation of the inflammatory response reduced the influx of leukocytes from the bloodstream into lungs and liver, protecting both organs from leukocyte-mediated damage. All these beneficial effects of CD18-βA peptide collectively protected septic mice against death.
The indication of CD18-βA peptide as a potent endotoxin antagonist promises to be an effective therapeutic agent to alleviate the deleterious superinflammatory responses in patients with acute septic conditions.
Polymicrobial peritonitis model is a clinically relevant model that resembles sepsis resulting from endotoxemia. Endotoxin (e.g. LPS) is a structural component of the Gram-negative bacteria cell wall, and once been liberated to circulation, can stimulate the innate immunity to release pro-inflammatory cytokines, culminating in systemic inflammatory response syndrome and sepsis.
This is a paper that evaluates the prophylactic properties of integrin CD18, and the results are very important. The authors show integrin CD18 suppressed circulating endotoxin activity and levels of tumor necrosis factor-α and interleukin-6.
Peer reviewer: Aldo Torre Delgadillo, Professor, MD, MSc, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, 14000 México City, México
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH
| 2. | Luk JM, Lind SM, Tsang RS, Lindberg AA. Epitope mapping of four monoclonal antibodies recognizing the hexose core domain of Salmonella lipopolysaccharide. J Biol Chem. 1991;266:23215-23225. |
| 3. | Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776-787. |
| 4. | Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699-1713. |
| 5. | Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42-49. |
| 6. | Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216-1221. |
| 7. | Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161-167. |
| 8. | Wong KF, Luk JM, Cheng RH, Klickstein LB, Fan ST. Characterization of two novel LPS-binding sites in leukocyte integrin betaA domain. FASEB J. 2007;21:3231-3239. |
| 9. | Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hültner L, Heumann D, Männel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164-170. |
| 10. | Sun S, Poon RT, Lee NP, Yeung C, Chan KL, Ng IO, Day PJ, Luk JM. Proteomics of hepatocellular carcinoma: serum vimentin as a surrogate marker for small tumors (<or=2 cm). J Proteome Res. 2010;9:1923-1930. |
| 11. | Lee NP, Tsang S, Cheng RH, Luk JM. Increased solubility of integrin betaA domain using maltose-binding protein as a fusion tag. Protein Pept Lett. 2006;13:431-435. |
| 12. | Villa P, Shaklee CL, Meazza C, Agnello D, Ghezzi P, Senaldi G. Granulocyte colony-stimulating factor and antibiotics in the prophylaxis of a murine model of polymicrobial peritonitis and sepsis. J Infect Dis. 1998;178:471-477. |
| 13. | Ho DW, Fan ST, To J, Woo YH, Zhang Z, Lau C, Wong J. Selective plasma filtration for treatment of fulminant hepatic failure induced by D-galactosamine in a pig model. Gut. 2002;50:869-876. |
| 14. | Luk JM, Kumar A, Tsang R, Staunton D. Biotinylated lipopolysaccharide binds to endotoxin receptor in endothelial and monocytic cells. Anal Biochem. 1995;232:217-224. |
| 15. | Luk JM, Tung PH, Wong KF, Chan KL, Law S, Wong J. Laparoscopic surgery induced interleukin-6 levels in serum and gut mucosa: implications of peritoneum integrity and gas factors. Surg Endosc. 2009;23:370-376. |
| 16. | Yu XM, Lo CY, Lam AK, Leung P, Luk JM. Serum vascular endothelial growth factor C correlates with lymph node metastases and high-risk tumor profiles in papillary thyroid carcinoma. Ann Surg. 2008;247:483-489. |
| 17. | Luk JM, Wang PP, Lee CK, Wang JH, Fan ST. Hepatic potential of bone marrow stromal cells: development of in vitro co-culture and intra-portal transplantation models. J Immunol Methods. 2005;305:39-47. |
| 18. | Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253-1267. |
| 19. | Lee NP, Leung KW, Wo JY, Tam PC, Yeung WS, Luk JM. Blockage of testicular connexins induced apoptosis in rat seminiferous epithelium. Apoptosis. 2006;11:1215-1229. |
| 20. | Steinberg D, Khoo JC, Glass CK, Palinski W, Almazan F. A new approach to determining the rates of recruitment of circulating leukocytes into tissues: application to the measurement of leukocyte recruitment into atherosclerotic lesions. Proc Natl Acad Sci USA. 1997;94:4040-4044. |
| 21. | Liu LX, Lee NP, Chan VW, Xue W, Zender L, Zhang C, Mao M, Dai H, Wang XL, Xu MZ. Targeting cadherin-17 inactivates Wnt signaling and inhibits tumor growth in liver carcinoma. Hepatology. 2009;50:1453-1463. |
| 22. | Luk JM, Lai W, Tam P, Koo MW. Suppression of cytokine production and cell adhesion molecule expression in human monocytic cell line THP-1 by Tripterygium wilfordii polysaccharide moiety. Life Sci. 2000;67:155-163. |
| 23. | Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, Zender P, Kubicka S, Luk JM, Schirmacher P. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135:852-864. |
| 24. | Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301-314. |
| 25. | Wong KF, Luk JM. Endotoxin-neutralizing peptides as gram-negative sepsis therapeutics. Protein Pept Lett. 2009;16:539-542. |
| 26. | Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517-524. |
| 27. | Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med. 2001;29:S121-S125. |
| 28. | Fisher CJ Jr, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271:1836-1843. |
| 29. | Bone RC. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457-469. |
| 30. | Menger MD, Vollmar B. Adhesion molecules as determinants of disease: from molecular biology to surgical research. Br J Surg. 1996;83:588-601. |
| 31. | Chan KL, Wong KF, Luk JM. Role of LPS/CD14/TLR4-mediated inflammation in necrotizing enterocolitis: pathogenesis and therapeutic implications. World J Gastroenterol. 2009;15:4745-4752. |
| 32. | Warren HS, Amato SF, Fitting C, Black KM, Loiselle PM, Pasternack MS, Cavaillon JM. Assessment of ability of murine and human anti-lipid A monoclonal antibodies to bind and neutralize lipopolysaccharide. J Exp Med. 1993;177:89-97. |
| 33. | Carlsson M, Lipcsey M, Larsson A, Tano E, Rubertsson S, Eriksson M, Sjölin J. Inflammatory and circulatory effects of the reduction of endotoxin concentration in established porcine endotoxemic shock--a model of endotoxin elimination. Crit Care Med. 2009;37:1031-1034. |