Published online Jan 14, 2010. doi: 10.3748/wjg.v16.i2.217
Revised: November 27, 2009
Accepted: December 4, 2009
Published online: January 14, 2010
AIM: To test the hypothesis that liver cirrhosis is associated with mobilization of hematopoietic progenitor cells.
METHODS: Peripheral blood samples from 72 patients with liver cirrhosis of varying etiology were analyzed by flow cytometry. Identified progenitor cell subsets were immunoselected and used for functional assays in vitro. Plasma levels of stromal cell-derived factor-1 (SDF-1) were measured using an enzyme linked immunosorbent assay.
RESULTS: Progenitor cells with a CD133+/CD45+/CD14+ phenotype were observed in 61% of the patients. Between 1% and 26% of the peripheral blood mononuclear cells (MNCs) displayed this phenotype. Furthermore, a distinct population of c-kit+ progenitor cells (between 1% and 38 % of the MNCs) could be detected in 91% of the patients. Additionally, 18% of the patients showed a population of progenitor cells (between 1% and 68% of the MNCs) that was characterized by expression of breast cancer resistance protein-1. Further phenotypic analysis disclosed that the circulating precursors expressed CXC chemokine receptor 4, the receptor for SDF-1. In line with this finding, elevated plasma levels of SDF-1 were present in all patients and were found to correlate with the number of mobilized CD133+ progenitor cells.
CONCLUSION: These data indicate that in humans, liver cirrhosis leads to recruitment of various populations of hematopoietic progenitor cells that display markers of intrahepatic progenitor cells.
- Citation: Gehling UM, Willems M, Schlagner K, Benndorf RA, Dandri M, Petersen J, Sterneck M, Pollok JM, Hossfeld DK, Rogiers X. Mobilization of hematopoietic progenitor cells in patients with liver cirrhosis. World J Gastroenterol 2010; 16(2): 217-224
- URL: https://www.wjgnet.com/1007-9327/full/v16/i2/217.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i2.217
Since 2000, several studies have suggested that after transplantation of bone-marrow-derived hematopoietic stem and progenitor cells, such cells have the capacity to migrate into the liver and differentiate into functional hepatocytes[1-4]. However, recent data have indicated that cell fusion, but not transdifferentiation, is the principle mechanism by which bone-marrow-derived cells acquire the features of hepatocytes[5-7], and that the myelomonocytic progeny rather than the bone marrow stem cells are the major fusion partners of hepatocytes[8,9].
In a previous study, we have demonstrated that partial hepatectomy (PH) in healthy human liver donors induces a significant mobilization of CD133+ hematopoietic progenitor cells into the peripheral blood[10]. Besides their hematopoietic potential, these cells can differentiate into cells with a hepatocytic morphology and phenotype in vitro, which suggests that the mobilized progenitor cells participate in liver repair in vivo. The released progenitor cells display a uniform monocytic phenotype, and with respect to their hematopoietic differentiation capacity, represent myelomonocytic precursor cells. Hence, these cells might be endowed with a high fusion potential. It has been shown that a subset of peripheral blood CD14+ cells can differentiate into multiple cell lineages of all three germ layers[11,12], therefore, it is also conceivable that the CD133+ progenitor cells identified in our study are the ancestor cell of this subset, with a similar differentiation plasticity.
By hypothesizing that PH-induced CD133+ cells also occur in other clinical situations of liver injury, we analyzed peripheral blood samples of patients with liver cirrhosis for the presence of these precursors. As in our previous study, we used antibodies against the stem cell markers c-kit and breast cancer resistance protein-1 (Bcrp-1) to characterize further the phenotype of circulating CD133+ cells. Using this approach, we found different populations of hematopoietic progenitor cells, including the CD133+ population observed after PH.
Phycoerythrin (PE)-conjugated anti-CD133 monoclonal antibody (MoAb; clone AC141), anti-CD133 MoAb-conjugated super paramagnetic beads, anti-c-kit MoAb-conjugated super paramagnetic beads, and anti-IgG2b secondary microbeads were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Anti-BCRP-1 MoAb and PE-anti-Bcrp-1 were purchased from eBioscience (Vienna, Austria). PE-anti-c-kit MoAb was from DAKO Cytomation (Hamburg, Germany). Fluorescein isothiocyanate (FITC)-conjugated anti-CD34 MoAb, anti-CD45 MoAb, anti-CD14 MoAb, as well as PE- and FITC-conjugated isotype-matched mouse immunoglobulins were from BD Pharmingen (Heidelberg, Germany), and FITC-anti-CXC chemokine receptor 4 (CXCR4) MoAb from R&D Systems (Wiesbaden, Germany). The stromal cell-derived factor-1 (SDF-1) enzyme linked immunosorbent assay (ELISA) kit was purchased from R&D Systems. Fibronectin was from Gibco Life Technologies (Karlsruhe, Germany), and chamber slides were from Becton Dickinson. Methylcellulose and methylcellulose supplemented with hematopoietic growth factors were purchased from Cell Systems (St. Katharinen, Germany). Paraformaldehyde and methanol were from Sigma (Deisenhofen, Germany). Anti-multi-cytokeratin antibody (rabbit anti-human polyclonal) was from Novocastra (Newcastle, UK). Anti-rabbit immuno alkaline-phosphatase polymer (N-Histofine) and the New Fuchsin substrate (N-Histofine Substrate Kit) were purchased from Medac (Hamburg, Germany).
The study involved peripheral blood samples from 72 patients with liver cirrhosis of varying etiology. All patients were enrolled into University Hospital Hamburg-Eppendorf Liver Transplant Protocols and gave informed consent for their blood samples to be used for experimental purposes. The study protocol was approved by the University Hospital’s Research Committee and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. All samples were collected in heparinized tubes and immediately prepared for analysis.
After lysis with hemolytic buffer (0.155 mol/L NH4Cl, 0.012 mol/L NaHCO3, 0.1 mmol/L EDTA, pH 7.2) for 5 and 2 min, 1 × 106 cells were incubated with PE-conjugated anti-CD133 MoAb in combination with either FITC-conjugated anti-CD34 MoAb, anti-CD45 MoAb, anti-CD14 MoAb or anti-CXCR4 MoAb. Other combinations included FITC-anti-CD34 MoAb, FITC-anti-CD14 MoAb, or FITC-anti-CXCR4 with either PE-anti-Bcrp-1 or PE-anti-c-kit. Isotype-matched mouse immunoglobulins (BD Pharmingen) served as controls. All incubations were performed at 4°C in the presence of normal goat serum. Two-color flow cytometry was accomplished using a FACSCalibur flow cytometer (Becton Dickinson) and CellQuest software (Becton Dickinson). Each analysis included at least 50 000 events. By using isotype controls for PE and FITC, gates for analysis were set such that the lower left panel contained at least 98% of the total cells. The percentage of positive cells was assessed after correction for the percentage of cells that was reactive with the respective isotype control.
Cells were incubated with either anti-CD133 MoAb, anti-c-kit MoAb conjugated super paramagnetic beads or with anti-Bcrp-1 MoAb and anti-IgG2b secondary microbeads, washed, and processed through a MACS magnetic separation column (Miltenyi Biotec), as previously described[13]. An aliquot of the purified cells was analyzed by flow cytometry.
The plasma levels of SDF-1 were measured using a sandwich ELISA (R&D Systems) according to the manufacturer’s instructions. Absorbance at 450 nm was determined by an automated ELISA reader (THERMOmax; Molecular Devices, Ismaning, Germany). Regression curve was used to convert OD units to picograms per milliliter of SDF-1. Patients’ samples were run in triplicate.
Immunoselected cells were cultured in fibronectin-coated chamber slides at a density of 2 × 106 cells/mL in stem cell growth medium, as previously described[13]. Hepatocytic differentiation was induced as previously described[10]. Cells were incubated for up to 28 d at 37°C in 5% CO2. Additional feeding was performed on alternate days. The supernatant was then removed by gentle pipetting and replaced with fresh medium.
Purified cells were plated at 1 × 103 cells/mL in semisolid growth medium (methylcellulose) that was supplemented with various hematopoietic growth factors, as previously described[13]. All cultures were performed in duplicate, incubated at 37°C in 5% CO2 and 95% humidity, and scored after 14 d culture, using an inverted microscope.
Cultured CD133-derived cells were fixed with 4% paraformaldehyde for 10 min at room temperature followed by fixation with methanol for 2 min at -20°C or with ice-cold acetone for 10 min. After blocking with 10% goat serum for 20 min, specimens were incubated with an anti-multi-cytokeratin antibody overnight at room temperature. The reactivity was detected by using an anti-rabbit immuno alkaline-phosphatase polymer in combination with the New Fuchsin substrate. Specimens were counterstained with hematoxylin. Negative controls included replacement of primary antibody with isotypes or PBS, as well as staining of peripheral blood smears. Positive controls were performed using cytospins with freshly thawed primary human hepatocytes (own preparations from resected liver tissue).
Distribution of data was tested with the Kolmogorov-Smirnov test. Variables are expressed as mean ± SE. Bivariate correlations were analyzed with Spearman’s rho as indicated. P < 0.05 was considered significant. For all statistical analysis, SPSS version 13.0 was used.
The characteristics of the patients studied are shown in Table 1. Cirrhosis was caused by autoimmune inflammation (11 patients), alcoholic liver disease (ALD, 30 patients), hepatitis B (9 patients), hepatitis C (13 patients), or to non-specified etiology. Autoimmune-mediated cirrhosis was observed more frequently in female patients, whereas male patients were predominantly affected by ALD and viral hepatitis. The subgroups didnot significantly differ in age. With respect to the Child score, advanced liver cirrhosis was most prominent in the ALD group.
| AIH | ALD | Hepatitis B | Hepatitis C | Other | |
| n | 11 | 30 | 9 | 13 | 9 |
| Sex (M/F) | 3/8 | 21/9 | 7/2 | 8/5 | 5/4 |
| Age (yr, ± SE) | 47.4 ± 7 | 52.9 ± 8 | 52.5 ± 5 | 50.5 ± 8 | 51.8 ± 9 |
| Child A | 6 | 11 | 7 | 6 | 4 |
| Child B | 3 | 17 | 2 | 6 | 4 |
| Child C | 2 | 4 | 0 | 1 | 1 |
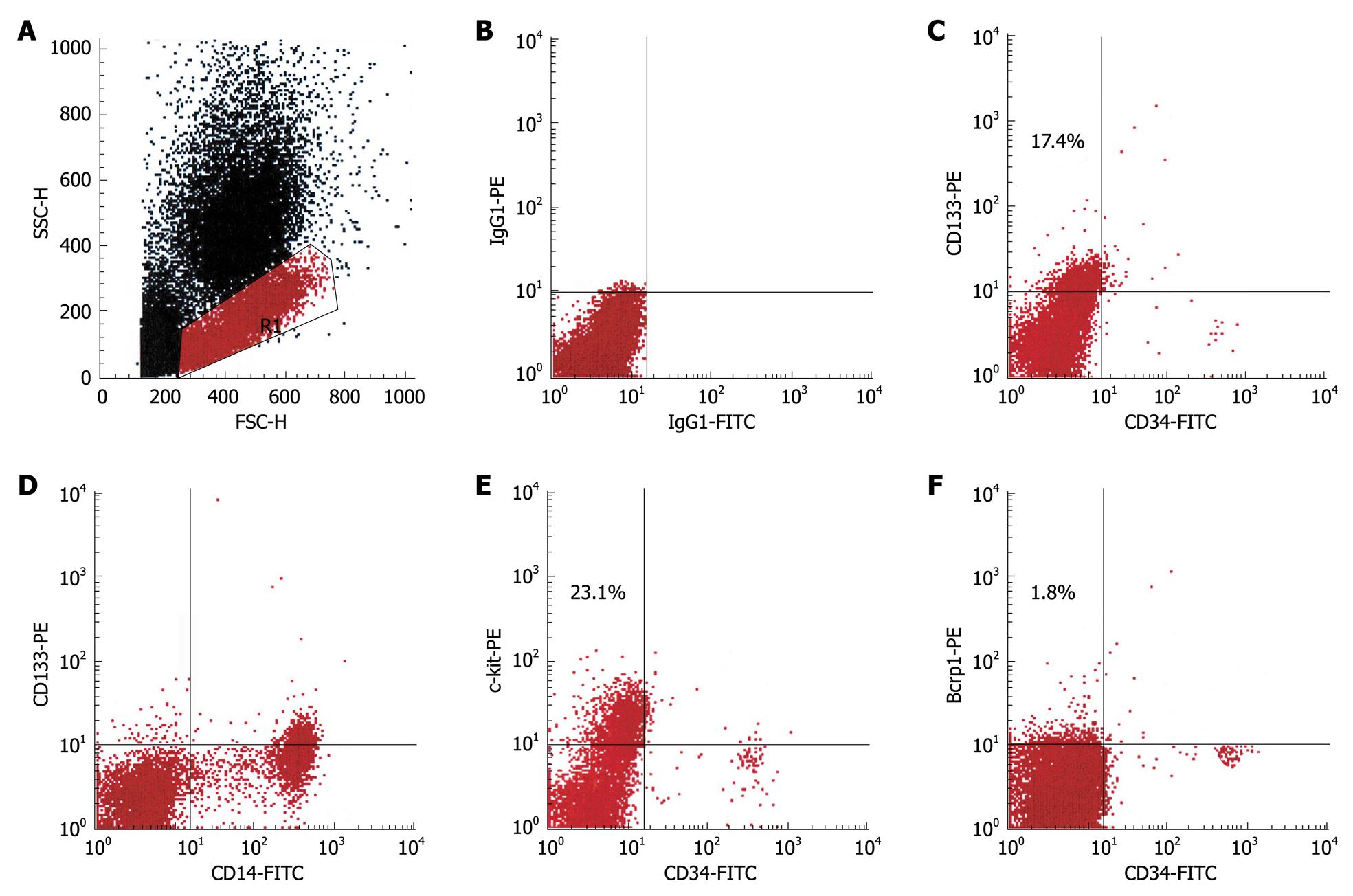
As revealed by flow cytometry, CD133+ cells were observed in 61% (44/72) of all patients (Table 2). On average, 5.8% of the peripheral blood mononuclear cells (MNCs) expressed this marker. Further phenotypical characterization showed that the vast majority of these cells coexpressed CD14 (Figure 1) and CD45 but not CD34 (data not shown), which indicated that this population was identical to PH-induced progenitor cells. Unexpectedly, a distinct population of c-kit+ cells was found in > 90% of the patients studied. Between 1% and 38% of the MNCs displayed this phenotype. In 12 patients, an additional population of Bcrp-1+ cells was detectable, which on average, represented 4.1% of the MNCs (Table 2). All three subsets coexpressed CD45, whereas coexpression of CD34 and/or CD14 was variable in c-kit+ and Bcrp-1+ populations (data not shown).
| Cell subset | Positive/total number of patients | % of the MNCs |
| CD133+ | 44/72 (61.2%) | 5.8 ± 4.9 |
| c-kit+ | 65/72 (90.9%) | 9.2 ± 3.2 |
| Bcrp-1+ | 12/72 (17.9%) | 4.1 ± 3.7 |
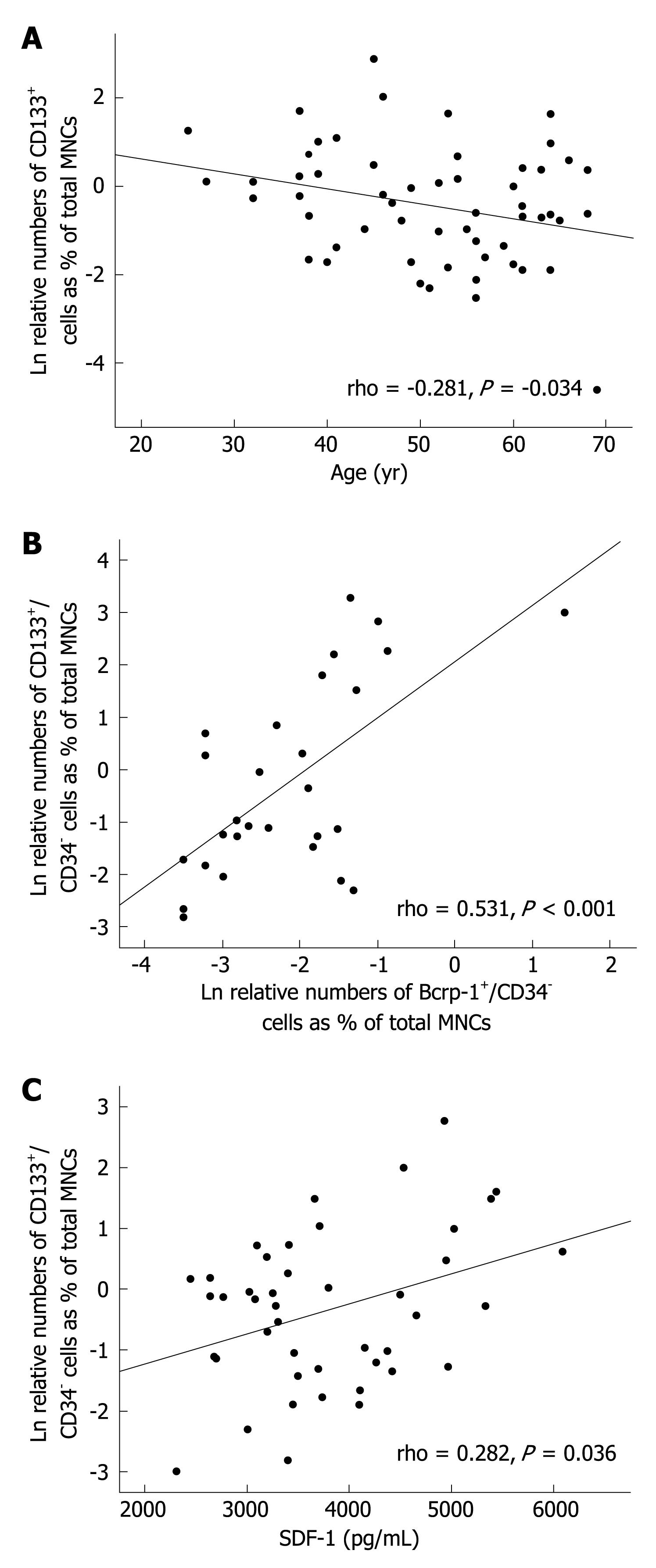
Analysis of peripheral blood samples from the same patient at different time points showed that progenitor cell mobilization is not a permanent phenomenon. The phenotypes and numbers of circulating progenitors varied in the same patient in an irregular timely manner. Thereby, no correlation was found with any clinical parameters, such as liver enzymes, bilirubin, serum albumin, leukocyte count, and platelet count or with the etiology or stage of disease (data not shown). However, numbers of circulating CD133+ progenitor cells inversely correlated with patients’ age (Figure 2A). In addition, there was a significant positive correlation between the numbers of CD133+/CD34- and Bcrp-1+/CD34- peripheral blood cells (Figure 2B).
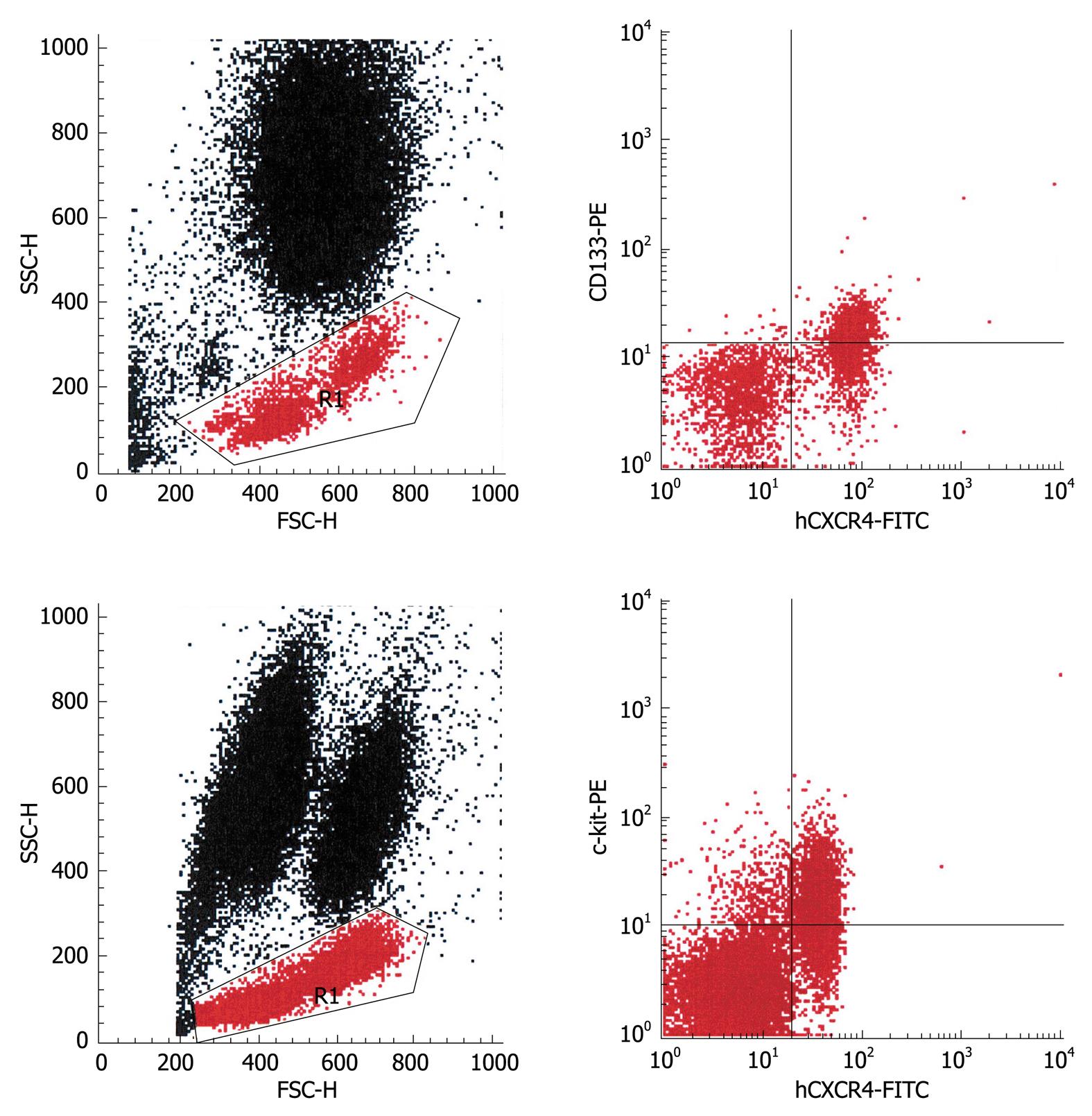
To investigate the molecular mechanisms that mediate progenitor cell mobilization, peripheral blood progenitor cells were analyzed for the expression of CXCR4, the receptor for SDF-1. Virtually all mobilized CD133+ cells coexpressed this receptor, whereas in the c-kit+ populations, on average, half of the cells stained positive for CXCR4 (Figure 3). As mentioned before, Bcrp-1+ populations were only observed in a few patients. In the set of experiments in which coexpression of CXCR4 was studied, no patient showed elevated numbers of Bcrp-1+ cells, therefore, the expression of CXCR4 on these cells remains to be explored. In view of the finding that the CD133+ and c-kit+ population were found to exhibit CXCR4, the plasma levels of SDF-1 were measured (n = 44). Elevated SDF-1 levels were noted in all patients studied. Statistical analysis revealed a significant positive correlation of the plasma levels with the number of mobilized CD133+/CD34- cells (Figure 2C).
To evaluate the clonogenic potential of the cirrhosis-induced progenitor cells, each subset was enriched by immunoselection and transferred to a standard colony assay for hematopoietic stem and progenitor cells. As shown in Table 3, c-kit+ populations and Bcrp-1+ cells had the capacity to produce colonies of the erythroid, granulocytic, and macrophage/monocytic lineage, as well as mixed colonies. In line with our previous study, CD133+ populations solely gave rise to colonies of the granulocytic and macrophage/monocytic lineage. The three progenitor cell populations were also evaluated for their potential to differentiate into the hepatocytic lineage, using culture conditions that were suitable for stimulating hepatocytic differentiation of PH-induced CD133+ progenitor cells[10]. CD133+ populations reproducibly generated an adherent layer of cytokeratin-expressing cells, while c-kit+ and Bcrp-1+ progenitor cells didnot differentiate towards the hepatocytic lineage and could only be maintained in culture for 5 d (data not shown).
| Cells | BFU-E | CFU-E | CFU-GEMM | CFU-GM | CFU-G | CFU-M |
| c-kit | 5 ± 2 | 13 ± 5 | 3 ± 3 | 19 ± 8 | 53 ± 11 | 27 ± 9 |
| Bcrp-1 | 8 ± 3 | 9 ± 2 | 4 ± 1 | 9 ± 7 | 37 ± 14 | 13 ± 5 |
| CD133 | 0 | 0 | 0 | 5 ± 3 | 46 ± 13 | 63 ± 15 |
We demonstrated in this study that liver cirrhosis is associated with mobilization of CD133+, c-kit+, and Bcrp-1+ populations of hematopoietic progenitor cells. Our previous studies have shown that liver resection leads to a mobilization of a unique population of CD133+ progenitor cells with a myelomonocytic phenotype and hematopoietic as well as hepatocytic differentiation potential in vitro. The finding that identical cells are present in the peripheral blood of patients with liver cirrhosis is highly indicative of a certain role of these cells in liver regeneration. In this context, there is increasing evidence that the intrahepatic compartment of progenitor cells, which are referred to as “oval cells” in rodents and as “ductular reactions” in humans, and which can only be observed as proliferates in diseased liver, expresses CD133[14-18]. A recent study has suggested that the human liver contains CD133+ stem cells that differ from the “ductular reactions” with respect to functional properties, and the fact that the CD133+ stem cells can be isolated from healthy livers[19]. The demonstration of circulating c-kit+ and Bcrp-1 progenitor cell populations in patients with liver cirrhosis might also be of functional significance, because both markers also have been shown to be expressed on human intrahepatic progenitor cells[20,21]. However, functional analyses in vitro have suggested that the c-kit+ and Bcrp-1+ populations identified in this study are multi-lineage hematopoietic progenitor cells without hepatocytic potential, whereas the CD133+ subset represents hematopoietic progenitor cells committed to the myelomonocytic lineage, which possess hepatocytic potential. Alternatively, it is possible that the c-kit+ and Bcrp-1+ cells require other stimuli than used to differentiate into hepatocytic cells.
Progenitor cell mobilization in patients with liver cirrhosis was found not to be a permanent phenomenon. Only a minority of patients showed all three populations at the same time point. Unexpectedly, the occurrence of mobilized progenitor cells didnot correlate with any clinical parameter. This led us to the hypothesis that mobilization and recruitment, respectively, of progenitor cells in these patients is triggered by intra-organ hypoxia. It has been shown that: (1) hypoxia induces mobilization of bone-marrow-derived endothelial progenitor cells for neovascularization[22] and the production of SDF-1 in endothelial cells[23], and (2) SDF-1 is involved in stress-induced stem cell recruitment to the liver[24,25]. Therefore, we investigated expression of the SDF-1 receptor CXCR4 on the progenitor cell populations and assayed the plasma levels of SDF-1 in these patients as a first approach to test our hypothesis. Virtually all mobilized CD133+ cells coexpressed CXCR4, whereas only a portion of c-kit+ cells displayed this receptor. Coexpression of CXCR4 by Bcrp-1+ cells could not be evaluated because the patients included in this set of experiments didnot mobilize Bcrp-1+ cells. However, the number of circulating CD133+ cells was found to correlate positively with SDF-1 plasma levels, which were elevated in all patients studied, and the numbers of circulating CD133+/CD34- cells correlated with the numbers of Bcrp-1+/CD34- cells, which indicated that the numbers of Bcrp1+/CD34- cells might also correlate with SDF-1 plasma levels. Hence, we hypothesize that within the Bcrp-1+ population, Bcrp1+/CD34- cells coexpress CXCR4. Interestingly, the numbers of c-kit+/CD34- cells didnot show a correlation with SDF-1 plasma levels. This finding is line with the observation that not all c-kit+ cells coexpressed CXCR4.
There are many hints in the literature that suggest that hypoxia-inducible factor 1α (HIF-1α) plays a major role in progenitor cell recruitment to injured liver tissue. Using a mouse model of soft tissue ischemia, Ceradini et al[26] have shown that HIF-1α regulates progenitor cell trafficking in a hypoxic-dependent manner via induction of SDF-1 expression in endothelial cells of the ischemic tissue. The authors have hypothesized that hypoxia is a fundamental requirement for progenitor cell trafficking and function. We do not believe that hypoxia is a prerequisite for progenitor cell recruitment in all situations of tissue injury, because numerous studies have shown that expression of HIF-1α can be induced independently of hypoxia by many cytokines, growth factors and hormones[27]. Nevertheless, we believe that intrahepatic hypoxia is the initial event that triggers progenitor cell recruitment in liver injury. Hence, progenitor cell mobilization after PH could arise via an acute state of intrahepatic hypoxia, because large parts of the hepatic endothelium are removed. Liver cirrhosis most likely causes intermittent hypoxia each time when progressive fibrosis ligates relatively large intrahepatic blood vessels. This would explain the intermittent occurrence of mobilized progenitor cells in these patients.
The origin of mobilized progenitor cells after PH and in patients with liver cirrhosis remains to be determined. The finding that the number of circulating CD133+ cells correlated inversely with patient age suggests that at least this population is mobilized from the bone marrow, because it is known that the reserve pool of hematopoietic bone marrow stem and progenitor cells decreases with age[28]. No correlation was found between the number of c-kit+ cells and age, which indicates that the c-kit+ cells do not solely derive from the bone marrow. The c-kit+ population might also comprise liver-derived angioblasts[19] that occur in the circulation because of hypoxia-induced proliferation of these cells in the liver. With respect to Bcrp-1+ cells, the number of patients that showed an increased number of these cells didnot suffice to calculate the statistical significance of the correlation with age.
In summary, we have shown that liver cirrhosis is associated with an intermittent mobilization of various populations of hematopoietic progenitor cells into the circulation. One of the populations, characterized by expression of CD133, is identical to the progenitor cell population observed in peripheral blood after liver resection. Coexpression of CXCR4 on CD133+ cells and c-kit+ cells suggests their origin from the bone marrow. Furthermore, our data indicate an involvement of the SDF-1/CXCR4 chemokine receptor system in the mobilization process. Animal studies are needed to evaluate the exact role of each progenitor cell population in liver regeneration.
The liver is known to possess enormous regenerative potential. Nevertheless, the cellular mechanisms that govern human liver regeneration are not understood completely. There is an increasing body of evidence to suggest that various types of stem and progenitor cells play a role in this process.
The authors have demonstrated recently that liver resection in humans leads to a significant increase in the number of circulating cells that express the hematopoietic stem cell marker CD133 and the leukocyte markers CD45 and CD14. This was the first study to indicate that CD133+ progenitor cells might contribute to liver regeneration. Meanwhile, several studies have shown that the human liver contains various populations of CD133+ stem and progenitor cells. In addition, these cells are believed to be involved in hepatocarcinogenesis, a process that results from chronic liver regeneration.
The present study further supports the hypothesis that the CD133+ hematopoietic precursor cells observed after liver resection contribute to liver regeneration, because circulating cells with an identical phenotype were found in a large number of patients with liver cirrhosis of varying etiology. Furthermore, this study is believed to be the first to demonstrate the presence of two additional populations of hematopoietic progenitor cells in the peripheral blood of these patients. Both populations are characterized phenotypically by the expression of stem cell markers that are also known to be expressed on human intrahepatic progenitor cells.
Understanding the cellular mechanisms of liver regeneration is a prerequisite for the development of novel strategies for treating liver disease. Further studies are needed to define the exact role of the three identified progenitor cell populations in liver regeneration and to evaluate their therapeutic potential.
CD133 is an antigen that is expressed on hematopoietic stem and progenitor cells, endothelial progenitor cells, neural progenitor cells, intrahepatic stem and progenitor cells, epithelial cells and various cancer stem cells. The physiological function of this molecule is not known yet. The antigen c-kit is the receptor for the hematopoietic cytokine stem cell factor and is expressed on hematopoietic stem and progenitor cells, endothelial and intrahepatic progenitor cells, as well as on monocytes, mast cells and dermal melanocytes. Breast cancer resistance protein-1 is a membrane protein, which was initially shown to be expressed on breast cancer cells. Functionally, it belongs to the ATP-binding cassette transporters that serve as protective efflux pumps for many cells, including hematopoietic stem cells and intrahepatic progenitor cells.
The article is well written, with interesting results that provide sufficient experimental evidence to draw the conclusions. The figures reflect the major findings of the study, and are presented appropriately. The discussion is well organized. References are appropriate, relevant and up to date.
Peer reviewer: Maria Concepción Gutiérrez-Ruiz, PhD, Departamento de Ciencias de la Salud, Universidad Autónoma Metropolitana-Iztapalapa, DCBS, Av San Rafael Atlixco 186, Colonia Vicentina, México, DF 09340, México
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
| 1. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. |
| 2. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. |
| 3. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. |
| 4. | Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201-4208. |
| 5. | Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897-901. |
| 6. | Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901-904. |
| 7. | Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968-973. |
| 8. | Camargo FD, Finegold M, Goodell MA. Hematopoietic myelomonocytic cells are the major source of hepatocyte fusion partners. J Clin Invest. 2004;113:1266-1270. |
| 9. | Willenbring H, Bailey AS, Foster M, Akkari Y, Dorrell C, Olson S, Finegold M, Fleming WH, Grompe M. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med. 2004;10:744-748. |
| 10. | Gehling UM, Willems M, Dandri M, Petersen J, Berna M, Thill M, Wulf T, Müller L, Pollok JM, Schlagner K. Partial hepatectomy induces mobilization of a unique population of haematopoietic progenitor cells in human healthy liver donors. J Hepatol. 2005;43:845-853. |
| 11. | Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc Natl Acad Sci USA. 2003;100:2426-2431. |
| 12. | Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, Kawakami Y, Ikeda Y. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol. 2003;74:833-845. |
| 13. | Gehling UM, Ergün S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106-3112. |
| 15. | Roskams T, van den Oord JJ, De Vos R, Desmet VJ. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol. 1990;137:1019-1025. |
| 16. | Yovchev MI, Grozdanov PN, Joseph B, Gupta S, Dabeva MD. Novel hepatic progenitor cell surface markers in the adult rat liver. Hepatology. 2007;45:139-149. |
| 17. | Rountree CB, Barsky L, Ge S, Zhu J, Senadheera S, Crooks GM. A CD133-expressing murine liver oval cell population with bilineage potential. Stem Cells. 2007;25:2419-2429. |
| 18. | Laurson J, Selden C, Clements M, Mavri-Damelin D, Coward S, Lowdell M, Hodgson HJ. Putative human liver progenitor cells in explanted liver. Cells Tissues Organs. 2007;186:180-191. |
| 19. | Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973-1987. |
| 20. | Crosby HA, Kelly DA, Strain AJ. Human hepatic stem-like cells isolated using c-kit or CD34 can differentiate into biliary epithelium. Gastroenterology. 2001;120:534-544. |
| 21. | Vander Borght S, Libbrecht L, Katoonizadeh A, van Pelt J, Cassiman D, Nevens F, Van Lommel A, Petersen BE, Fevery J, Jansen PL. Breast cancer resistance protein (BCRP/ABCG2) is expressed by progenitor cells/reactive ductules and hepatocytes and its expression pattern is influenced by disease etiology and species type: possible functional consequences. J Histochem Cytochem. 2006;54:1051-1059. |
| 22. | Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434-438. |
| 23. | Heidemann J, Ogawa H, Rafiee P, Lügering N, Maaser C, Domschke W, Binion DG, Dwinell MB. Mucosal angiogenesis regulation by CXCR4 and its ligand CXCL12 expressed by human intestinal microvascular endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1059-G1068. |
| 24. | Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160-169. |
| 25. | Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339-351. |
| 26. | Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858-864. |
| 27. | Déry MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37:535-540. |