Published online May 7, 2010. doi: 10.3748/wjg.v16.i17.2183
Revised: February 4, 2010
Accepted: February 11, 2010
Published online: May 7, 2010
We describe a 77-year-old man with refractory gastric ulcer that worsened after Helicobacter pylori eradication therapy. Pathology showed marked infiltration of IgG4-positive plasma cells in the gastric lesions, which led us to suspect IgG4-related sclerosing disease. To the best of our knowledge, this is the first report of IgG4-related gastric ulcer without the main manifestation of autoimmune pancreatitis.
- Citation: Fujita T, Ando T, Sakakibara M, Hosoda W, Goto H. Refractory gastric ulcer with abundant IgG4-positive plasma cell infiltration: A case report. World J Gastroenterol 2010; 16(17): 2183-2186
- URL: https://www.wjgnet.com/1007-9327/full/v16/i17/2183.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i17.2183
Gastric ulcer is now recognized as an infectious disease that results from infection with Helicobacter pylori (H. pylori). H. pylori causes continuous gastric inflammation with abundant neutrophil infiltration, and eradication therapy is standard treatment in gastric ulcer[1]. We encountered a 77-year-old man with gastric ulcer of 5 years’ duration, which worsened after H. pylori eradication therapy. Pathologically, gastric lesions show marked infiltration of plasma cells, and immunohistochemical study revealed frank IgG4-positive plasma cell infiltration. This clinical picture suggested refractory gastric ulcer that was suspected of being IgG4-related sclerosing disease.
A 72-year-old man visited the gastroenterology department of our hospital in June 2004 complaining of abdominal discomfort and appetite loss. He had been well until 1 mo earlier, when he experienced discomfort in the upper abdomen and decreased appetite. He had no abdominal pain, diarrhea, fevers, night sweats or body weight loss. He had no allergies, and was not taking any medication. He had undergone appendectomy at age 21 years. He had no history of tobacco smoking and drank alcohol socially. There was no family history of cancer or autoimmune disease. On examination, he appeared comfortable, with a temperature of 37.3°C, blood pressure 110/70 mm Hg, and pulse 128 beats/min. The abdomen was soft, without tenderness or distension. The remainder of the examination was normal.
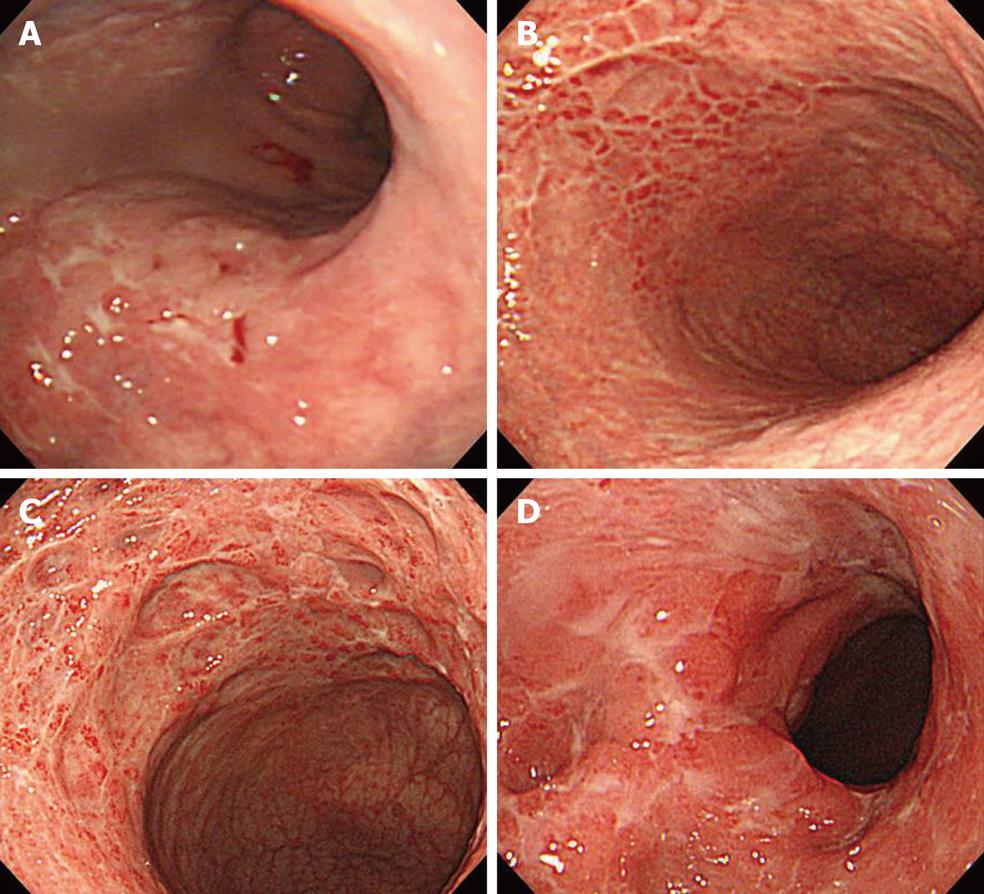
Esophagogastroduodenoscopy (EGD) revealed multiple ulcers in the stomach, while the rapid urease test (RUT) was negative (Figure 1A). Biopsy specimens revealed no malignant cells. Treatment with famotidine was initiated at a single daily dose of 20 mg, which resulted in the gradual resolution of symptoms. EGD at 2 mo revealed multiple gastric ulcer scars. At 14 mo, he remained well, and EGD revealed multiple gastric ulcer scars and biopsy revealed gastritis.
At 26 mo, he remained well, and again EGD revealed multiple gastric ulcer scars and biopsy specimens revealed gastritis (Figure 1B). However, urea breath testing was positive for H. pylori infection. Elimination with lansoprazole (30 mg twice daily), amoxicillin (750 mg twice daily) and clarithromycin (200 mg twice daily) for 7 d was successful, and was maintained with rabeprazole (10 mg daily).
At 37 mo, he remained well, but EGD revealed severe active inflammation (edematous mucosa with exudates, multiple ulcers and stricture) at the body of the stomach (Figure 1C). Biopsy revealed no neoplastic infiltrate and RUT was negative. Laboratory results in serum included lactate dehydrogenase 122 IU/L (reference range: 124-226 IU/L), soluble interleukin 2 receptor 325 U/mL (reference range: 190-650 U/mL), carcinoembryonic antigen 3.3 ng/mL (normal value: ≤ 5.0) and amylase 181 IU/L (reference range: 58-167 IU/L). Serum Epstein-Barr antiviral-capsid-antigen IgG titer was 160. Cytomegalovirus (CMV) antigenemia assay and the Treponema pallidum hemagglutination test were negative. Additional investigation on reference to other facilities precluded the presence of malignant tumor and inflammatory bowel disease, whereas ultrasonography of the abdomen showed slight enlargement of the pancreatic head. Serum IgG4 at this time was 165 mg/dL (reference range: 4.8-105 mg/dL). The presence of autoimmune pancreatitis (AIP) was suspected.
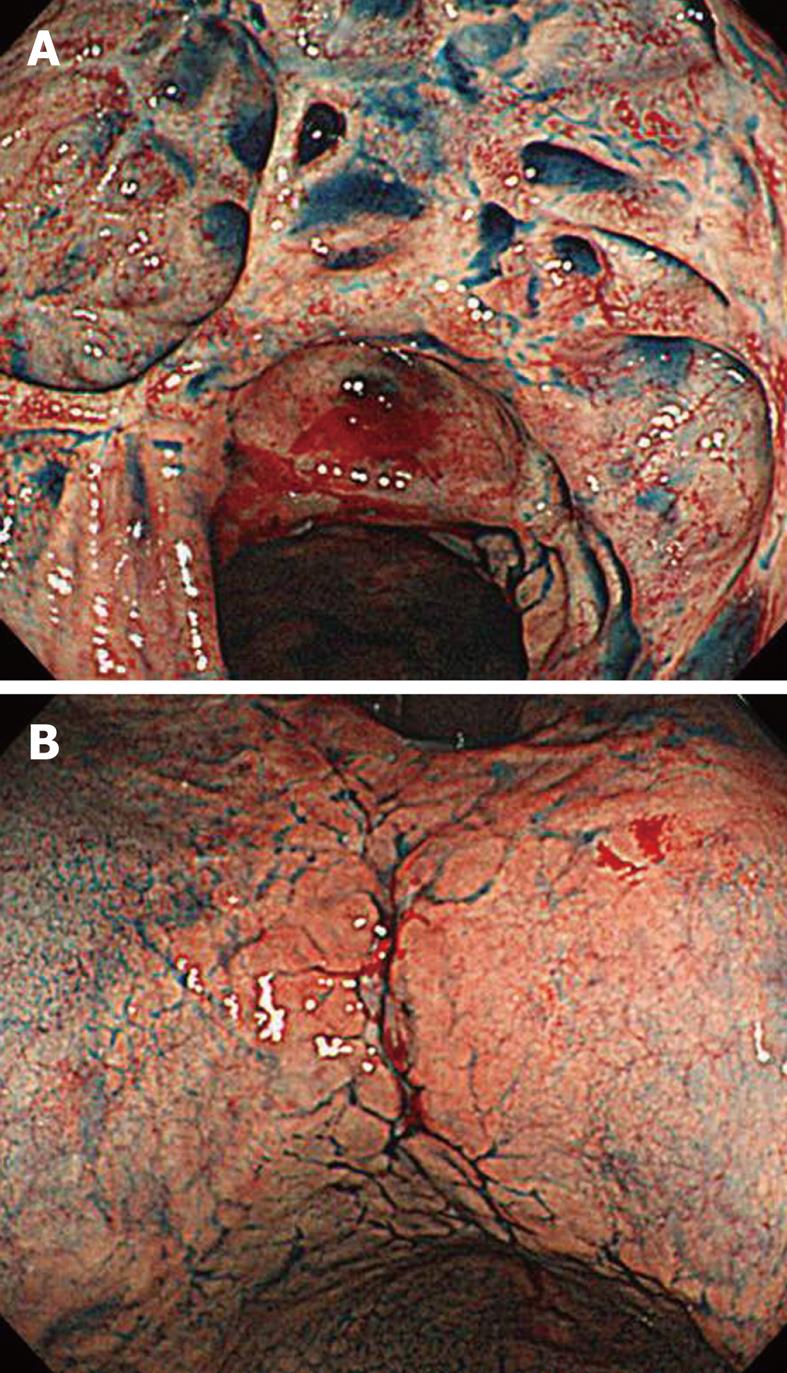
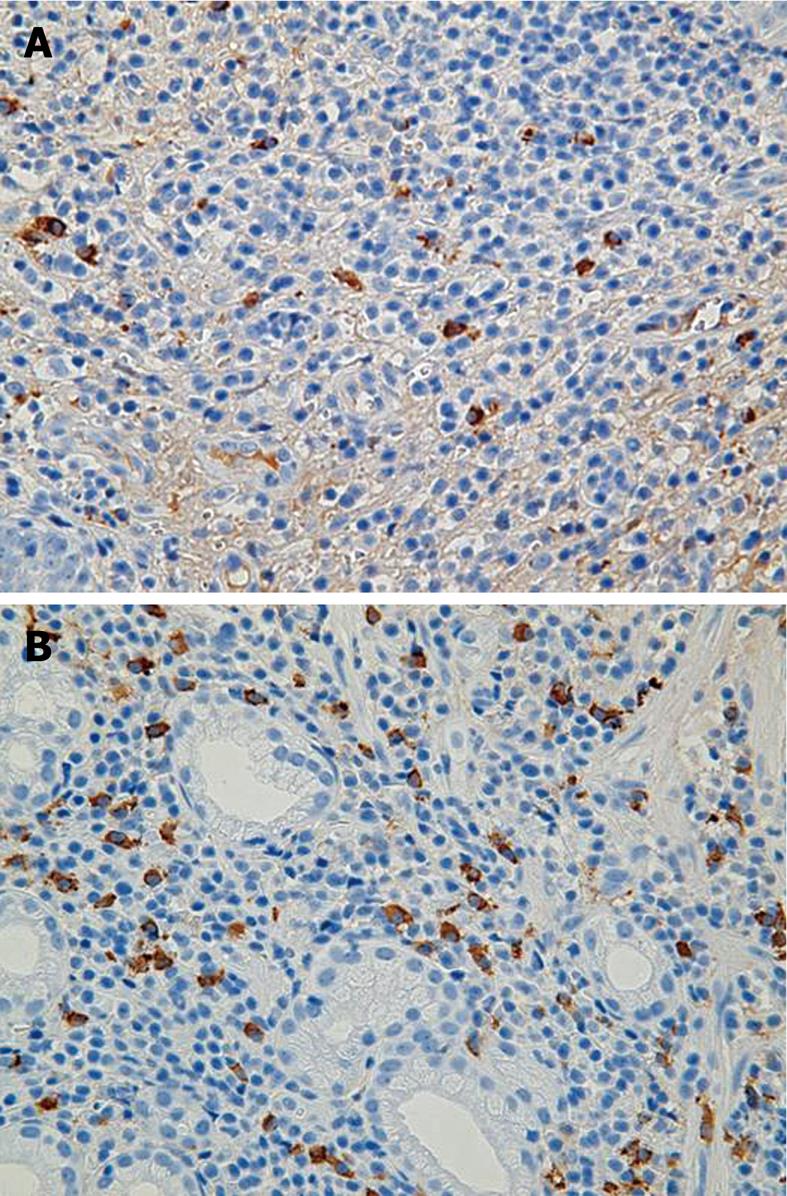
At 49 mo, he remained well on maintenance treatment with rabeprazole. EGD revealed multiple ulcers and ulcer scars, and worsening of the stricture of the upper stomach (Figure 1D). At 54 mo, in February 2009, he remained well and completely asymptomatic. Repeated EDG disclosed multiple ulcer scars with stenosis of the upper body and well-defined ulcers at the lesser curvature (Figure 2). Pathological examination of gastric lesion biopsies revealed intense infiltration of plasma cells that contained IgG4 (Figure 3). Serum IgG was 1909 mg/dL (reference range: 870-1700 mg/dL); IgG4 was 203 mg/dL (reference range: 4-108 mg/dL); complement component C3 was 114 mg/dL (reference range: 80-140 mg/dL); complement component C4 was 25.9 mg/dL (reference range: 11.0-34.0 mg/dL); and amylase was 167 IU/L (reference range: 58-167 IU/L). Antinuclear antibody, rheumatoid factor, anti SS-A antibody, and anti SS-B antibody were negative. Computed tomography scanning of the abdomen showed that the pancreas was normal, with no evidence of enlargement. Magnetic resonance imaging of the abdomen with magnetic resonance cholangiopancreatography revealed normal biliary and pancreatic ducts, and confirmed the lack of pancreatic enlargement.
Although the high serum IgG4 level strongly suggested AIP, the clinical imaging studies showed no pancreatic enlargement. Corticosteroid therapy and the management of side effects were discussed, but the patient, who was asymptomatic, declined this option. At 64 mo (December 2009), he remains well and stable under maintenance therapy with a proton-pump inhibitor.
AIP is a specific type of pancreatitis that is thought to have an autoimmune etiology, and typically shows infiltration of IgG4-positive plasma cells and increased levels of serum IgG4[2,3]. IgG4-positive plasma cells may involve not only the pancreas, but also other organs including the bile duct, gallbladder, salivary gland, thyroid gland, lungs, stomach, colon, liver, retroperitoneum, kidney, prostate and lymph nodes. In 2003, Kamisawa et al[4,5] proposed a new clinicopathological entity, IgG4-related sclerosing disease, and suggested that AIP is a pancreatic lesion that reflects this systemic disease. Shinji et al[6] described a relationship between gastric ulcer and AIP in 2004, and detected gastric ulcer in eight of 23 AIP patients, and significantly more abundant IgG4-positive plasma cell infiltration in the gastric lesions of AIP patients. They have concluded that AIP is closely associated with gastric ulcer with abundant IgG4-bearing plasma cell infiltration.
A common cause of gastric ulcer is H. pylori infection. However, the gastric lesions in the present patient worsened after successful eradication of H. pylori with triple therapy. He had no history of non-steroidal anti-inflammatory drug use, which is another important cause of gastric ulcer. Pathological and serological findings precluded the possibility of gastric cancer of the scirrhous type, malignant lymphoma arising from mucosa-associated lymphoid tissue, Crohn’s disease, reactivation of CMV infection, chronic active EBV infection, and syphilis. Given his elevated serum levels of IgG4 and abundant infiltration of IgG4-positive plasma cells, we consider that the gastric lesions in this patient were associated with infiltration of IgG4-positive plasma cells, and that the most likely diagnosis is IgG4-related sclerosing disease. Since some IgG4-related sclerosing disease patients relapse and improve spontaneously, worsening of this gastric lesion after H. pylori eradication was assumed to be independent of H. pylori infection. From recent reports that suggest that IgG4-related sclerosing disease is associated with cancer, our treatment goals in this asymptomatic 77-year-old man are the prevention of gastric cancer and gastric obstruction[7,8]. Since corticosteroid therapy is effective in IgG4-related sclerosing disease, it may be considered the first treatment option, albeit with consideration to its many side effects, particularly in elderly patients, and uncertainty over optimal dosages. This patient, who was asymptomatic, declined this option and was placed instead on maintenance therapy with a proton-pump inhibitor. Treatment of this rare gastric ulcer will be advanced by multicenter trials in patients with gastric ulcer and IgG4-related sclerosing disease.
Peer reviewer: Dr. Joao Rocha, Toxicological Biochemistry, Universidade Federal De Santa Maria, Santa Maria, 97105-900, Brazil
S- Editor Wang YR L- Editor Kerr C E- Editor Zheng XM
| 1. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. |
| 2. | Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670-2676. |
| 3. | Okazaki K, Uchida K, Fukui T. Recent advances in autoimmune pancreatitis: concept, diagnosis, and pathogenesis. J Gastroenterol. 2008;43:409-418. |
| 4. | Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982-984. |
| 5. | Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006;41:613-625. |
| 6. | Shinji A, Sano K, Hamano H, Unno H, Fukushima M, Nakamura N, Akamatsu T, Kawa S, Kiyosawa K. Autoimmune pancreatitis is closely associated with gastric ulcer presenting with abundant IgG4-bearing plasma cell infiltration. Gastrointest Endosc. 2004;59:506-511. |
| 7. | Kamisawa T, Chen PY, Tu Y, Nakajima H, Egawa N, Tsuruta K, Okamoto A, Hishima T. Pancreatic cancer with a high serum IgG4 concentration. World J Gastroenterol. 2006;12:6225-6228. |
| 8. | Chiba H, Kubota K, Yoneda M, Abe Y, Inamori M, Saito S, Nakajima A, Nomura N, Shimada H, Oshiro H. Autoimmune pancreatitis associated with gastric cancer. Gastroenterol Endosc. 2008;50:1736-1742. |