INTRODUCTION
The incidence of acute pancreatitis is in the range of 300 or more patients per million annually[1,2]. Using the Atlanta classification on severity, about 10% of acute pancreatitis patients are classified as severe[3]. About one-third of all deaths from acute pancreatitis has been reported to occur prior to admission to hospital, and in most cases, is associated with acute lung injury (ALI)[4]. Hospital deaths occur within the first week after admission in 35%-50%[1,5,6] and the cause of death is related to single or multiple organ failure in a majority of cases[7]. In elderly patients, up to 60% of all deaths within the first week are considered to be caused by pancreatitis-associated ALI and acute respiratory distress syndrome (ARDS)[8]. Independently, ALI is a consequence of a pronounced systemic inflammatory response with increased endothelial and epithelial barrier permeability, with leakage of a protein-rich exudate into the alveolar space and interstitial tissues, thus compromising oxygenation and gas exchange[9]. The magnitude of the systemic inflammatory response determines the concomitant clinical course and outcome[10,11] and this also is true for the severity of the acute-pancreatitis-associated ALI[12] (Figure 1). Respiratory complications are frequent in acute pancreatitis, and respiratory dysfunction, presenting as ALI or ARDS, is a major component of multiple organ dysfunction syndrome (MODS), with a frequent need for ventilatory support[8,13], which contributes to early death in severe acute pancreatitis[14] (Figure 2).
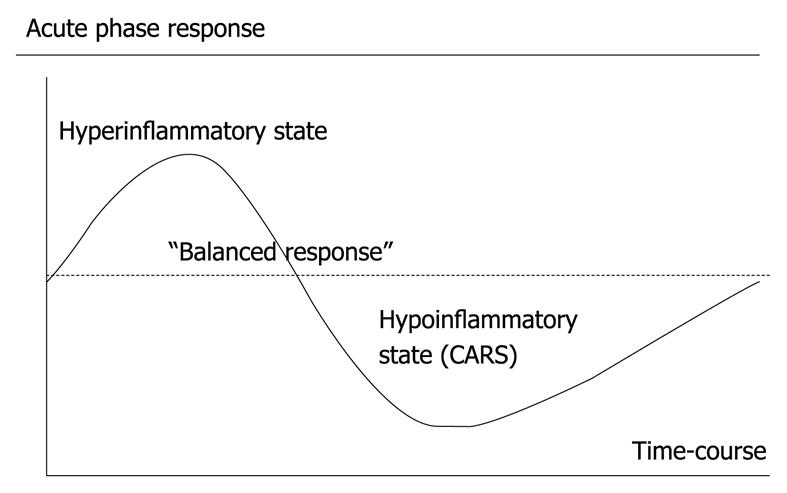
Figure 1 The acute phase response as seen in critical illness, e.
g. severe acute pancreatitis.
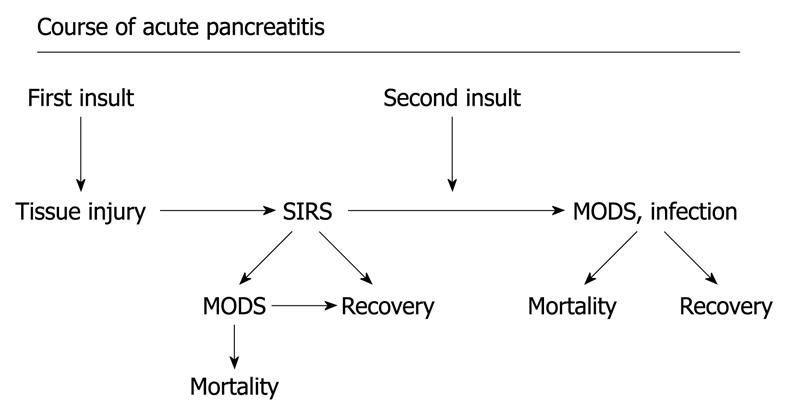
Figure 2 Course of acute pancreatitis.
A potential development in severe acute pancreatitis with the first “insult” resulting in a pronounce systemic inflammatory response and potential development of organ dysfunction, and in the worst scenario early mortality. Later during the course, combination of organ dysfunction and infection, potentially pronounced after the second “insult” (translocation from the gut, burst of proinflammatory cytokines, surgery, etc.) may result in late mortality. MODS: Multiple organ dysfunction syndrome.
The mortality in ALI has been reported as 30%-60%, and is higher in elderly patients[15,16]. In those who survive, the quality of life is impaired[17]. Overall, ALI and ARDS represent the most common and earliest organ dysfunction in the development of MODS, in which mortality is related to the number of involved organs[18]. This type of secondary ALI, a dominant part of MODS, is also found in severe acute pancreatitis, in which lung injury has been reported to account for a high percentage of deaths.
Acute respiratory failure, including ALI and the more severe form, ARDS, has radiological findings with bilateral pulmonary infiltrates and physiological changes, normal cardiac filling pressures, and a ratio of arterial oxygen pressure and inspiratory oxygen concentration (PaO2/FiO2 < 300 mmHg for ALI and < 200 mmHg for ARDS, which reflects pronounced morphological changes)[19]. ALI and ARDS frequently occur in critically ill patients, although the exact incidence in acute pancreatitis has not been stated. If we extrapolate Scandinavian data on ALI and ARDS patients, mortality in the United States is about 36 000 patients per year[20]. More recent mortality rates have also been reported to be 30%-40% and higher in elderly patients[21].
MECHANISMS
Two different phases in ALI and ARDS have been described. Initially, an exudative phase during the first days with diffuse alveolar damage, microvascular injury, type I pneumocyte necrosis, and influx of inflammatory cells and fluid to the pulmonary interstitium has been seen, followed by a fibro-proliferative phase during days 3-7, during which type II pneumocyte hyperplasia, proliferation of fibroblasts and lung repair occur[22]. As a consequence of a pronounced and complex systemic net pro-inflammatory response, both endothelial and epithelial injury is involved in ALI and ARDS (Figure 3). Mediators are several, including cytokines and chemokines, pro-inflammatory mediators and a variety of cells, which regulate the migration and pulmonary infiltration of neutrophils into the interstitial tissue, where they cause injury and breakdown of the pulmonary parenchyma[23]. In order to give a sense of the significance of acute pancreatitis as an etiological factor for ARDS in ICU patients, almost one out of seven patients have acute pancreatitis as a primary cause[24].
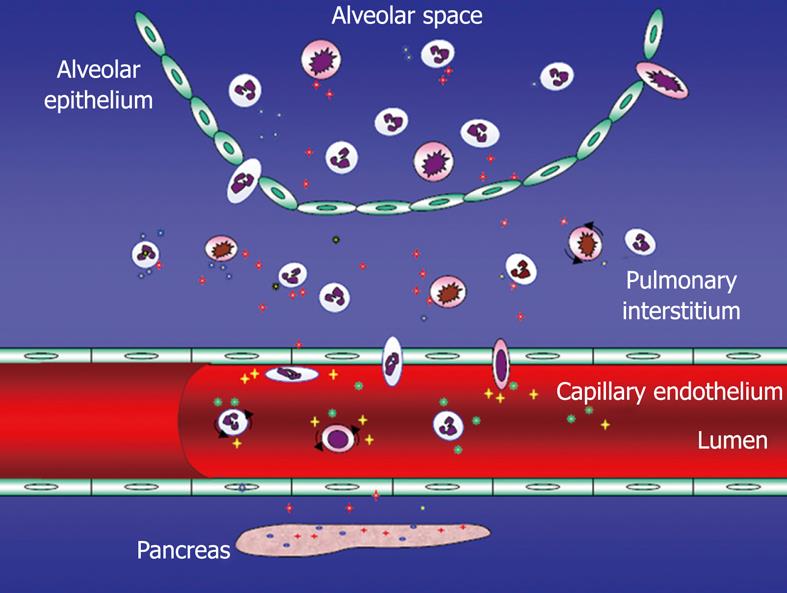
Figure 3 Acute pancreatitis-associated acute lung injury (ALI) - potential mechanisms including endothelial barrier dysfunction.
Several adhesion molecules [selectins, intercellular adhesion molecule-1 (ICAM-1), platelet endothelial cell adhesion molecule-1 (PECAM-1) among others] involved in the extravasation of not at least polymorphonuclear neutrophils (PMNs). Tissue injury by not at least these PMNs.
PATHOPHYSIOLOGICAL MECHANISMS IN SECONDARY ALI
ALI and ARDS may occur secondary to acute pancreatitis, with similar appearances. In general, controlling the source of what is actually fuelling the ALI is important. The features of secondary ALI/ARDS thus involve an initial exudative phase with diffuse alveolar damage, microvascular injury, type-I pneumocyte necrosis and influx of inflammatory cells, followed by a fibro-proliferative phase with lung repair and type-II pneumocyte hyperplasia and proliferation of fibroblasts[13]. Both endothelial and epithelial injury is involved. These changes in ALI, which involve endothelial barrier dysfunction, neutrophil and monocyte/macrophage activation, adhesion molecule expression and intracellular signaling, can to a great extent be executed by proteases derived from polymorphonuclear neutrophils (PMNs), and the process seems driven by tumor necrosis factor (TNF)-α and monocyte chemoattractant protein (MCP)-1, with involvement of mast cells, at least during the initiation of leukocyte activation[25-27]. These complex mechanisms that underlie the ALI associated with acute pancreatitis, and the variety of cells involved, which contribute to neutrophil recruitment, adhesion and activation, as well as signal transduction pathways such as tyrosine kinase activation, local transcription of nuclear factor-κB, and expression of multiple inflammatory genes, have been described in a number of experimental studies and reviews[9,13,28,29]. It thus seems well established that inflammatory mediators play a key role in the pathogenesis of ALI and ARDS. These mediators include TNF-α, interleukins-1β, -6, and -10, transforming growth factor-β, granulocyte-macrophage colony-stimulating factor, platelet-activating factor (PAF), selectin and adhesion molecules, complement component C5a, neuropeptide substance P, and chemokines such as MCP-1, and macrophage inflammatory protein-1α. Moreover, one of the results seems to be the production of reactive oxygen and nitrogen species with potential deleterious effects on pulmonary endothelial and epithelial functions[12,29,30]. The neuropeptide substance P possesses pro-inflammatory action that increases vascular permeability, evidently acting through neurokinin-1 receptors. The complement component C5a is a pro-inflammatory chemoattractant that, at least in the experimental setting, seems to increase lung injury, as does the CD40 receptor found on lymphocytes, monocytes and dendritic cells[30]. All of these represent potential targets for future intervention. It is thus the net magnitude of the pro-inflammatory response and the ratio between pro- and anti-inflammatory activity during the process that ultimately determine the outcome of pancreatitis-associated ALI/ARDS. The means of activating leukocytes may be complex and different, and also involve mast cells during the initiation of leukocyte activation. Pulmonary macrophages are likely to be involved in pancreatitis-induced endothelial barrier dysfunction, compromise of type II pneumocytes, and tissue injury, and furthermore, the release of matrix metalloproteinases seems essential, not at least, that derived from mast cells[31,32].
Pancreatitis-associated ALI has been reported to be related to the effects of pancreatic enzymes, and in particular, phospholipase A2 is thought to play a role in ALI by damaging pulmonary surfactant, which is a substrate for phospholipase A2[33]. Furthermore, patients with severe acute pancreatitis have been found to have elevated serum concentrations of phospholipase A2, which are correlated with the extent of pulmonary complications[34], and a correlation between lung injury score and serum concentration of phospholipase A2 has been identified[35]. It should be mentioned that the increased systemic levels of phospolipase A2 that have been reported in this study may be derived not only from the pancreas, but also be of non-pancreatic origin[35]. With all these mechanisms involved, including a variety of different mediators and cells, there have been high expectations on the use of various types of pancreatic protease inhibitors.
DOES THE GUT PLAY A ROLE IN ALI?
The gut barrier not only represents a mechanical line of defense, i.e. a border between the gut lumen and its contents and the rest of the body, but also includes a variety of immunocompetent cells both within the intestinal wall and in associated lymph nodes, which are represented as mucosa-associated lymphoid tissue and gut-associated lymphoid tissue. Preserving an intact gut barrier is thus a complex and meticulous process that is impaired in critical illness such as severe acute pancreatitis, in which the compromised barrier allows for increased intestinal permeability and translocation of bacteria derived from the intestinal lumen and toxins, as well as a gut inflammatory state[10,36-39] (Figure 4).
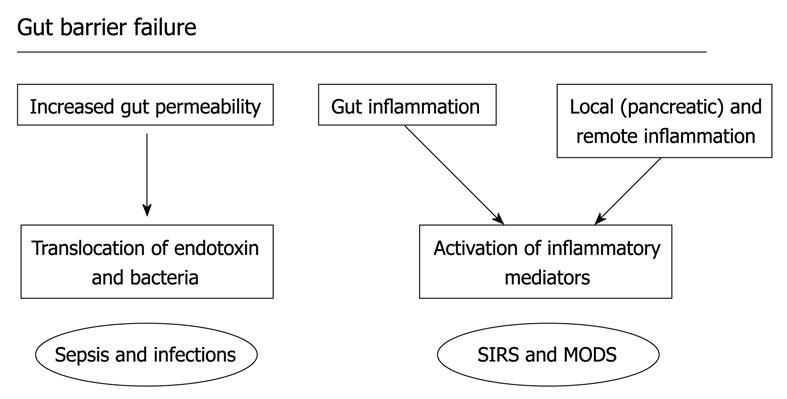
Figure 4 Gut barrier failure.
The increase in permeability of the gut barrier from the intestinal lumen may allow translocation of endotoxin and bacteria and there is also activation of immunocompetent cells in the gut wall and gut associated lymphoid tissue, contributing to the inflammatory response, infection, and potentially the development of organ dysfunction.
Mesenteric lymphatics have been found to carry gut-derived factors that contribute to ALI in various experimental models[40-42]. Interruption of the flow of mesenteric lymph leads to amelioration of ALI, but still we have not identified the exact factors derived from the gastrointestinal tract that are responsible for the pulmonary and systemic effects. This observation represents one potential tool to achieve control of the systemic response and decrease pulmonary injury by modulating factors derived from the gut[43,44].
OUTCOME OF EXPERIMENTAL STUDIES AND POTENTIAL CLINICAL IMPLICATIONS
Our improved understanding of the underlying pathophysiological mechanisms involved in ALI in critical illness has led to a corresponding expectation about potential clinical interventions. This concerns the role of the inflammatory response and signaling mechanisms, such as the protein kinase C pathway[30-32]. Pretreatment and early treatment in experimental acute pancreatitis with, for example, a PAF antagonist and monoclonal antibodies against adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and platelet endothelial cell adhesion molecule-1 (PECAM-1) have been successful[26,27,45]. When evaluating clinical trials with a variety of non-antibiotic interventions in acute pancreatitis, outcome has been less favorable with contradictory results for octreotide and its analogs, as well as the use of the intracellular protease inhibitor gabexate[46]. High expectations have been raised for the use of the highly specific PAF antagonist lexipafant, which has been shown to reduce organ failure and the inflammatory response in patients with predicted severe acute pancreatitis, when administered early[47,48]. A concomitant major study was less convincing, although it did report decreased organ failure inflammatory mediators[49].
FUTURE ASPECTS
Cross-talk between coagulation and inflammation evidently seems to exist, as exemplified by treatment with recombinant human activated protein C in patients with severe acute pancreatitis, in whom a reduction in mortality has been reported[50]. Other components of the coagulation cascade seem to possess inflammatory properties to various degrees. For example, blockers of tissue factor or factor VIIa in experimental severe acute pancreatitis have been shown to ameliorate the associated ALI and decrease neutrophil influx, both when administered as pretreatment and as early treatment[51]. The role of anticoagulants as anti-inflammatory agents in ALI may represent a novel therapeutic option and should be further investigated[52].
The epithelium is involved early in the development of ALI, and produces pro-inflammatory chemokines and triggers neutrophil migration. Furthermore, the epithelium interacts with pulmonary macrophages, which may exacerbate production of pro-inflammatory mediators, thereby increasing recruitment of PMNs from the circulation to the pulmonary interstitial tissue and alveolar lumen. The blocking of chemokines, for example, MCP-1, may thus represent an interesting mode of intervention[53].
Gram-negative infections may be an important predisposing factor for ARDS in acute pancreatitis and endotoxin might potentiate ALI[54]. This emphasizes translocation from the gastrointestinal tract to the systemic circulation and remote organs, as well as the role of the gut-lymph-lung axis. Toll-like receptor 4 (TLR4) compromises the innate immune response and initiates complex signaling pathways when interacting with lipopolysaccharide, which ultimately results in a pro-inflammatory response. Amelioration of the severity of acute pancreatitis and reduced lung injury has been noted in mice that lack TLR4[55], and the lung injury decreases in severity in experimental severe acute pancreatitis treated with nitric oxide, which affects TLR4 gene expression[56]. Therefore, TLR4 has been emphasized as a potential future therapeutic target against inflammatory processes[57].
Heparan sulfate derived from the extracellular matrix or the surface of epithelial cells induces a pro-inflammatory response and has, in its soluble form, been suggested to activate TLR4, thus triggering an endogenous pathway to initiate cytokine production[58]. In experimental acute pancreatitis, heparan sulfate aggravates pancreatic inflammation, and promotes the progression from a local to a systemic inflammatory response, which implies that blocking of heparan sulfate is a potential route of intervention[59]. It has also been suggested that heparan sulfate is important during the initiation of acute pancreatitis, by aggravating the local inflammatory response within the pancreas and thereby potentially promoting the development of a more pronounced systemic inflammatory response, in which heparan sulfate is cleaved off from the epithelial lining of the pancreatic ducts[60]. The role of heparan sulfate and its inhibition thus represents an interesting future line of research, both for the initiation of acute pancreatitis, as well as the development of a systemic inflammatory response.
CONCLUSION
ALI and ARDS in acute pancreatitis remains a major challenge that requires substantial resources. Presently, there is a lack of interventions directed at the underlying pathophysiological mechanisms, although improved understanding may provide us with novel tools for prevention and intervention. Ongoing research on acute pancreatitis and acute respiratory injury gives hope of improvement in the management of this severe and resource-demanding complication, that is still associated with substantial mortality.
Peer reviewers: Udayakumar Navaneethan, MD, Department of Internal Medicine, University of Cincinnati College of Medicine, 231 Albert Sabin Way, Cincinnati, OH 45267, United States; Naoaki Sakata, MD, PhD, Division of Hepato-Biliary Pancreatic Surgery, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8574, Japan
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM