Published online Apr 14, 2010. doi: 10.3748/wjg.v16.i14.1788
Revised: January 20, 2010
Accepted: January 27, 2010
Published online: April 14, 2010
AIM: To investigate the effects of interleukin-8 (IL-8), macrophage migration inhibitory factor (MIF) gene polymorphisms, Helicobacter pylori (H. pylori) infection, on the risk of developing severe chronic atrophic gastritis (SCAG) and intestinal metaplasia (IM).
METHODS: A total of 372 cases were selected from a cohort study in Linqu County, a high risk area for gastric cancer (GC) in northern China. To obtain a sufficient group size, patients with normal or superficial gastritis were included. Based on an average follow-up period of 56 mo, the 372 cases were divided into no progression group (no histological progression from normal or superficial gastritis, n = 137), group I (progressed from normal or superficial gastritis to SCAG, n = 134) and group II (progressed from normal or superficial gastritis to IM, n = 101). IL-8, MIF gene polymorphisms were detected by polymerase chain reaction-based denaturing high-performance liquid chromatography analysis and DNA sequencing.
RESULTS: An increased risk of SCAG was found in subjects with IL-8-251 AA genotype [odds ratio (OR) = 2.62, 95% CI: 1.23-5.72] or IL-8-251 A allele carriers (AA + AT) (OR = 1.81, 95% CI: 1.06-3.09). An elevated risk of IM was found in subjects with IL-8-251 AT genotype (OR = 2.27, 95% CI: 1.25-4.14) or IL-8-251 A allele carriers (OR = 2.07, 95% CI: 1.16-3.69). An increased risk of SCAG was found in subjects with MIF-173 GC genotype (OR = 2.36, 95% CI: 1.38-4.02) or MIF-173 C allele carriers (GC + CC) (OR = 2.07, 95% CI: 1.21-3.55). An elevated risk of IM was found in subjects with MIF-173 CC genotype (OR = 2.27, 95% CI: 1.16-4.46) or MIF-173 C allele carriers (OR = 3.84, 95% CI: 1.58-9.34). The risk of SCAG and IM was more evident in subjects carrying IL-8-251 A allele (OR = 6.70, 95% CI: 1.29-9.78) or MIF-173 C allele (OR = 6.54, 95% CI: 2.97-14.20) and positive for H. pylori infection.
CONCLUSION: IL-8-251 and MIF-173 gene polymorphisms are significantly associated with the risk of SCAG and IM in a population with a high risk of GC in Linqu County, Shandong Province, China.
- Citation: Li ZW, Wu Y, Sun Y, Liu LY, Tian MM, Feng GS, You WC, Li JY. Inflammatory cytokine gene polymorphisms increase the risk of atrophic gastritis and intestinal metaplasia. World J Gastroenterol 2010; 16(14): 1788-1794
- URL: https://www.wjgnet.com/1007-9327/full/v16/i14/1788.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i14.1788
Atrophic gastritis (AG) and intestinal metaplasia (IM) are two important precursor lesions of intestinal type gastric cancer (GC)[1]. These precursor lesions may significantly elevate the risk of intestinal type GC[2,3].
Some bacterial factors, such as the pathogenic island of Helicobacter pylori (H. pylori) including cagA, sIm1 vacA, babA2, sabA, and oipA, are correlated with the severity of atrophic gastritis and occurrence of IM[4-9]. However, bacterial factors alone are not sufficient to explain the diverse results of H. pylori-related diseases. Our previous study has shown that the proportion of cagA + H. pylori strains in children living in Linqu County, an area with a high risk of GC in China, is very high (88.5%)[10]. It has also been demonstrated that almost 100% of H. pylori strains isolated from Chinese population are cagA positive[11].
There is increasing evidence that host inflammation-related cytokines and their gene polymorphisms are related with atrophic gastritis and IM[12,13]. Interleukin-8 (IL-8), a member of Cys-X-Cys (CXC) chemokine family, is an activator and chemoattractant of neutrophils and lymphocytes[14]. Gastric mucosal levels of IL-8 increase significantly after H. pylori infection and parallel to the severity of gastritis[15]. Macrophage migration inhibitory factor (MIF), an important activator of T lymphocytes and macrophages, plays a pivotal role in inflammatory and immune diseases[16,17]. H. pylori infection is associated with an increased expression of MIF mRNA and protein in gastric epithelial and inflammatory cells. Increased expression of the MIF protein correlates with histological severity of GC and its precursor[18].
As these inflammatory cytokines and their gene polymorphisms may potentially influence the outcome of H. pylori infection, a few studies have investigated the association of gene polymorphisms in these inflammatory cytokines with the risk of atrophy and IM[15,19,20].
However, these studies were limited by their single time-point assessment for pathological diagnosis. Therefore, we conducted a prospective study to investigate the association of IL-8 gene polymorphisms with the risk of atrophic gastritis, and MIF gene polymorphisms with IM, showing that the high expressing genotypes of IL-8 are significantly associated with the increased risks of severe chronic atrophic gastritis, and MIF is significantly associated with IM.
The initial study included over 3399 people from Linqu County, a rural area of Shandong Province, China, which has one of the highest GC mortality rates in the world (70/10 000 males and 25/10 000 females per year)[21]. In brief, we launched an endoscopic-pathological screening program for GC and precancerous lesions of GC in 3399 residents from 14 randomly selected villages of Linqu County in autumn of 1989 or in spring of 1990. In 1994, a follow-up endoscopic screening was performed in 83% of eligible members. People, diagnosed as normal or superficial gastritis in 1989 or in 1990, were subsequently genotyped for IL-8 and MIF. We analyzed the relation between genotypes and progression of gastric disease. The study design received approval from the Institutional Review Board of Peking University School of Oncology, and was conducted in accordance with the Helsinki Declaration. Informed consent was obtained from all participants.
Seven biopsies were taken from standard locations in each subject. The procedures and histopathologic criteria have been described elsewhere[22,23]. We reviewed all slides of 372 patients in our study according to the updated Sydney system[24]. Each subject was assigned a global diagnosis based on the most severe diagnosis among the seven biopsies. Independent examinations were performed by three experienced gastroenterologists. If there was any disagreement, the final decision was made based on the majority or subsequent discussion of the case.
After the first examination in 1989, 372 of the 3399 subjects including 172 males and 200 females with a mean age of 42.2 years (range: 24-65 years) were enrolled in this study. All these 372 subjects were initially diagnosed as normal or as superficial gastritis (baseline). After a 56-mo follow-up, theses 372 subjects were subdivided into group I (n = 134), group II (n = 101) and no progression group (n = 137). Lesions in patients of group I were progressed from baseline to severe chronic atrophic gastritis (SCAG). According to the updated Sydney system, recognition of minor degrees of atrophy without intestinal metaplasia in the antrum was difficult, marked degrees of atrophy without intestinal metaplasia in the antrum were selected as the presence of atrophic gastritis. Lesions in patients of group II were progressed from baseline to IM, and lesions in patients of no progression group did no progress from baseline lesion.
In 1994, blood samples were collected and allowed to clot for 30-40 min at room temperature in the dark. Serum was harvested, the clot was frozen immediately at -20°C, and stored at -70°C for 2 or 3 d. During the transfer, dry ice was used.
The detailed serologic assay has been described elsewhere[22]. Serum level of H. pylori-specific IgG and IgA in all samples was measured by enzyme-linked immunosorbent assay (ELISA). Quality-control samples were assayed at Vanderbilt University (Nashville, TN). An optical density ratio (ODR) value > 1.0 and < 1.0 was considered seropositive and negative, respectively.
The presence of H. pylori was further confirmed by immunohistochemistry (IHC). Briefly, paraffin-embedded tissue sections were stained using IHC with an avidin-biotin complex immunoperoxidase kit. Polyclonal rabbit anti-H. pylori (ab7788, Abcam, Cambridge, UK) was used as a primary antibody as previously described[25].
Blood clots were thoroughly washed with Tris-EDTA (TE) buffer containing 50 mmol/L Tris-HCl (pH 8.0) and 1 mmol/L EDTA. After centrifugation, pellets were incubated with rotation in a lysis buffer (TE buffer containing 2 g/L SDS and 200 μg/mL proteinase K) overnight at 37°C. Lysate was then extracted with phenol and precipitated with isopropanol. The precipitate was washed with 70% ethanol, dried, and dissolved in TE buffer. Concentration and purity of DNA were determined by spectrophotometry at A260 nm and A280 nm. Then, DNA was aliquoted and stored at -80°C until use.
Polymerase chain reaction (PCR) was performed in a 25 μL reaction mixture containing 100ng of DNA, 0.1 μmol/L of each primer, 0.2 mmol/L of deoxynucleosidetriphosphate, 1.0 U of Taq DNA polymerase (Promega, Madison, WI), and 1 × reaction buffer. The primer sequences of MIF-173, IL-8-251 have been described elsewhere[26]. PCR-based denaturing high-performance liquid chromatography (DHPLC) and single nucleotide polymorphisms (SNP) were further confirmed by direct sequencing.
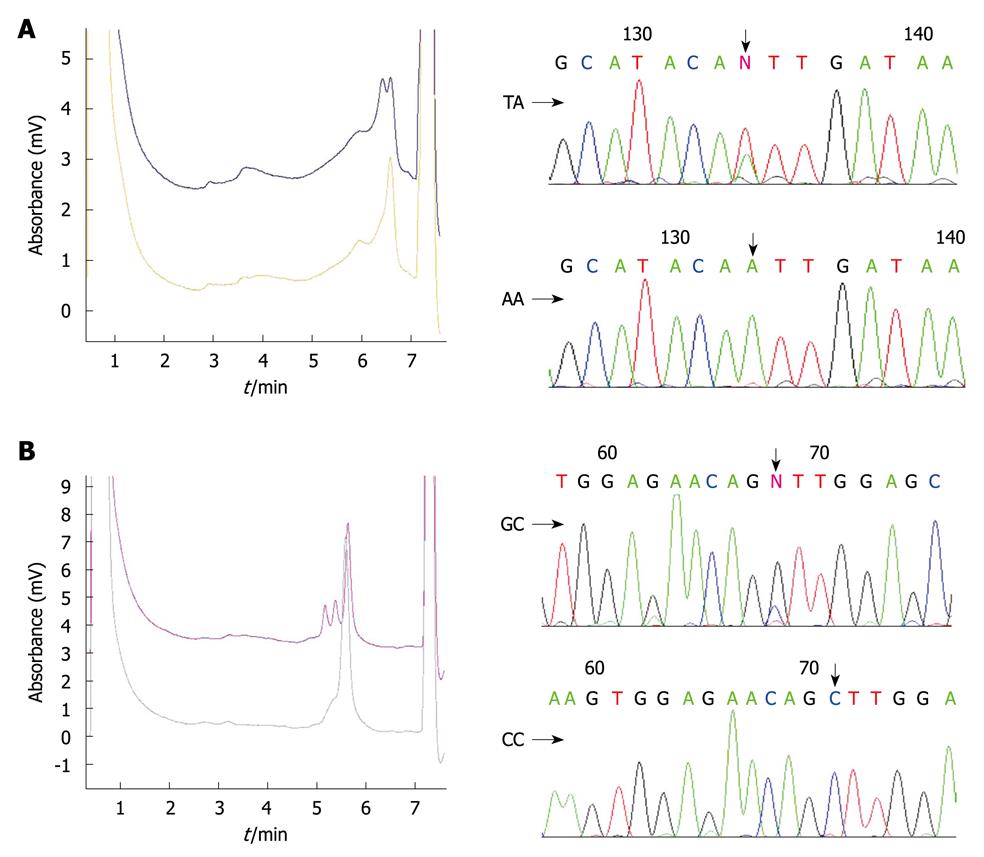
DHPLC analysis was performed on a transgenomic WAVE system (Transgenomic Inc., Omaha, NE). The detailed genotyping process has been described elsewhere[27]. In brief, PCR products were denatured for 1 min at 94°C and then gradually re-annealed by decreasing the sample temperature from 94°C to 45°C for 30 min to form homo- and/or hetero-duplexes. The PCR products were then applied to the DHPLC column at an optimal oven temperature and eluted with a linear acetonitrile gradient at a flow rate of 0.9 mL/min. The results of DHPLC were further confirmed by DNA sequencing with an ABI Prism 377 DNA sequencer (Applied Biosystems, Foster City, CA) (Figure 1).
Data were analyzed using the SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Multiple linear regression analyses were performed with gender, age, smoking, and drinking as explanatory variables to determine which factors influence the progression of gastric lesions. Genotypes of IL-8-251 and MIF-173 loci were also included as explanatory variables when difference in groups I and II and no progression group was detected. Odds ratios (OR) with 95% confidence interval (CI) were computed. P < 0.05 was considered statistically significant.
Demographic characteristics and H. pylori infection with progression and no progression are listed in Table 1. The number of patients under the age of 40 years was greater in groups I and II than in no progression group. The percentage of H. pylori infection was significantly higher in group I than in no progression group.
| No progression group (n = 137) | Group I (n = 134) | P value | Group II (n = 101) | P value | |
| Age (yr) | |||||
| ≤ 40 | 32 (23.4) | 45 (33.6)a | 0.045 | 35 (34.7)b | 0.027 |
| 40-50 | 76 (55.5) | 60 (44.8) | 42 (41.6) | ||
| ≥ 50 | 29 (21.1) | 29 (21.6) | 0.330 | 24 (23.7) | 0.200 |
| mean ± SD | 44.2 ± 0.7 | 43.8 ± 0.6 | 46.7 ± 0.7 | ||
| Sex | 0.600 | 0.260 | |||
| Male | 59 (43.1) | 62 (45.9) | 51 (50.5) | ||
| Female | 78 (56.9) | 72 (54.1) | 50 (49.5) | ||
| H. pylori infection | < 0.001 | 0.100 | |||
| Negative | 59 (43.1) | 30 (22.4) | 33 (32.7) | ||
| Positive | 78 (56.9) | 104 (77.6)c | 68 (67.3) | ||
The patients expressed Alleles at the individual loci were expressed in patients showing no progression of the lesions, with no significant χ2 values.
Multivariate analysis showed that the frequencies of IL-8-251 in groups I and II were significantly different from those in no progression group (Table 2). Compared with IL-8-251 TT genotype, IL-8-251 AA genotype and IL-8-251 A allele carriers exhibited a significantly increased risk for progression from baseline lesions to SCAG (Table 3). The patients with IL-8-251 TA genotype or IL-8-251 A allele carriers had an increased risk for progression from baseline lesions to IM.
| No progression group (n = 137) | Group I (n = 134) | Group II (n = 101) | |
| IL-8-251 | |||
| TT | 59 (43.1) | 39 (29.1) | 25 (24.8) |
| TA | 64 (46.7) | 70 (52.2) | 65 (64.4) |
| AA | 14 (10.2) | 25 (18.7) | 11 (10.9) |
| MIF-173 | |||
| GG | 100 (73.0) | 71 (53.0) | 62 (60.4) |
| GC | 34 (24.8) | 54 (40.3) | 29 (28.7) |
| CC | 3 (2.2) | 9 (6.7) | 10 (10.9) |
| Genotype | Group I (n = 134) OR (95% CI) | Group II (n = 101) OR (95% CI) |
| IL-8-251 | ||
| TT | 1.00 | 1.00 |
| TA | 1.64 (0.96-2.79) | 2.27 (1.25-4.14) |
| AA | 2.62 (1.23-5.72) | 1.20 (0.76-1.90) |
| TA + AA | 1.81 (1.06-3.09) | 2.07 (1.16-3.69) |
| MIF-173 | ||
| GG | 1.00 | 1.00 |
| GC | 2.36 (1.38-4.02) | 1.50 (0.57-3.94) |
| CC | 1.92 (0.95-3.87) | 2.27 (1.16-4.46) |
| GC + CC | 2.07 (1.21-3.55) | 3.84 (1.58-9.34) |
Multivariate analysis showed that the MIF-173 GC genotype or MIF-173 C allele carriers were significantly associated with an increased risk for progression from baseline lesions to SCAG and IM (Table 3).
The risk for SCAG in association with IL-8-251 and MIF-173 genotypes was further examined with stratification by H. pylori infection. The OR for development of SCAG in subjects carrying IL-8-251 A allele or with H. pylori infection alone was 2.34 (95% CI: 0.95-2.83) or 3.28 (95% CI: 1.09-9.78), respectively. However, the OR was significantly elevated in subjects carrying the AA genotype and with H. pylori infection (OR = 6.70, 95% CI: 1.29-9.78) (Table 4). There was an interaction between IL-8-251 A allele carriers and H. pylori infection, with a relative risk for development of SCAG due to the interaction of 6.70, and a synergy index of 1.57.
A similar trend to develop SCAG was observed between the MIF-173 C allele carriers and H. pylori infection. The OR of developing SCAG significantly increased in subjects carrying at least one MIF-173 C allele and with H. pylori infection (OR = 6.54, 95% CI: 2.97-14.20) (Table 4). An interaction between the MIF-173 C allele carriers and H. pylori infection was observed (OR = 2.26, synergy index = 3.15).
The association of IM and IL-8-251 with MIF-173 genotypes was further examined with stratification by H. pylori infection. However, the OR for IM in subjects carrying MIF-173 C allele and with H. pylori infection was elevated significantly (OR = 2.93, 95% CI: 1.28-6.60) (Table 5). There was also an interaction between the MIF-173 C allele carriers and H. pylori infection (OR = 2.20, synergy index = 1.25).
In this study, all tested genetic polymorphisms in IL-8 and MIF increased the risk of SCAG and IM. IL-8 and MIF are inflammatory cytokines expressed in injured mucosa after H. pylori infection. IL-8 is an important mediator of the inflammatory response and increases mucosal injury in H. pylori infected patients because IL-8 is a major activator and chemokine for neutrophils which contribute to mucosal damage by secreting NO and H2O2[28] and significantly augments T helper 1 (Th1) immune response by inducing proinflammatory cytokines such as TNF-α, interferon-γ, and IL-1β secretion. It has been shown that Th1 predominant immune responses inhibit acid secretion from gastric glands, and cause gastric atrophy and metaplasia in a H. pylori infected mouse model[29,30].
The transcript activity is significantly higher in the IL-8-251 A promoter than in the IL-8-251 T promoter[31]. Furthermore, the DNA sequence around the IL-8-251 A allele region may produce a potential binding site for C/EBP, and induce IL-8 expression through the nickel subsulphide dependent pathway[32].
In this study, the risk of progression from baseline lesions to SCAG and IM was significantly increased in patients carrying the IL-8-251 AA and IL-8-251 AT genotype or the IL-8-251 A allele, which is consistent with the reported findings[19,31]. Furthermore, SCAG occurred due to the interaction between IL-8-251 A allele carriers and H. pylori infection. A previous study on the same population also demonstrated that the IL-8-251 AA genotype significantly increases the risk of GC[33]. In the present study, IL-8-251 A allele carriers were positively correlated with the development of SCAG and IM, implying that IL-8-251 gene polymorphism plays an important role in the development of GC.
MIF promotes the recognition of Gram-negative bacteria by the innate immune system[34]. The MIF gene appears to be a strong candidate susceptibility gene for H. pylori -related diseases. Xia et al[17] reported that both mRNA and protein levels of MIF are up-regulated in H. pylori -infected patients and parallel to the severity of gastritis. Moreover, the expression level of MIF protein is markedly different in patients with gastritis, IM, DYS, GC[17,18].
Functional studies, both in vivo and in vitro, demonstrated that the mutant allele MIF-173 C is associated with an increased MIF protein production[31,35]. The presence of MIF-173 C allele stimulates protein 4 (AP-4) transcription factor binding site that may up-regulate MIF expression[36].
In our study, the MIF-173 C allele was found to be associated with the high risk of SCAG and IM. Moreover, an interaction occurred between MIF-173 C allele carriers and H. pylori infection, thus promoting progression from baseline lesions to SCAG and IM. Other studies found that MIF not only modulates the expression of proinflammatory mediators such as TNF-α, IL-1β, IL-8, IFN-γ, but also regulates the activation of T cells[35,37].
Therefore, we hypothesize that variants of MIF gene polymorphism may contribute to the different outcomes of H. pylori -related gastritis. Moreover, MIF gene polymorphisms may be another important candidate gene marker for the outcomes of patients infected with H. pylori.
The fact that the population in our study lived in a relatively closed society and had similar living conditions or habits, may minimize the effects of other mixed factors such as intake of fresh vegetables, salt consumption, water intake. Furthermore, the subjects in our study were followed up for an average period of 56 mo, and the final pathological diagnosis was made in 1994. Although more recent pathological assessments may provide additional insights, an average follow-up period of 4-5 years provides a more dynamic process for the assessment of risks than a single time point analysis[15,19,20].
In summary, H. pylori infection and variants in IL-8-251 or MIF-173 polymorphisms influence the occurrence of SCAG and IM. Because of the high prevalence of H. pylori infection, antibiotic resistance, and some potential drawbacks associated with H. pylori eradication therapy (e.g. reflux esophagitis), our study may provide a reasonable basis for therapeutic decisions even at the early stage of GC.
Atrophic gastritis and intestinal metaplasia are two important precursory lesions of intestinal type gastric cancer (GC), except for factors of Helicobacter pylori (H. pylori). Host gene polymorphisms also play a very important role in the development of GC.
To date, a few studies are available on the relation between gene polymorphisms of inflammatory cytokines and risk of atrophy and intestinal metaplasia (IM).
In this study, the authors conducted a prospective study using the data obtained during the 56-mo follow-up (including both gastroscopic and histopathological information). The relation between interleukin-8 (IL-8), migration inhibitory factor (MIF) gene polymorphisms and risk of atrophic gastritis and IM was also studied.
Because of the high prevalence of H. pylori infection, antibiotic resistance, and some potential drawbacks in H. pylori eradication therapy (e.g. reflux esophagitis), the result of this study may provide some new evidence for the selection of patients infected with H. pylori and for the prevention of progression of H. pylori-related gastritis.
IL-8, a member of Cys-X-Cys (CXC) chemokine family, is an activator and chemoattractant of neutrophils and lymphocytes, thus playing an important role in the development of gastritis and GC. Macrophage MIF, an important activator of T lymphocytes and macrophages, plays a pivotal role in inflammatory and immune diseases. The expression of MIF is increased after H. pylori infection and related with histological severity of GC and its precursor.
The authors studied the role of different IL-8 and MIF genotypes in the development of precancerous gastric lesions, severe atrophic gastritis or intestinal metaplasia, in a cohort of 372 patients. The study defined H. pylori-infected patients at the risk for GC.
Peer reviewer: Dr. Lea Veijola, Consultant Gastroenterologist, Herttoniemi Hospital, Health Care of City of Helsinki, Kettutie 8, Helsinki, 00800, Finland
S- Editor Wang YR L- Editor Wang XL E- Editor Lin YP
| 1. | Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554-3560. |
| 2. | Inoue M, Tajima K, Matsuura A, Suzuki T, Nakamura T, Ohashi K, Nakamura S, Tominaga S. Severity of chronic atrophic gastritis and subsequent gastric cancer occurrence: a 10-year prospective cohort study in Japan. Cancer Lett. 2000;161:105-112. |
| 3. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. |
| 4. | Furuta T, El-Omar EM, Xiao F, Shirai N, Takashima M, Sugimura H. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology. 2002;123:92-105. |
| 5. | Hansson LE, Nyrén O, Hsing AW, Bergström R, Josefsson S, Chow WH, Fraumeni JF Jr, Adami HO. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335:242-249. |
| 6. | Parkin DM. The global burden of cancer. Semin Cancer Biol. 1998;8:219-235. |
| 7. | Yu J, Leung WK, Go MY, Chan MC, To KF, Ng EK, Chan FK, Ling TK, Chung SC, Sung JJ. Relationship between Helicobacter pylori babA2 status with gastric epithelial cell turnover and premalignant gastric lesions. Gut. 2002;51:480-484. |
| 8. | Parsonnet J, Harris RA, Hack HM, Owens DK. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. Lancet. 1996;348:150-154. |
| 9. | Broutet N, Moran A, Hynes S, Sakarovitch C, Mégraud F. Lewis antigen expression and other pathogenic factors in the presence of atrophic chronic gastritis in a European population. J Infect Dis. 2002;185:503-512. |
| 10. | You WC, Zhang L, Pan KF, Jiang J, Chang YS, Perez-Perez GI, Liu WD, MA JL, Gail MH, Blaser MJ. Helicobacter pylori prevalence and CagA status among children in two counties of China with high and low risks of gastric cancer. Ann Epidemiol. 2001;11:543-546. |
| 11. | Zhou J, Zhang J, Xu C, He L. cagA genotype and variants in Chinese Helicobacter pylori strains and relationship to gastroduodenal diseases. J Med Microbiol. 2004;53:231-235. |
| 12. | Gao L, Weck MN, Nieters A, Brenner H. Association between a pro-inflammatory genetic profile and the risk of chronic atrophic gastritis among older adults from Germany. Eur J Cancer. 2009;45:428-434. |
| 13. | Tahara T, Arisawa T, Shibata T, Nakamura M, Yamashita H, Yoshioka D, Okubo M, Maruyama N, Kamano T, Kamiya Y. Effect of RANTES promoter genotype on the severity of intestinal metaplasia in Helicobacter pylori-infected Japanese subjects. Dig Dis Sci. 2009;54:1247-1252. |
| 14. | Zhang QB, Etolhi G, Dawodu JB, Gemmell CG, Russell RI. Relationship between mucosal levels of Helicobacter pylori-specific IgA, interleukin-8 and gastric inflammation. Clin Sci (Lond). 1999;96:409-414. |
| 15. | Taguchi A, Ohmiya N, Shirai K, Mabuchi N, Itoh A, Hirooka Y, Niwa Y, Goto H. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev. 2005;14:2487-2493. |
| 16. | Calandra T, Spiegel LA, Metz CN, Bucala R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc Natl Acad Sci USA. 1998;95:11383-11388. |
| 17. | Xia HH, Lam SK, Huang XR, Wong WM, Leung SY, Yuen ST, Lan HY, Wong BC. Helicobacter pylori infection is associated with increased expression of macrophage migratory inhibitory factor--by epithelial cells, T cells, and macrophages--in gastric mucosa. J Infect Dis. 2004;190:293-302. |
| 18. | He XX, Yang J, Ding YW, Liu W, Shen QY, Xia HH. Increased epithelial and serum expression of macrophage migration inhibitory factor (MIF) in gastric cancer: potential role of MIF in gastric carcinogenesis. Gut. 2006;55:797-802. |
| 19. | Ohyauchi M, Imatani A, Yonechi M, Asano N, Miura A, Iijima K, Koike T, Sekine H, Ohara S, Shimosegawa T. The polymorphism interleukin 8 -251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut. 2005;54:330-335. |
| 20. | Arisawa T, Tahara T, Shibata T, Nagasaka M, Nakamura M, Kamiya Y, Fujita H, Nakamura M, Yoshioka D, Arima Y. Functional polymorphisms in the promoter region of macrophage migration inhibitory factor and chronic gastritis. Int J Mol Med. 2007;20:539-544. |
| 21. | You WC, Blot WJ, Li JY, Chang YS, Jin ML, Kneller R, Zhang L, Han ZX, Zeng XR, Liu WD. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res. 1993;53:1317-1321. |
| 22. | You WC, Zhang L, Gail MH, Chang YS, Liu WD, Ma JL, Li JY, Jin ML, Hu YR, Yang CS. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J Natl Cancer Inst. 2000;92:1607-1612. |
| 23. | Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, Brooks EG, Graham DY, Reyes VE, Ernst PB. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482-492. |
| 24. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. |
| 25. | Li Z, Li J. Local expressions of TGF-beta1, TGF-beta1RI, CTGF, and Smad-7 in Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 2006;41:1007-1012. |
| 26. | Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hültner L, Heumann D, Männel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164-170. |
| 27. | Li WQ, Zhang L, Ma JL, Zhang Y, Li JY, Pan KF, You WC. Association between genetic polymorphisms of DNA base excision repair genes and evolution of precancerous gastric lesions in a Chinese population. Carcinogenesis. 2009;30:500-505. |
| 28. | Barchowsky A, Soucy NV, O'Hara KA, Hwa J, Noreault TL, Andrew AS. A novel pathway for nickel-induced interleukin-8 expression. J Biol Chem. 2002;277:24225-24231. |
| 29. | Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631-636. |
| 30. | Fox JG, Sheppard BJ, Dangler CA, Whary MT, Ihrig M, Wang TC. Germ-line p53-targeted disruption inhibits helicobacter-induced premalignant lesions and invasive gastric carcinoma through down-regulation of Th1 proinflammatory responses. Cancer Res. 2002;62:696-702. |
| 31. | Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci USA. 1996;93:7849-7854. |
| 32. | Donn R, Alourfi Z, De Benedetti F, Meazza C, Zeggini E, Lunt M, Stevens A, Shelley E, Lamb R, Ollier WE. Mutation screening of the macrophage migration inhibitory factor gene: positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2402-2409. |
| 33. | Barton A, Lamb R, Symmons D, Silman A, Thomson W, Worthington J, Donn R. Macrophage migration inhibitory factor (MIF) gene polymorphism is associated with susceptibility to but not severity of inflammatory polyarthritis. Genes Immun. 2003;4:487-491. |
| 34. | Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920-924. |
| 35. | Abe R, Peng T, Sailors J, Bucala R, Metz CN. Regulation of the CTL response by macrophage migration inhibitory factor. J Immunol. 2001;166:747-753. |
| 36. | Benigni F, Atsumi T, Calandra T, Metz C, Echtenacher B, Peng T, Bucala R. The proinflammatory mediator macrophage migration inhibitory factor induces glucose catabolism in muscle. J Clin Invest. 2000;106:1291-1300. |
| 37. | Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry. 1994;33:14144-14155. |