Published online Apr 14, 2010. doi: 10.3748/wjg.v16.i14.1772
Revised: January 21, 2010
Accepted: January 28, 2010
Published online: April 14, 2010
AIM: To identify the brain loci that process human biliary sensation.
METHODS: In 6 patients (age range: 42-74 years; 4 men), who underwent percutaneous transhepatic biliary drainage (PTBD), the distal biliary tract was stimulated by repeatedly inflating the balloon of the PTBD catheter so that it reached volumes that produced a definite painless sensation. The functional magnetic resonance imaging (fMRI) of the cortical response to biliary sensation was examined.
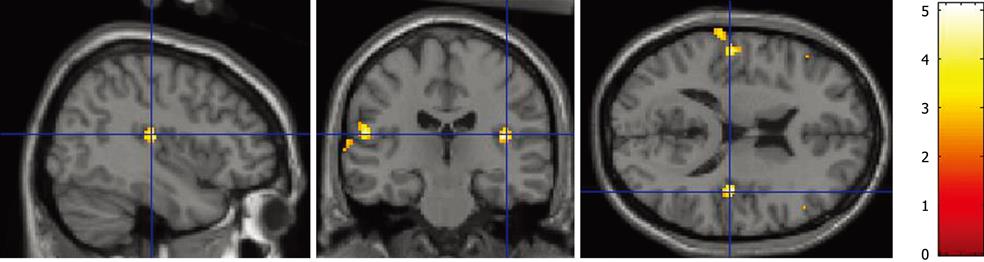
RESULTS: Biliary balloon stimulation elicited activation of the insular cortex, prefrontal cortex, and somatosensory cortex (P < 0.001).
CONCLUSION: Biliary balloon stimulation evoked a cerebral cortical response detectable by fMRI.
- Citation: Sai JK, Suyama M, Kubokawa Y, Matsumura Y, Inami K, Watanabe S, Kirino E. Identification of cerebral response to balloon distention of the bile duct. World J Gastroenterol 2010; 16(14): 1772-1775
- URL: https://www.wjgnet.com/1007-9327/full/v16/i14/1772.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i14.1772
Elevated pressure of the extrahepatic bile duct may elicit a variety of symptoms ranging from various degrees of abdominal distention, discomfort, dyspepsia, and pain. However, there is no information concerning the cerebral cortical response to sensory signals that originate in the biliary tract in humans. On the other hand, the cortical response to esophageal, stomach or rectal stimulation has been studied using several different modalities including evoked potentials[1], functional magnetic resonance imaging (fMRI)[2], and positron emission tomography[3].
In the present study, we used fMRI to investigate the cerebral cortical response to biliary stimulation in humans.
Six patients who underwent percutaneous transhepatic biliary drainage (PTBD) participated in this study after the study protocol was approved by the Institutional Review Board of Juntendo University, and the patients provided their informed consent. The study population consisted of 4 men and 2 women with an average age of 58 years (range: 42-74 years). The indication for PTBD in these patients was the presence of intrahepatic stones. A balloon-affixed 18 French PTBD catheter was used in every patient to perform lithotripsy of intrahepatic stones under percutaneous transhepatic cholangioscopy.
Bile duct distention was achieved using the balloon-affixed 18 French PTBD catheter. Before each study, the balloons were inflated in 1 mL increments to determine the volume of air necessary to induce a definite but painless sensation, and the condition was confirmed by magnetic resonance cholangiopancreatography beforehand. The balloon catheter was located at 4 cm proximal to the papilla of Vater and inflated to the desired diameter by manually injecting the predetermined volume of air. Balloon distention was done by the same investigator in all studies. The subjects were asked to confirm the sensation of distention before and after the fMRI study.
Functional MRI has been shown to accurately detect cortical activity with the use of the blood oxygenation level-dependent (BOLD) technique. The BOLD technique is based on the fact that deoxygenated and oxygenated blood have subtly different magnetic susceptibilities. This change in magnetic susceptibility alters the microscopic distribution of the magnetic field within a single MRI pixel and results in a change in signal intensity, greater oxygenation being associated with a brighter pixel contrast. Thus, the time course of gradient echo MRI images shows subtle changes in brightness in regions of altered oxygenation rate. Neuronal activity is associated with increased venous oxyhemoglobin levels; consequently, the anatomic location of the cortical regions that increase in brightness during application of a stimulus is associated with the sensation of that stimulus[2,4].
Magnetic resonance images were acquired using a 1.5-Tesla Toshiba VISART EX system with a standard quadrature head coil. BOLD imaging was performed using a field-echo echo planar imaging (EPI) sequence [repetition time (TR) = 2000 ms, echo time (TE) = 45 ms, matrix = 96 × 96, flip angle 70° and field of view (FOV) = 26 cm]. EPI-MRI images were acquired in the axial plane for 13 contiguous slices, 8 mm in thickness, covering the whole brain.
After positioning the subject, echo planar MRI images were captured as follows: for a total of 180 s were captured over a period of 180 s with three 60 s cycles (30 s rests alternated with 30 s periods of sustained balloon distention). In the same subjects, sham biliary balloon distention was performed for control.
The functional data processing was performed on personal computer (PC) workstations (Dynabook, Toshiba, Tokyo) using Statistical Parametric Mapping (SPM99) (Wellcome Department of Imaging Neuroscience) implemented in Matlab (Mathworks). We used SPM99 software to perform realignment for motion correction, normalization or deformation using the standard brain template from the Montreal Neurological Institute and conversion to the standard stereotaxic atlas of Talairach space and performing data analysis (using a threshold of P < 0.01) for individual subjects. A typical boxcar model convolved with a hemodynamic response of SPM was used. Data analysis was performed using the amplitude estimates (percent signal change) from the 6 patients.
The volume threshold of bile duct balloon distention at which a sensation of abdominal discomfort was induced was 4.5 ± 0.8 mL; the mean diameter of the balloon was 15.0 ± 1.7 mm, which was 159.0% ± 11.6% greater than the bile duct diameter before balloon dilation. All subjects reported they felt a definite painless sensation when the balloon was inflated, before and after fMRI scanning. Bile duct balloon distention produced a cortical fMRI response in all subjects. At the corrected P level of less than 0.01, the cortical response was mostly observed in the insular cortex, prefrontal cortex, and somatosensory cortex (Table 1 and Figure 1). The fMRI signal increased to an average maximum value of 4.1% ± 0.6% above the average baseline signal intensity.
| Subjects | Regions of activation | ||||
| OFC | Ins | PFC | S | POC | |
| 1 | B | L | |||
| 2 | B | R | |||
| 3 | B | ||||
| 4 | B | ||||
| 5 | L | B | B | ||
| 6 | B | R | |||
With sham biliary balloon distention, all subjects felt no sensation during, before and after fMRI scanning, and the fMRI signal was not observed in all subjects.
In this study we characterized the cerebral cortical response to bile duct balloon distention. The technique used in this study is based on measurable changes in magnetic signal intensity due to changes in cerebral blood oxygenation, and the percent average signal change in our study was 4.1% ± 0.6%; the percent change in signal intensity accepted as significant has been reported for simple motor tasks (4.3% ± 0.32%)[5], visual stimulation (3%-5%)[6], and psychotropic drug effects (2%-3%)[4].
The findings of the present study indicated that balloon stimulation of the bile duct at the definite sensation was relayed to the cerebral cortex and induced neuronal activity detectable by fMRI. Functional MRI technique has been previously used to study the cortical response to balloon dilation of the esophagus, stomach, and rectum[2,7-9]. The present findings are the first step in the evaluation of cerebral response to bile duct balloon distention, and show the possibility of the presence of pressure sensitive vagal afferents in the biliary tract and the cortical relay of these afferents beyond the brainstem.
The cerebral cortical response to biliary balloon distention was mostly observed in the insular cortex, prefrontal cortex, and somatosensory cortex. The spread of activation around these regions was comparable to that observed in a meta-analysis of studies on noxious somatic stimulation[10]. Furthermore, insular activation was found to be the most consistent finding in visceral stimulation research[9,11]. As described by Neafsey et al[12], electrical stimulation of the insula in rats, cats, dogs, monkeys, and human elicits changes in blood pressure, heart rate, respiration, piloerection, papillary dilation, gastric motility, peristaltic activity, salivation, and adrenalin secretion, and the insula is regarded as a key integrative visceral sensory area, mediating affective response to visceral stimulation[11].
Visceral hypersensitivity, a condition characterized by lower thresholds for discomfort, pain, or other sensations during intraluminal balloon distention, has been demonstrated by fMRI in patients with different functional gastrointestinal disorders including functional dyspepsia, irritable bowel syndrome, and noncardiac chest pain[8,9,13]. The present study suggests the possibility to detect hypersensitive conditions in functional biliary disorder using fMRI.
Potential weaknesses of this study include, first, the small number of patients. The current data need to be confirmed in larger groups of patients. Secondly, manual control of the volume used to induce biliary distension rather than barostat pressure controlled inflations was used due to difficulties with the use of a barostat in the MRI scanner room. A third limitation of this study was the introduction of the PTBD catheter that may cause not only emotional distress, but also vagal activation. The possible impact of this on registered brain activation patterns was minimized by allowing at least 2 wk between introduction of the PTBD catheter and the MRI scan. However, a certain amount of stimulation at baseline by the presence of the catheter is unavoidable[9,14].
In summary, biliary balloon stimulation, inducing painless distention, evokes a cerebral cortical response detectable by fMRI. Further functional imaging studies of biliary sensation are required to improve the confidence of interpretation of results.
Elevated pressure of the extrahepatic bile duct may elicit a variety of symptoms ranging from various degrees of abdominal distention, discomfort, dyspepsia, and pain. However, there is no information concerning the cerebral cortical response to sensory signals that originate in the biliary tract in humans.
In the present study, the authors used functional magnetic resonance imaging (fMRI) to investigate the cerebral cortical response to biliary stimulation in humans.
The present findings are the first step in the evaluation of cerebral response to bile duct balloon distention.
The present study suggests the possibility to detect hypersensitive conditions in functional biliary disorder using fMRI.
It is an interesting study. The authors are congratulated on making the first observation on the relay of mechanosensitive information from the biliary tract presumably via vagal afferents, the brain stem and on to specific regions of the cerebral cortex.
Peer reviewers: Jackie Wood, PhD, Department of Physiology and Cell Biology, College of Medicine and Public Health, The Ohio State University, 304 Hamilton Hall, 1645 Neil Avenue, Columbus, OH 43210-1218, United States; Dr. Kaye M Reid Lombardo, MD, Assistant Professor, Department of General Surgery, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, United States
S- Editor Wang JL L- Editor O’Neill M E- Editor Ma WH
| 1. | Castell DO, Wood JD, Frieling T, Wright FS, Vieth RF. Cerebral electrical potentials evoked by balloon distention of the human esophagus. Gastroenterology. 1990;98:662-666. |
| 2. | Kern MK, Birn RM, Jaradeh S, Jesmanowicz A, Cox RW, Hyde JS, Shaker R. Identification and characterization of cerebral cortical response to esophageal mucosal acid exposure and distention. Gastroenterology. 1998;115:1353-1362. |
| 3. | Aziz Q, Andersson JL, Valind S, Sundin A, Hamdy S, Jones AK, Foster ER, Långström B, Thompson DG. Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology. 1997;113:50-59. |
| 4. | Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155:1009-1015. |
| 5. | Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161-173. |
| 6. | DeYoe EA, Bandettini P, Neitz J, Miller D, Winans P. Functional magnetic resonance imaging (FMRI) of the human brain. J Neurosci Methods. 1994;54:171-187. |
| 7. | Kern MK, Shaker R. Cerebral cortical registration of subliminal visceral stimulation. Gastroenterology. 2002;122:290-298. |
| 8. | Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595-1601. |
| 9. | Vandenberghe J, Dupont P, Van Oudenhove L, Bormans G, Demyttenaere K, Fischler B, Geeraerts B, Janssens J, Tack J. Regional cerebral blood flow during gastric balloon distention in functional dyspepsia. Gastroenterology. 2007;132:1684-1693. |
| 10. | Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin. 2000;30:263-288. |
| 11. | Derbyshire SW. A systematic review of neuroimaging data during visceral stimulation. Am J Gastroenterol. 2003;98:12-20. |
| 12. | Neafsey EJ, Terreberry RR, Hurley KM. Anterior cingulate cortex in rodents: Connections, visceral control functions, and implications for emotion. Neurobiology of cingulate cortex and limbic thalamus: A comprehensive treatise. Boston: Birkhauser 1993; 206-223. |
| 13. | Bernstein CN, Frankenstein UN, Rawsthorne P, Pitz M, Summers R, McIntyre MC. Cortical mapping of visceral pain in patients with GI disorders using functional magnetic resonance imaging. Am J Gastroenterol. 2002;97:319-327. |
| 14. | Mohamed MA, Yousem DM, Tekes A, Browner N, Calhoun VD. Correlation between the amplitude of cortical activation and reaction time: a functional MRI study. AJR Am J Roentgenol. 2004;183:759-765. |