Published online Apr 14, 2010. doi: 10.3748/wjg.v16.i14.1735
Revised: January 20, 2010
Accepted: January 27, 2010
Published online: April 14, 2010
AIM: To observe the regional distributions and morphological features of nesfatin-1/nucleobindin-2 (NUCB2) immunoreactive (IR) cells in the rodent digestive system.
METHODS: Paraffin-embedded sections of seven organs (pancreas, stomach, duodenum, esophagus, liver, small intestine and colon) dissected from sprague-dawley (SD) rats and institute of Cancer Research (ICR) mice were prepared. The regional distributions of nesfatin-1/NUCB2 IR cells were observed by immunohistochemical staining. The morphological features of the nesfatin-1/NUCB2 IR cells were evaluated by hematoxylin and eosin (HE) staining. Fresh tissues of the seven organs were prepared for Western blotting to analyze the relative protein levels of NUCB2 in each organ.
RESULTS: Immunohistochemical staining showed that the nesfatin-1/NUCB2 IR cells were localized in the central part of the pancreatic islets, the lower third and middle portion of the gastric mucosal gland, and the submucous layer of the duodenum in SD rats and ICR mice. HE staining revealed that the morphological features of nesfatin-1/NUCB2 IR cells were mainly islet cells in the pancreas, endocrine cells in the stomach, and Brunner’s glands in the duodenum. Western blotting revealed that NUCB2 protein expression was higher in the pancreas, stomach and duodenum than in the esophagus, liver, small intestine and colon (P = 0.000).
CONCLUSION: Nesfatin-1/NUCB2 IR cells are expressed in the pancreas, stomach and duodenum in rodents. These cells may play an important role in the physiological regulation of carbohydrate metabolism, gastrointestinal function and nutrient absorption.
- Citation: Zhang AQ, Li XL, Jiang CY, Lin L, Shi RH, Chen JD, Oomura Y. Expression of nesfatin-1/NUCB2 in rodent digestive system. World J Gastroenterol 2010; 16(14): 1735-1741
- URL: https://www.wjgnet.com/1007-9327/full/v16/i14/1735.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i14.1735
Nesfatin-1, an anorexigenic peptide derived from nucleobindin-2 (NUCB2), is the latest addition to the surprisingly large number of chemical messengers telling the body that the “tank (appetite) is full”[1]. Nesfatin-1 injected directly into the brain of rats promotes anorexia, whereas an antibody specific to nesfatin-1 injected into the brain potently stimulates feeding[1]. Previous studies have shown that nesfatin-1/NUCB2 immunoreactive (IR) cells are present in a number of discrete neuronal populations or nuclei, including the hypothalamic arcuate nucleus (ARC), paraventricular nucleus (PVN), supraoptic nucleus, lateral hypothalamic area, and the dorsal motor nucleus of the vagus and nucleus tractus solitarius in the brain stem[1-5]. Energy homeostasis-regulating circuits are found within and connect these nuclei. NUCB2 gene expression and the nesfatin-1 concentration in the PVN were reduced after a fast of 24 h[1]. Nesfatin-1 alters the membrane potential of different subpopulations of neurons within the PVN[6]. In the ARC, nesfatin-1 exerts its anorexigenic effects by inhibiting the orexigenic neuropeptide Y neurons[7]. In mice, 5-hydroxytryptamine (5-HT) systems, via 5-HT2C receptors, upregulate the expression of hypothalamic NUCB2 and induce anorexia via a leptin-independent pathway[8]. These observations indicate a physiological role of central nesfatin-1 in the regulation of food intake.
Two different groups have demonstrated that peripherally injected nesfatin-1 crosses the blood-brain barrier in a non-saturable way to reach brain tissues[9,10]. Central and peripheral administration of nesfatin-1 resulted in a reduced food intake[1,11]. These observations, and the fact that various centrally active regulatory neuropeptides are also produced in the periphery, particularly the digestive system[12], raise the question as to whether peripheral sites also express nesfatin-1/NUCB2.
Therefore, in the present study, we investigated the regional distributions and morphological features of nesfatin-1/NUCB2 IR cells in various organs of the digestive system of sprague-dawley (SD) rats and institute of Cancer Research (ICR) mice, to lay a foundation for the further investigation of its functions in the digestive system.
All procedures strictly adhered to the guidelines of the Institution Council of Animal Care and were approved by the Ethics Committee of Nanjing Medical University.
Adult male SD rats, weighing 220-250 g, and adult ICR mice, weighing 22-25 g, were obtained from the Experimental Animal Center of Nanjing Medical University (China). Animals were housed in groups of three per cage under controlled illumination (12:12 h light/dark cycle, lights on/off: 6 h/18 h), humidity (60%) and temperature (22 ± 2°C). Animals were fed a standard rodent diet and tap water ad libitum.
Mouse anti-nesfatin-1 monoclonal antibody [1:40 000, diluted in 0.3% Triton X-100 in phosphate-buffered saline (PBS) for immunohistochemistry and 1:1000, diluted in Tris-buffered saline (TBS) in 5% (g/L) nonfat milk for Western blotting], was purchased from Alexis Biochemicals Company, USA. The SP Eivision™ plus kit used for immunohistochemistry, was purchased from Maixin_Bio Fuzhou, Co., Ltd., China. Mouse anti-β-actin monoclonal antibody [1:5000, diluted in TBS/5% nonfat milk for Western blotting], was purchased from Sigma-Aldrich, Co., Inc., USA. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody [1:5000, diluted in TBS/5% nonfat milk for Western blotting], was purchased from KPL Gaithersbug, MD, USA.
Animals (fed ad libitum) were euthanized at 09:00 am by intraperitoneal injection of 10% chloral hydrate anesthetic and the pancreas, stomach, duodenum, esophagus, liver, small intestine and colon were quickly resected and transferred to 4% paraformaldehyde and fixed at 4°C for 24 h. After being dehydrated through an ethanol-xylene series, the specimens were embedded in paraffin. Sections were cut (5 μm thick) and mounted on polylysine-coated slides.
Each representative section was immunostained using the streptavidin-peroxidase method[13]. Sections were de-waxed in xylene and dehydrated with a gradient alcohol series, then incubated for 20 min at 100°C in citrate buffer (pH 6.0) for antigen retrieval[14]. Endogenous peroxidase was blocked by incubating the sections with 0.3% hydrogen peroxide for 15 min and non-specific binding was reduced by pretreatment with 3% normal goat serum for 20 min. The sections were then incubated with primary antibody (mouse anti-nesfatin-1 monoclonal antibody) overnight at 4°C. After washing in PBS, the sections were incubated for 20 min at room temperature (RT) with polymer enhancer and then incubated with the secondary antibody (polymerized HRP-conjugated anti-mouse IgG antibody) supplied with the SP Eivision™ plus kit for 30 min at RT. The peroxidase reaction was carried out in 3,3’-diaminobenzidine tetrachloride solution (MaiXin_Bio, Fuzhou, Co., Ltd., China) for 2 min and regularly checked under a light microscope. After being lightly counterstained with Mayer’s hematoxylin for 1 min, the section was mounted on a slide, dehydrated with absolute alcohol followed by xylene, air-dried and coverslipped with Permount. Sections were observed and photomicrographed under a confocal microscope (Zeiss, AxioCam MRc5, Germany).
To investigate the specificity of the nesfatin-1 antibody under our conditions, the same protocol was applied for immunostaining after pre-absorption of the anti-nesfatin-1-antibody. Recombinant rat nesfatin-1 (50 μg ALX-522-116 Alexis Biochemical, USA) was incubated with mouse monoclonal anti-nesfatin-1 antibody at 1:40 000 in 0.3% Triton × in PBS for 2 h at RT followed overnight at 4°C. The solution was centrifuged for 15 min at 12 000 ×g and the supernatant was used for staining as described above, as a negative control. For the positive control, we used tissue sections obtained from the hypothalamus of three ad libitum-fed male SD rats, which had been confirmed to express the peptides of interest[1]. Cells expressing these peptides were colored brown.
The paraffin specimens used for immunohistochemical staining were also used for hematoxylin and eosin (HE) staining. Each section was de-waxed in xylene and dehydrated with a graded alcohol series. After being washed with distilled water for 3 min, the sections were counterstained with Mayer’s hematoxylin for 15 min, washed in distilled water for 3 min and incubated in the eosin staining solution for 3 min. Finally, the sections were mounted on a slide, dehydrated with absolute alcohol followed by xylene, air-dried and coverslipped with Permount. All specimens were observed and photomicrographed under a confocal microscope.
Tissue specimens from seven digestive organs of SD rats and ICR mice were kept on ice and ultrasonicated in the presence of one tablet of protease split cocktail [20 mmol/L Tris buffer pH 6.8, 4 mmol/L ethylene diamine tetraacetic acid pH 8.0 and 2% sodium dodecylsulfate (SDS)]. The crude protein fractions were obtained by centrifugation of the homogenates in a Sorvall centrifuge at 12 000 ×g for 20 min at 4°C to remove cell debris and nuclei. The supernatant was used as the protein fraction. The final protein concentrations of the protein fractions were determined using a BCA protein assay (Pierce Biotechnology, Rockford, IL, USA).
Gel samples were prepared by mixing protein samples with gel sample buffer [10% SDS, 0.05% bromophenol blue (g/L), 25% glycerol (g/L), 10% mercaptoethanol (mL/L) in 0.1 mmol/L Tris buffer pH 6.8]. The samples were boiled at 100°C for 5 min before gel electrophoresis. Forty micrograms of each protein sample was loaded on a 5%-10% SDS polyacrylamide gel and run in a running buffer [25 mmol/L Tris-HCl, 192 mmol/L glycine, 0.1% SDS (g/L)]. After SDS-polyacrylamide gel electrophoresis, the proteins were transferred by electrophoresis to polyvinylidene difluoride membranes (Roche Diagnostics Indianapolis, IN, USA) in a transfer buffer [48 mmol/L Tris-HCl, 39 mmol/L glycine, 0.037% SDS] for 60 min on ice. The membranes were washed twice with TBS [10 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.05% Tween-20 (mL/L)]. Non-specific binding sites were blocked by incubation in TBS containing 5% nonfat milk (Guangming, Co., Ltd., Shanghai, China) for 1 h at RT. The membranes were then incubated with the primary antibodies [mouse anti-nesfatin-1 monoclonal antibody (1:1000) and mouse anti-β-actin monoclonal antibody (1:5000)] overnight at 4°C. β-actin, a house-keeping protein, served as an internal control for the Western blotting. The next day, the membranes were washed three times with TBS and then incubated with the secondary antibody [HRP-conjugated goat anti-mouse IgG antibody (1:5000)] for 2 h at 37°C. After the membranes were washed three times in TBS, enhanced chemiluminescence detection of the target protein was performed using the ECL plus Western blotting detection system (Pierce Biotechnology, Rockford, IL, USA) and exposed using a Kodak autoradiography system (Kodak, Image Station 2000 mm, USA). Densitometry was performed using Kodak Molecular Imaging software. The relative protein levels of NUCB2 in each organ were represented as the density ratio vsβ-actin (NUCB2/β-actin). All Western blotting analyses were repeated at least three times.
Statistical analysis was performed using SPSS 11.5 software (SPSS Inc., Chicago, IL, United States). Data were expressed as means ± SD. Comparisons between groups were made using one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls (SNK) post hoc tests for multiple comparisons. P values less than 0.05 were considered statistically significant.
Nesfatin-1/NUCB2 IR, visualized as brown granules, was exclusively localized in the cytoplasm, but not in the nucleus or cell membrane. No brown granules were detected in the negative control sections stained with pre-absorbed anti-nesfatin-1-antibody, indicating that the immunostaining was specific for the target protein.
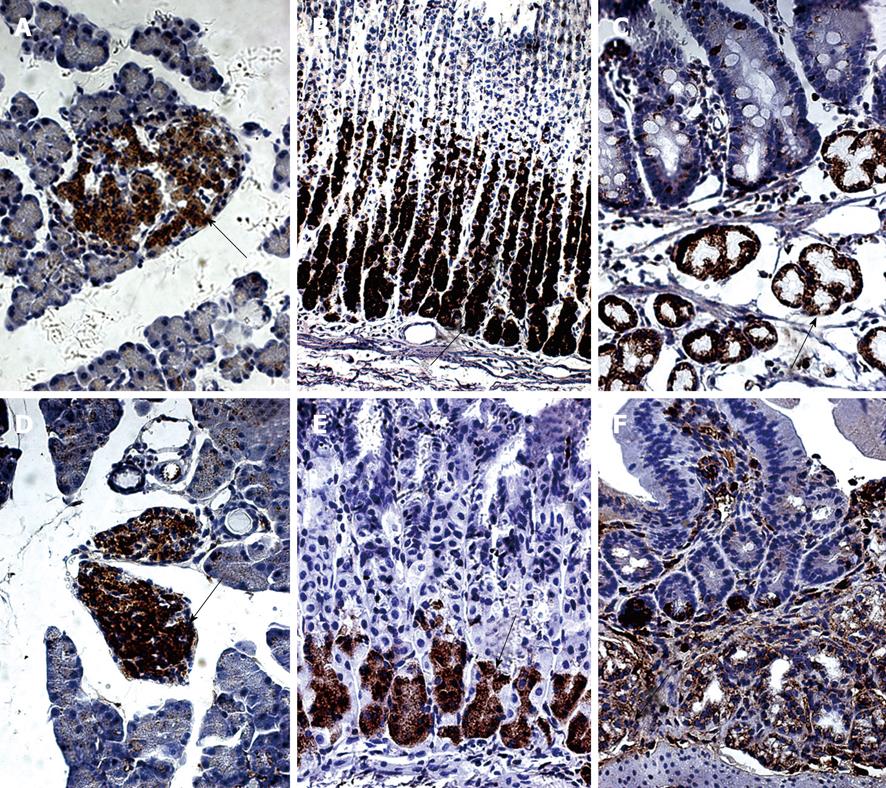
In the SD rats, nesfatin-1/NUCB2 IR cells were detected in the center of the pancreatic islets, but no immunopositive cells were detected in the pancreatic exocrine cells (Figure 1A). Nesfatin-1/NUCB2 IR cells were also found in the lower third and the middle portion of the gastric oxyntic glands (Figure 1B) and the submucosa of the duodenum (Figure 1C).
In the ICR mice, nesfatin-1/NUCB2 IR cells were also detected in the center of the pancreatic islets (Figure 1D). However, in the stomach, they were only localized in the lower third of the gastric oxyntic glands (Figure 1E). In the duodenum, nesfatin-1/NUCB2 IR cells were also expressed in the submucosal layer glands (Figure 1F). No nesfatin-1/NUCB2 IR cells were detected in the esophagus, liver, small intestine or colon in either the SD rats or in the ICR mice (data not shown).
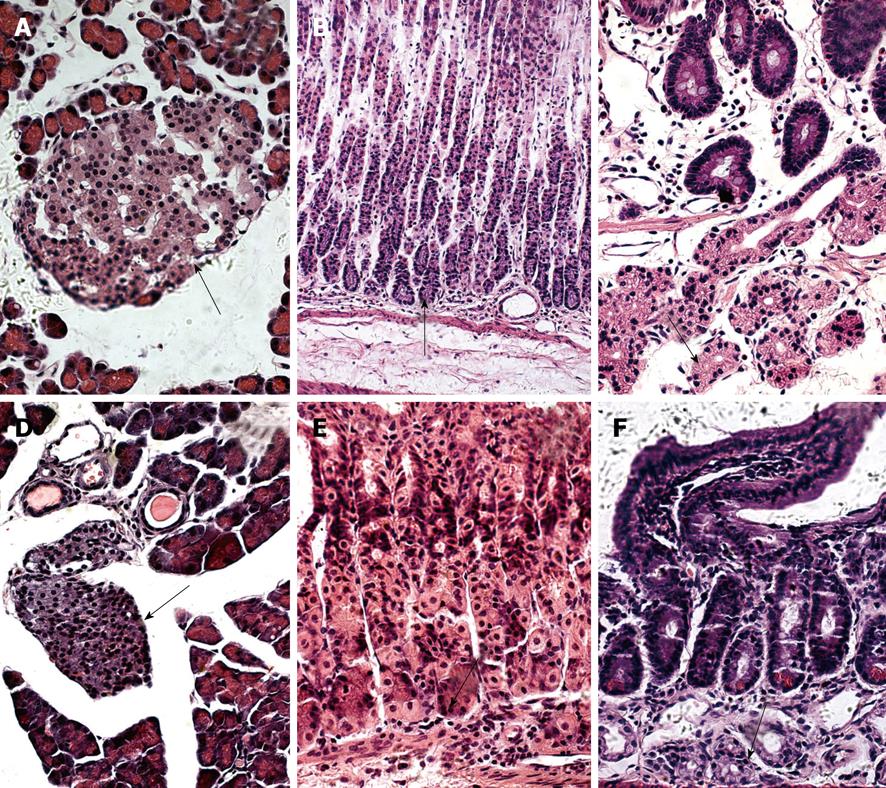
We further observed the features of nesfatin-1/NUCB2 IR cells in the SD rats (Figure 2A-C) and the ICR mice (Figure 2D-F) using HE staining. The IR cells in the pancreas were islet cells. In histological sections of the pancreas, the islets were seen as relatively pale-staining groups of cells embedded in a region of darker-staining exocrine tissues (Figure 2A and D). In the stomach, they were endocrine cells. These cells were round, oval, triangular, spindle-, shuttle- or flask-like in shape and located between the glandular cells and the basement membrane at the basal portion of the gastric oxyntic glands (Figure 2B and E). The submucosal glands in the duodenum were Brunner’s glands, which were compound tubular submucosal glands consisting of epithelial tubules with frequent distal branches (Figure 2C and F).
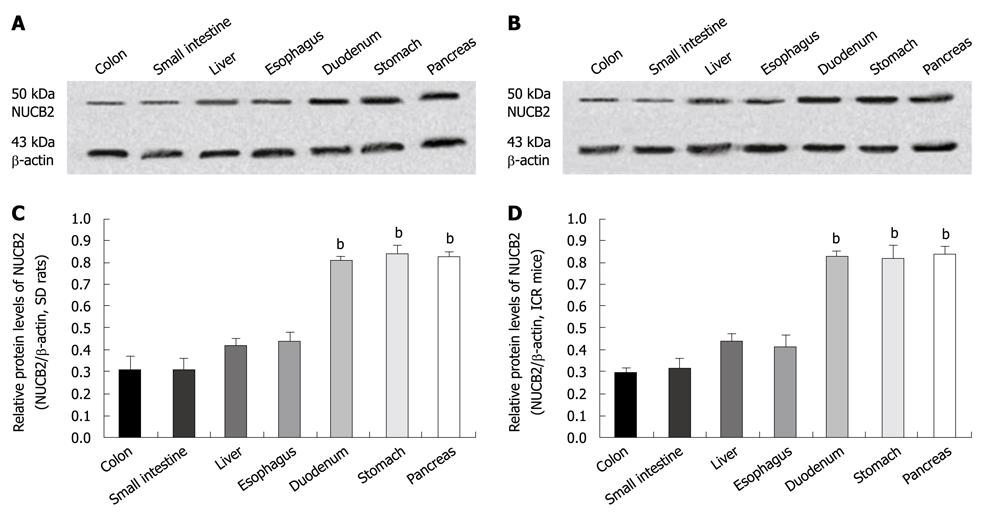
Western blotting of crude proteins of the seven digestive organs with the mouse anti-nesfatin-1 monoclonal antibody, which targets nesfatin-1 and its precursor NUCB2, showed a band corresponding to full-length NUCB2 protein (50 kDa). By contrast, no band corresponding to nesfatin-1 (9.7 kDa) was detected in either SD rats (Figure 3A) or the ICR mice (Figure 3B). In SD rats (n = 6), NUCB2 protein was highly expressed in the pancreas (relative expression; 0.82 ± 0.03), stomach (0.84 ± 0.04) and duodenum (0.80 ± 0.02), with lower expression in the esophagus (0.43 ± 0.05), liver (0.42 ± 0.03), small intestine (0.31 ± 0.05) and colon (0.31 ± 0.06) (Figure 3C). A similar pattern of expression was found in the ICR mice (n = 6), with high expression of NUCB2 in the pancreas (0.84 ± 0.03), stomach (0.82 ± 0.06) and duodenum (0.83 ± 0.02), and lower expression in the esophagus (0.42 ± 0.05), liver (0.43 ± 0.04), small intestine (0.31 ± 0.05) and colon (0.29 ± 0.02) (Figure 3D). The relative protein levels of NUCB2 in each immunopositive organ (pancreas, stomach and duodenum) were significantly higher than that in the immunonegative organs (esophagus, liver, small intestine and colon) in the SD rats and ICR mice (P = 0.000; ANOVA followed by SNK test).
Although the function of nesfatin-1/NUCB2 remains largely unknown, its sequence is highly conserved from rats and mice to humans, indicating an important biological role[1]. NUCB2 is proteolytically processed by pro-hormone convertase to nesfatin-1 (residues 1-82), nesfatin-2 (residues 85-163) and nesfatin-3 (residues 166-396). However, of these, only nesfatin-1 was effective in the reduction of food intake[1]. The initial reports by several researchers have described a wide distribution of nesfatin-1 in the central nervous system of rats. In the present study, our findings expand these reports to the digestive system of SD rats and ICR mice. We found an abundant distribution of nesfatin-1/NUCB2 IR cells in the pancreatic islets, gastric endocrine cells and the duodenal Brunner’s glands of the rodents. In particular, our results have revealed that nesfatin-1/NUCB2 IR cells are located in the submucosal Brunner’s glands of the duodenum, which has not been previously reported in rats[15]. Because the number of Brunner’s glands in the duodenum is relatively small, which are restricted to a small region of the duodenum, they may have been overlooked in the earlier studies. Thus, to the best of our knowledge, this is the first report to show the presence of nesfatin-1/NUCB2 IR cells in the Brunner’s glands of the duodenum. The Brunner’s glands are primarily confined to the duodenal bulb and their number gradually decreases to absent in the duodenojejunal flexure[16]. The main function of Brunner’s glands is to produce an alkaline secretion containing bicarbonate and mucus to: (1) protect the duodenum from the acidic content of chyme, which is introduced into the duodenum from the stomach; (2) provide an alkaline condition for the intestinal enzymes to be active, thus enabling absorption to take place; and (3) lubricate the intestinal walls[17]. Although the functional role of nesafatin-1/NUCB2 in the duodenum is currently unknown, the abundant expression of nesfatin-1/NUCB2 IR cells in the Brunner’s glands found in this study suggests that nesfatin-1/NUCB2 may be involved in the regulation of intestinal enzyme activation, nutrition absorption and preservation of the intestinal walls. To verify this hypothesis, further studies should focus on the molecular mechanism underlying nesfatin-1/NUCB2 IR cells in the Brunner’s glands to gain a basic understanding of their pathobiology.
The immunohistochemical analysis also showed abundant nesfatin-1/NUCB2 IR cells in the center of the pancreatic islets, but none in the pancreatic exocrine cells. The islets of Langerhans in the pancreas regulate blood glucose levels. Islets consist of four types of secretary cells: glucagon-producing α-cells, insulin-producing β-cells, somatostatin-producing δ-cells, and PP cells, which contain pancreatic polypeptide. Interestingly, the different cell types within an islet are not randomly distributed. β-cells occupy the central portion of the islet and are surrounded by a layer of α- and δ-cells[18]. In our experiment, we found that the nesfatin-1/NUCB2 IR cells were principally concentrated in the central region of the islets. Therefore, based on this localization, we speculated that they were β-cells. This is consistent with a recent study, which demonstrated that insulin-producing β-cells in mice and rats co-express nesfatin-1 immunoreactivity[19]. These findings suggest possible roles of nesfatin-1/NUCB2 in carbohydrate metabolism.
A recent study has shown the presence of nesfatin-1/NUCB2 IR cells in the stomach of rats[15]. Furthermore, the expression of NUCB2 mRNA in gastric endocrine cells is significantly down-regulated after a 24-h fast[15]. That study suggested a regulatory anorexigenic role of peripheral nesfatin-1/NUCB2 in energy homeostasis. Similarly, in the present study, we found abundant nesfatin-1/NUCB2 IR cells in the lower third and middle portion of the gastric oxyntic glands in SD rats but only in the lower third of the gastric oxyntic glands in ICR mice. These results suggest fine distinctions in species-specific expression patterns of nesfatin-1/NUCB2 in the rodent stomach. The morphological features of these cells were endocrine cells. Therefore, it is assumed that nesfatin-1/NUCB2 in the stomach may play an important role in the regulation of gastric functions.
In the present study, the 50-kDa NUCB2 protein was detected ubiquitously in all digestive organs, but the protein was only detected in appreciable amounts in the pancreas, stomach and duodenum by immunohistochemistry, which is consistent with the higher levels of NUCB2 protein detected in these three tissues by Western blotting. Nesfatin-1 consists of 82 amino acids and has a predicted molecular weight of 9.7 kDa[1]. Although the antibody we used in the Western blotting is targeted against the nesfatin-1 and its precursor NUCB2, the Western blottings of all tissues studied here only detected a single band of approximately 50 kDa representing NUCB2, but failed to show a band at 9.7 kDa. Therefore, as described by Stengel et al[15], the protein identified in this study was called nesfatin-1/NUCB2.
Taken together, the findings of this study demonstrate the presence of nesfatin-1/NUCB2 IR cells in the pancreas, stomach and duodenum but not in the esophagus, liver, small intestine or colon of the SD rats and the ICR mice and the relative protein levels of NUCB2 were significantly higher in pancreas, stomach and duodenum. Like many other feeding behavior regulatory peptides, nesfatin-1 was expressed both centrally and in the periphery. The broad distributions of nesfatin-1/NUCB2 in the digestive system suggest a possible regulatory role of peripheral nesfatin-1/NUCB2 in carbohydrate metabolism, gastrointestinal function and nutrition absorption. Going forward, it will be crucial to understand the site and mechanism of action for the anorectic signal mediated by nesfatin-1. Identifying a receptor or non-classical binding partner for nesfatin-1 will be an important first step in this journey.
In 2006, Oh-I and his colleagues discovered a new feeding inhibitory molecule, and named it nesfatin-1. They initially found the novel anorexigenic peptide expressed in a number of discrete neuronal populations or nuclei, which are involved in feeding behavior. Subsequently, several researchers have expanded the distributions of nesfatin-1 in the central nervous system. It is well known that various centrally active food regulatory neuropeptides are also produced in the periphery, particularly the digestive system. The authors investigated the regional distributions of nesfatin-1/ nucleobindin-2 (NUCB2) in various organs of the digestive system of sprague-dawley (SD) rats and institute of Cancer Research (ICR) mice.
Although the function of nesfatin-1/NUCB2 remains largely unknown, its sequence is highly conserved from rats and mice to humans, indicating an important biological role. The nesfatin-1 signaling pathway might be associated with the melanocortin signaling pathway in the hypothalamus. The hypothalamic leptin signaling pathway does not exist downstream of the pathway by which nesfatin-1 causes anorexia. Nesfatin-1 can cross the blood-brain barrier without saturation. Nesfatin/NUCB2-immunoreactivity was recently identified in the rat gastric mucosa. Systemic or local administration of nesfatin-1-related drugs may improve metabolic disorders by reducing the body weight of patients with obesity and metabolic syndrome.
This is the first study to report the expression of nesfatin-1/NUCB2 in the duodenal Brunner’s glands. Furthermore, a quantitative analysis with Western blotting studies showed higher protein levels of NUCB2 in the immunopositive organs than in the immunonegative organs.
By understanding where nesfatin-1/NUCB2 is expressed and by quantitatively analyzing its expression in the digestive system, this study may lay a foundation for the further investigation of its functions in the digestive system. In addition, this may help understand the relationship between the peripheral and the central nervous system.
The authors examined the expression of nesfatin-1/NUCB2 in seven organs of the digestive system in SD rats and ICR mice. The study not only confirmed that nesfatin-1/NUCB2 is expressed in the pancreas and stomach, but is also for the first time to reveal that nesfatin-1/NUCB2 is expressed in the duodenal Brunner’s gland. The authors conducted a quantitative analysis to determine the protein levels of NUCB2 in the seven organs. The results are interesting and may lay a foundation for the further investigations of its functions in the digestive system.
Peer reviewer: Naofumi Mukaida, MD, PhD, Chairperson and Professor, Division of Molecular Bioregulation, Cancer Research Institute, Kanazawa University, 13-1 Takara-machi, Kanazawa 920-0934, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
| 1. | Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709-712. |
| 2. | Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088-5094. |
| 3. | Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology. 2008;149:1295-1301. |
| 4. | Fort P, Salvert D, Hanriot L, Jego S, Shimizu H, Hashimoto K, Mori M, Luppi PH. The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience. 2008;155:174-181. |
| 5. | Foo KS, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563-579. |
| 6. | Price CJ, Hoyda TD, Samson WK, Ferguson AV. Nesfatin-1 influences the excitability of paraventricular nucleus neurones. J Neuroendocrinol. 2008;20:245-250. |
| 7. | Price CJ, Samson WK, Ferguson AV. Nesfatin-1 inhibits NPY neurons in the arcuate nucleus. Brain Res. 2008;1230:99-106. |
| 8. | Nonogaki K, Ohba Y, Sumii M, Oka Y. Serotonin systems upregulate the expression of hypothalamic NUCB2 via 5-HT2C receptors and induce anorexia via a leptin-independent pathway in mice. Biochem Biophys Res Commun. 2008;372:186-190. |
| 9. | Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides. 2007;28:2223-2228. |
| 10. | Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides. 2007;28:2372-2381. |
| 11. | Shimizu H, Oh-I S, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150:662-671. |
| 12. | Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116-2130. |
| 13. | Huang XG, Wu XB. Immunohistochemical study on gastrointestinal endocrine cells of four reptiles. World J Gastroenterol. 2005;11:5498-5505. |
| 14. | Shi SR, Cote RJ, Liu C, Yu MC, Castelao JE, Ross RK, Taylor CR. A modified reduced-temperature antigen retrieval protocol effective for use with a polyclonal antibody to cyclooxygenase-2 (PG 27). Appl Immunohistochem Mol Morphol. 2002;10:368-373. |
| 15. | Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Taché Y, Sachs G, Lambrecht NW. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232-238. |
| 16. | Gao YP, Zhu JS, Zheng WJ. Brunner’s gland adenoma of duodenum: a case report and literature review. World J Gastroenterol. 2004;10:2616-2617. |
| 17. | Krause WJ. Brunner’s glands: a structural, histochemical and pathological profile. Prog Histochem Cytochem. 2000;35:259-367. |
| 18. | Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334-2339. |
| 19. | Gonzalez R, Tiwari A, Unniappan S. Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem Biophys Res Commun. 2009;381:643-648. |