INTRODUCTION
Neuroendocrines tumors (NETs) of the small bowel account for 23%-28% of all gastroenteropancreatic (GEP) NETs and about 2% of all gastrointestinal (GI) tumors. Incidence rates of 0.28 to 0.8 per 100 000 population per year have been reported[1,2]. Their prevalence has increased in the last 3 decades as awareness and diagnostic techniques have improved[3]. A recent study showed a significant increase in their reported annual age-adjusted incidence from 1973 (1.09/100 000) to 2004 (5.25/100 000) in the US population[4]. Most of these tumors are well-differentiated and have an indolent course. As a consequence of the long delay between onset of symptoms and final diagnosis, many patients have advanced disease at the time of diagnosis. Jejuno-ileal (JI) NETs originate from the diffuse endocrine system (enterochromaffin cells), located in the GI tract that may produce serotonin. Clinical and biological features are heterogeneous[2]. This explains that classification is a critical point and is evolving. The recent World Health Organization (WHO) classification is based on clinical pathological criteria[5]. The WHO classification subdivides JI NETs, similar to other GEP endocrine tumors, into 3 general categories[5]: (1) well-differentiated endocrine tumor (carcinoid) of benign behavior (confined to the mucosasubmucosa, non-angioinvasive, < 1 cm in size) or uncertain behavior (non-functioning, confined to mucosasubmucosa, > 1 cm in size, or angioinvasive); (2) well-differentiated endocrine carcinoma (malignant carcinoma) with low-grade malignant behavior, deeply invasive (muscularis propria or beyond) or with metastases; and (3) poorly-differentiated endocrine carcinoma (small-cell carcinoma), high-grade malignant. However, because of its prognostic limits, a need for development of a TNM classification has been identified and such work is underway[6]. A proposal for a TNM classification for tumors of the lower jejunum and ileum, where T1-4 describes the size of the tumor (with T2 tumors > 1 cm) and the depth of involvement of the intestinal wall, was discussed. Stage I comprises T1 tumors with limited growth. Stage II identifies tumors that are larger in size or more invasive, i.e. T2 or T3, but without metastases. Stage III encompasses tumors invading surrounding structures (IIIA), T4, or the presence of regional lymph node metastases (IIIB), whereas stage IV indicates distant metastases. In the proposal, a grading system is also included, in which the G1 tumor category has a mitotic count < 2 or Ki-67 < 2%, G2 tumors a mitotic count 2-20 or Ki-67 2%-20%, and G3 tumors mitotic count > 20 or Ki-67 > 20%. Clinical features of JI NETs are heterogeneous: they can either remain asymptomatic for years, or occur with obstructive symptoms, or with liver metastases diagnosed by abdominal computed tomography (CT) or magnetic resonance imaging (MRI) for other purposes, or as incidental terminal ileum tumors by colonoscopy, or less frequently occur with symptoms related to hormonal hypersecretion (“functioning tumors”). The term “carcinoid” should be reserved for a characteristic clinical syndrome that results from the intermittent release of serotonin and other substances, such as tachykinins, prostaglandins and bradykinins, into the systemic circulation that occurs in about 10%-20% of patient with JI NETs[2]. Signs and symptoms of the carcinoid syndrome can include one or any of the following: flushing, diarrhea, carcinoid heart disease (CHD), and intermittent bronchoconstriction. These tumors are commonly metastases to the liver. Tumor size is an unreliable predictor of metastatic potential, and metastases can occur with primary tumors that are smaller than 1 cm in diameter[7].
Survival of JI NETs correlates closely with the stage of the disease at presentation with a 5-year survival of 65% in patients with localized or regional disease and 36% in those with distant metastases[8,9]. Pape et al[10] reported on 75 JI NETs, with more than 90% metastatic and 97% low-grade malignant; the 5-year survival rate was 90%. In addition, recent studies demonstrated the prognosis relevance of the WHO classification and the newly proposed TNM classification system for NETs[10-13].
Treatment should be highly individualized based on the diverse range of symptoms, general health of the patient, tumor burden, degree of uptake of radionuclide, histological features, and tumor growth. Critical assessment of every treatment option is difficult because of the complexity, heterogeneity, and rarity of GEP NETs. There is a paucity of relevant randomized trials. However, some different groups (Nordic NE Tumour Group[14,15], European Neuroendocrine Tumour Society[9,16], National Comprehensive Cancer Network guidelines in the United States, Canadian National Carcinoid Expert Group[17], United Kingdom NETwork[18]) have recently published guidelines for the management of NETs. The treatment of carcinoid syndrome by somatostatin analogs is the first priority. Then the minimally required imaging studies and biochemical tests to decide any treatment include chromogranin A and urinary 5-hydroxyindoleacetic acid (5-HIAA)[9]. Imaging studies are essential to localize the primary tumor as well as to guide management by staging, to monitor tumor growth, and for follow-up evaluation after therapy. The standard imaging procedures for JI NETs include abdominal ultrasonography, contrast-enhanced helical CT or MRI of the abdomen, pelvis and thorax, somatostatin receptor scintigraphy (SSRS), endoscopy, echocardiography, and bone scan or spine MRI to prove bone metastases if SSRS is negative[9]. Capsule endoscopy and double balloon enteroscopy can be useful to detect the intestinal primary tumors[9].
Management strategies include surgery for cure (which is rarely achieved) or cytoreduction, radiological interventions (by chemoembolization or radiofrequency ablation), chemotherapy and new biological agents, interferon, somatostatin analogs and peptide-receptor radionuclide therapy. Tumor growth is evaluated from clinical symptoms, repeated biological tests and imaging studies (every 3 to 6 mo), allowing decision-making between a curative treatment including aggressive treatment of metastatic disease, a palliative or symptomatic treatment, or a simple follow-up.
DIAGNOSIS AND TREATMENT OF CARCINOID SYNDROME
Presence of carcinoid syndrome, occurring in about 10%-20% of patients with JI NETs[2], must be treated as a priority. Urinary 5-HIAA has a sensitivity of 73% and a specificity of 100% in predicting the presence of a carcinoid syndrome in the midgut area[19]. 5-HIAA should be collected with strict dietary restrictions to avoid false positive levels. Somatostatin analogs remain the mainstay of symptomatic treatment for JI NETs. They could be started immediately in patients with inoperable disease or preoperatively in patients who have operable disease (liver resection with or without resection of the primary tumor). They are administered subcutaneously every 6-12 h. Long-acting formulations require infrequent administration and have contributed to an improved quality of life for patients. To date, the most effective formulations include long-acting octreotide (10, 20 or 30 mg) and lanreotide autogel (60, 90 or 120 mg), which are widely accepted as effective in controlling tumor-related symptoms in about 50%-80% of patients and in reducing serum concentration of tumor markers by 40%-60%[20]. These drugs are well tolerated and safe, with mild adverse effects and high tolerability after sustained use. However, tachyphylaxis and resistance to octreotide or lanreotide are known to occur. Toumpanakis et al[21] reported that in 17% of patients with loss of symptomatic response with the initial dose, symptoms were controlled by just an increase of somatostatin analogs dose, whilst the other patients required additional treatment (interferon, or transarterial hepatic embolization, etc.). Also, for all patients with a functioning carcinoid tumor, a potential carcinoid crisis should be prevented by prophylactic administration of octreotide, given by constant intravenous infusion at a dose of 50 mg/h for 12 h before and at least 48 h after surgery or other stress arising from invasive treatment (e.g. embolization, radiofrequency ablation)[22]. A phase II trial, reported in 2005, aimed to examine the efficacy of a novel somatostatin analog, pasireotide (SOM-230), in 45 patients with carcinoid syndrome who were refractory to octreotide therapy[23]. SOM-230 is a hexapeptide compound with 30 to 40 times higher binding affinity than octreotide to somatostatin receptors (SSR) subtype 1 and 5 and has a similar binding affinity to SSR 2[24,25]. Treatment with SOM-230 was well tolerated except for a few episodes of hyperglycemia. This trial established a 27% rate of symptom improvement in patients who switched from octreotide to SOM-230. A 2-stage, randomized vs placebo, multicenter study of SOM-230 followed by SOM-230 LAR in patients with malignant carcinoid tumors whose disease-related symptoms are inadequately controlled by somatostatin analogs is currently underway (http://www.clinicaltrials.gov).
In addition to the presence of SSR, the expression of dopamine D2 receptors in GEP NETs has recently been studied[26,27]. BIM23A760 is a new chimeric compound that selectively interacts with these receptors. The development of this drug is ongoing for the control of pituitary adenomas and Cushing’s syndrome[28,29] and a phase II study to assess the efficacy of this drug in patients with carcinoid syndrome is currently underway (http://http://www.clinicaltrials.gov). Another novel approach for the management of the carcinoid syndrome is LX1032, produced by Lexicon Pharmaceuticals. LX1032 is an orally bioavailable small molecule designed to inhibit peripheral serotonin synthesis[30]. Its use in a phase 1 clinical study of 87 subjects has been reported by the European Neuroendocrine Tumors Society[31]. A dose-dependent reduction in urinary 5-HIAA levels and whole blood serotonin concentration was observed. The development of LX1032 continues in a phase II clinical trial (http://http://www.clinicaltrials.gov). Patients not responding to somatostatin analogs may also be candidates for other therapeutic measures, such as debulking surgery, hepatic embolization, and radiofrequency ablation[32]. Other agents, such as loperamide or diphenoxylate for diarrhea and H1 or H2 blockers (or both) for histamine-secreting tumors may be administered as required.
Finally, patients with carcinoid syndrome should have an echocardiogram at diagnosis, permitting detection of cardiac involvement, which occurs in more than 50% of cases. The use of somatostatin analogs, titrated to manage symptoms or to normalize 5-HIAA levels, can help to prevent or minimize CHD, but CHD may continue to progress even if 5-HIAA is carefully controlled[33]. If CHD develops, heart failure rather than metastatic disease may be the cause of death. Medical therapy for heart failure should be introduced when necessary. Cardiac surgery with valvular replacement should be considered for patients with symptomatic CHD, which can significantly increase survival[34,35]. Cardiac surgery should be performed before major liver surgery or liver embolization.
ANTITUMOR TREATMENT
The aim of treatment should be curative whenever possible but is palliative in the majority of cases. These patients often maintain a good quality of life for a long period of time despite having metastases. Although the rates of growth and malignancy are variable, the aim should always be to maintain a good quality of life for as long as possible.
SURGICAL TREATMENT
The treatment of non-metastatic cases (stage I-III of the TNM classification) is based on a complete surgical resection to obtain a microscopic healthy margin (R0), the only way to significantly improve the 5-year survival rate[3]. However, in cases of retractile mesenteritis, metastatic disease or peritoneal carcinomatosis, removal of the primary tumor should still be considered as this might prevent subsequent local complications of small-bowel obstruction or mesenteric ischemia[9,36,37]. During laparotomy, a careful exploration of the entire abdominal cavity including the entire small bowel must be performed in order not to miss a second localization, which occurs in 30% of cases[3]. This surgery requires a lymphadenectomy as wide as possible toward the mesenteric artery origin, associated with a prophylactic cholecystectomy because of possible future treatment with somatostatin analogs or future arterial embolization of hepatic metastases. No positive phase III studies in an adjuvant situation are available. Given the rarity of these tumors and the poor results of anti-tumoral treatment in metastatic disease (chemotherapy, interferon), these tumors only necessitate forward monitoring, except in cases of trial inclusion.
Only 15% of patients had unilobar or bilobar liver metastases without extrahepatic spread. These patients should be assessed for the possibility of aggressive approaches with curative surgical resection. The 5-year survival rate is around 60% in patients who undergo resection of liver metastases compared with 30%-40% in patients with unresected liver metastases[38,39]. However, the recurrence risk is high, increasing from 55% to 84% within 5 years. Hepatic metastasis resection should be proposed for fit patients who have no extra-hepatic metastasis or tricuspid valve deficiency, and when complete resection of the primary tumor is possible. Concerning synchronous metastases, surgical management should be discussed according to several parameters including general health, number, size, and localization of liver metastases. Radiofrequency ablation can also be performed either before or during surgery[40]. A 2-stage hepatectomy can be proposed in patients with liver metastases in both lobes. The first step associates the resection of the primary tumor with the resection of the left liver metastases (+/- radiofrequency ablation) and the ligature of the right branch of the portal vein. The second step consists of a right hepatectomy 4 or 6 wk later, when left liver hypertrophy is obtained: the hepatectomy can be extended to segment I and IV[41]. As liver metastatic recurrences are nearly systematic in the course of the disease, major hepatectomy must be weighted against medical or isotopic therapy. In rare individuals such as young patients with no extra-hepatic metastasis and low Ki67, a liver transplantation may be proposed. The 5-year survival rate is about 45%, with a 5-year survival rate without recurrence of around 25%[42].
Palliative resection should also be considered in patients who remain symptomatic despite the use of medical therapy if more than 90% of the tumor load can be removed, as this can achieve survival benefit and good symptom control[16,38]. Liver metastasis cytoreduction can also be required in the case of a voluminous compressive mass (gastric compression).
RADIOLOGICAL TREATMENT
Hepatic artery embolization and transarterial chemoembolization
Many patients with JI NETs have liver metastases at the time of diagnosis, most of them being hypervascular. Selective hepatic trans-catheter arterial embolization (TAE) or chemoembolization (TACE) may be used to treat liver metastases in patients where surgery is not feasible. These modalities are effective in the control of symptoms and tumor growth. The intra-arterial injection of a cytotoxic drug, such as doxorubicin, streptozotocin, cisplatin or mitomycin C, is administered together with non-polar contrast[43-47]. Embolization with gelatine sponge particles or microspheres is used until evidence of a marked decrease in blood flow. Although TACE has been used for 20 years, no current evidence exists that TACE is superior to TAE. TACE has been proved to be effective in symptom relief in 63%-100% of patients[43,44,48-51]. Long-term palliation can be achieved with repeated TACE sessions[43]. Objective tumor response rates are noted in 33%-86% of patients[43,44,48-51]. Median time to progression is about 15 mo; 5-year survival is about 50%[40]. Contraindications for embolization are complete portal vein obstruction, liver insufficiency, and previous biliary reconstruction. Concomitant antibiotics and somatostatin analogs are used to avert a carcinoid crisis and diminish the possibility of hepatic abscess. Adverse events include a post-embolization syndrome (nausea, fever, elevated liver enzymes, and abdominal pain) which occurs in 90% of patients. Major side effects, including acute liver or renal failure, carcinoid crisis, cholecystitis, or bleeding peptic ulcers, occur in about 10% of patients. Treatment-related deaths are very rare. More recently, the radiocontrast agent lipiodol has become available with 131I instead of cold iodine, permitting embolization with radioactive material. Radioembolization using resin 90Y-microspheres is also currently increasingly used. It can deliver high doses of radiation preferentially to hepatic metastases of NETs. In comparison to published reports of other local treatments of liver metastases from NETs, radioembolization shows a similar safety profile and improvement in debulking of tumor and survival[52,53]. Another interesting modality of TACE is to use drug-eluting beads (DEBs). de Baere et al[54] reported in 20 patients that TACE plus DEBs loaded with 100 mg doxorubicin were well tolerated and appeared effective. This new procedure may increase the necrosis and reduce side effects from chemotherapy. Comparative studies with standard TAE, TACE, radioembolization and TACE with DEBs are warranted to define the best protocol for transarterial treatment of NET liver metastases. In addition, as not all patients are responsive to TAE or TACE, better selection criteria are still needed to obtain better responses to the procedure.
Radiofrequency ablation
Radiofrequency ablation has been used percutaneously or laparoscopically[55], with some effect in reducing tumor size, but randomized trials are lacking. Response rates from 80% to 95% are reported. It may be indicated in patients with inoperable bilobar metastases in whom hepatic artery embolization has failed[56]. Radiofrequency ablation can be used to reduce hormone secretion if at least 90% of the visible tumor can be destroyed or to reduce tumor burden. The main limitation for radiofrequency ablation is the size (only tumors < 3-5 cm in diameter should be treated) and number of tumors (less than 3-5 tumors should be treated per session), which are often small, and numerous in NETs. Radiofrequency ablation morbidity is low.
MEDICAL TREATMENT
Somatostatin analogs
Although the inhibitory effects of octreotide on hormonal secretion are well established, the effects on cell proliferation and tumor growth are somewhat controversial. However octreotide has not yet been registered in any country as an antitumor agent. Their effects on tumor growth are limited: less than 5% of patients have objective radiological tumor regression, although 35%-70% of patients have stabilization of tumor size[57-65]. The first placebo-controlled, double-blind, phase III study on the effect of octreotide LAR (long-acting release) in the control of tumor growth in patients with well-differentiated metastatic midgut NETs has been reported recently[66]. The median time to tumor progression in the octreotide LAR and placebo groups was 15.6 and 5.9 mo (hazard ratio = 0.34, P = 0.000072), respectively. However, only a limited group of patients-those with less than 10% tumor mass in the liver along with resected primary tumors-responded to treatment[67]. Therefore the effectiveness of octreotide LAR for control of growth in patients with a larger tumor burden is unproven. Also, we did not know whether patients had progressive disease at the beginning of the treatment. Another similar multicenter, placebo-controlled European Phase III study is currently underway to assess whether lanreotide autogel prolongs time to disease progression in patients with non functioning GEP-NETs (http://www.clinicaltrials. gov).
Interferon
Interferon-α is given for the same indications as somatostatin analogs, with the exception of carcinoid crisis. A biochemical response and symptomatic improvement could be noted in 40%-60% and 49%-70% of patients, respectively, whereas partial tumor size responses could be demonstrated in 10%-15%[9,68,69]. The duration of response was 12 to 36 mo. Because of more pronounced side effects, interferon is generally used as second-line therapy for symptomatic control[9]. Interferon, usually recombinant interferon-α, is given subcutaneously at 3-5 MU 3-5 times per week. A pegylated formulation, given once a week is available but not yet registered[70]. Minor side effects include flu-like symptoms, weight loss and fatigue. Major side effects include autoimmune reactions, depression and mental disturbances. Bone marrow toxicity is usually mild as is hepatotoxicity, which can be managed by dose adjustments. There is no evidence that addition of interferon to somatostatin analogs increases the tumor response[71,72]. However patients progressing on monotherapy of either drug may benefit from the addition of the other[9].
Peptide receptor radionuclide therapy
Most JI NETs express SSR, especially subtype 2. Targeting these receptors with radiolabeled somatostatin analogs may not only be used for imaging, but also for radiotherapy. Since the early 1990s, different radiolabeled analogs have been used for tumor-targeted therapy. For metastatic disease with evidence of avid uptake on Octreoscan™, radionuclide targeted therapies (for example, 131I metaiodobenzylguanidine, 90Y or 177Lu labeled somatostatin analogs for peptide receptor targeted therapy) show promising results[73]. Randomized studies of peptide receptor radionuclide therapy (PRRT) are lacking, making comparison of published data difficult. A symptomatic response in up to 80% of patients, a partial tumor response in up to 35% of patients and a disease stabilization in up to 56% of patients have been reported. Side effects are limited as long as radiation dose to the kidney and bone marrow are kept within dose limits; the use of kidney protection by co-infusion of amino acids (lysine and arginine) allows the administration of higher doses of the radiopharmaceuticals[9]. The most recent data indicate a partial or complete response in 28% of patients with a median time to progression of more than 36 mo for Lutate[74,75]. These radiopharmaceuticals are only available in a few centers. PRRT is recommended in SSRS-positive tumors in symptomatic patients refractory to medical treatment with inoperable disease.
External radiation therapy
Carcinoid tumors have often been regarded as being radioresistant. However, external beam radiotherapy is recommended for some brain and symptomatic bone metastases.
Traditional chemotherapy
Although pancreatic NETs seem to be moderately sensitive to systemic cytotoxic chemotherapy, well-differentiated JI NETs are generally considered to be chemoresistant. Single agents [5-fluorouracil (5-FU), doxorubicin, DTIC] and combinations (streptozotocin + doxorubicin or 5-FU) generally produce responses in less than 15% of patients[76-79]. Dahan et al[80] reported no significant difference in progression-free survival and overall survival between interferon and 5-FU plus streptozotocin (even a trend in favor of interferon) in a phase III randomized trial in patients with metastatic carcinoid tumors. Based on the activity of dacarbazine and 5-FU in NETs[81,82], there is a rationale for the study of the oral agents temozolomide, which is converted as dacarbazine into the active alkyling agent MTIC[83-85] and capecitabine (an oral pro-drug for 5-FU)[86]. Kulke et al[84] reported on the combination of temozolomide with thalidomide in a variety of NETs, documenting an objective response rate of 45% in pancreatic NETs vs only 7% in carcinoid tumors. Interestingly, the response to temozolomide-based therapy appeared to be correlated with 06-methylguanine DNA methyltransferase deficiency[87]. Combining capecitabine and temozolomide may be an interesting association in patients with NETs[88]. Oxaliplatin seems to be also an interesting drug in NETs[86,89]. Even if some new drugs are coming, there is a general consensus that chemotherapy with agents available today is not recommended in patients with well-differentiated JI NETs[9,36].
Angiogenesis inhibitors and other novel targets
Currently, there are a number of new drugs undergoing evaluation in the clinic. These could be divided into 3 groups as follows: (1) drugs targeting vascular endothelial growth factor (VEGF), such as the VEGF monoclonal antibody bevacizumab and a more recent related compound, VEGF-trap; (2) small molecules that inhibit the intracellular tyrosine kinase domain of vascular endothelial growth factor receptor or other growth factor receptors, such as sunitinib, sorafenib, imatinib and valatanib; and (3) other compounds inhibiting different signaling pathway components such as epithelial growth factor receptor, insulin-like growth factor 1 receptor, phosphoinositide-3-kinase, RAC-α serine/threonine-protein kinase (AKT), and mammalian target of rapamycin (mTOR).
The first reported phase II trial of bevacizumab in NETs was performed in 44 patients with advanced carcinoid tumors. Patients were randomly assigned to 18 wk of treatment with either octreotide plus bevacizumab or octreotide plus pegylated interferon[90]. At week 18, the progression-free survival rate was 96% in the group receiving bevacizumab vs only 68% in the group receiving pegylated interferon. Moreover, a rapid reduction in blood tumor perfusion measured by functional CT scan was demonstrated in the bevacizumab-treated patients. Based on the promising results in the bevacizumab study, a confirmatory randomized phase III trial is underway by the Southwest Oncology Group, comparing octreotide plus bevacizumab or interferon in advanced carcinoid tumors, with progression-free survival as the primary endpoint. The experience with bevacizumab in other solid tumors such as colorectal cancer has shown that its addition to chemotherapy can significantly improve outcome, whereas it has very little clinical activity as a single agent. The combination of temozolomide and bevacizumab has recently been reported in a small phase II trial with 0% of objective response rate in carcinoid tumors vs 24% in pancreatic NETs[91]. Currently, different combination are ongoing: a phase I-II trial of FOLFOX plus bevacizumab in refractory carcinoid and pancreatic endocrine tumors in the USA; a phase II trial of capecitabine plus bevacizumab in chemotherapy-naive patients with carcinoid tumors in France; a phase II trial of bevacizumab plus 2-methoxyestradiol (Panzem) in patients with locally advanced or metastatic carcinoid tumors (http://www.clinicaltrials.gov).
Similarly, a multi-institutional phase II study of sunitinib, a novel tyrosine kinase inhibitor with activity against VEGFR-1 to 3, PDGFR, FLT-3, c-Kit and RET, was also conducted among patients who had advanced NETs[92]. In this trial on 66 patients with pancreatic NETs and 41 patients with carcinoid tumors[92], the treatment was well tolerated and a partial response occurred in 11 (17%) of pancreatic NETs and 1 (2%) of carcinoid tumors. Rates of stabilized disease were high in both groups (68% in pancreatic NETs and 83% in carcinoid tumors). Raymond et al[93] reported the first phase III randomized, double-blind study, with the efficacy of sunitinib given daily as a continuous dose vs placebo in patients with advanced islet cell tumors. The median disease-free survival was 11.1 mo in the sunitinib group vs 5.5 mo in the placebo group. No similar phase III trial is ongoing for carcinoid tumors. In addition, a pending phase II trial will investigate the use of sunitinib after TAE in the treatment of NETs with liver-predominant metastases. The purpose of the trial will be to assess whether angiogenesis inhibition assists in prolonging the time to disease progression following liver embolization. Previously, use of sorafenib, an oral and multitarget agent with potent activities against VEGFGR-3, PDGFR-b, FLT-3, c-Kit and fibroblast growth factor receptor-1, has also been reported in 50 carcinoid tumors, but with more than 40% of grade 3-4 toxicity[94]. Other trials testing different tyrosine kinase inhibitors are currently underway (e.g. vatalanib).
The third approach for new drugs has focused on inhibiting different signaling pathway components. For example, mTOR is an intracellular serine/threonine kinase that acts as a central regulator of multiple signaling pathways (IGF-I, EGF, VEGF) that participates in the regulation of apoptosis, angiogenesis, proliferation and cell growth through modulation of cell cycle progression[95]. Two rapamycin derivatives have recently been evaluated in NETs: temsirolimus[96] and everolimus[97,98]. The results of a first phase II study combining everolimus (RAD001) at 5 or 10 mg orally daily and depot octreotide at 30 mg intramuscularly every 28 d in low-grade NETs have been recently reported[97]. Of the 60 patients, there were 30 with carcinoids and 30 with islet cell carcinomas. Toxicities were mild to moderate, including those expected from everolimus such as stomatitis and myelosuppression. Tumor response rates were higher in the group with RAD001 at the 10 mg dose level vs the 5 mg dose level, and in the islet cell carcinoma (27%) vs the carcinoid tumor (17%) group[97]. Tumor response was low, but control of disease was very promising (97% in the carcinoid tumor group). Based on these encouraging results, 3 large trials were sponsored by Novartis (RADIANT-1,-2,-3). RADIANT-1 is an open-label, stratified, single-arm phase II study of RAD001 in patients with advanced pancreatic NETs after failure of cytotoxic chemotherapy[98]. Two further phase III, placebo-controlled, randomized trials in patients receiving depot octreotide recently closed: RADIANT-2 in patients with advanced carcinoid tumors and RADIANT-3 in patients with advanced pancreatic islet cell tumors. In contrast, interest in EGF receptor inhibitors, such as gefitinib, on carcinoid tumors seems to be poor[99].
ALGORITHMS OF TREATMENT IN PATIENTS WITH JI WELL-DIFFERENTIATED ENDOCRINE CARCINOMAS
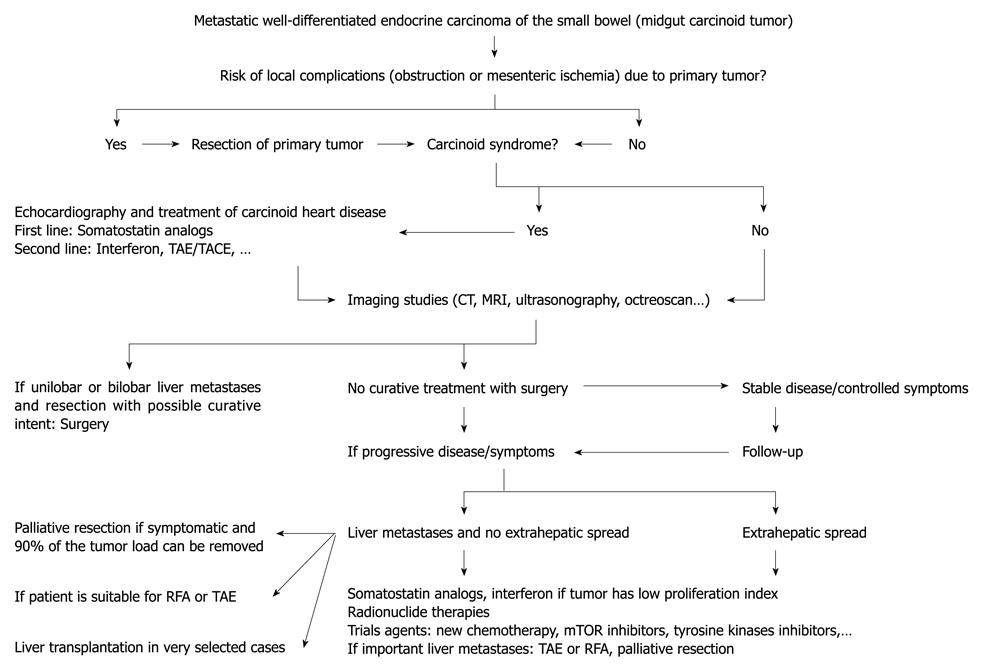
Recently, management algorithms have been proposed by several experts[16-18,37,100]. Treating these tumors needs collaboration of different types of physicians including endocrinologists, gastroenterologists, oncologists, interventional radiologists, pathologist, and surgeons. Only a complete surgical resection permits cure of localized well-differentiated endocrine carcinoma of the small bowel (stage I-III of TNM classification). After curative surgery, there is no indication for medical therapy other than pre- and peri-operative somatostatin analogs to avoid a carcinoid crisis (Figure 1). The strategy for treatment of metastatic tumors (stage IV of TNM classification) is more complicated and varies depending on tumor spread, general health of the patient, predominant symptoms, and tumor growth (Figure 2). Somatostatin analogs remain the primary treatment for carcinoid syndrome. Since the PROMID study, they may be also considered for asymptomatic patients with low hepatic tumor load. TAE, TACE, radioembolization or TACE with DEBs are indicated for patients with non-resectable multiple metastases and persistence of carcinoid syndrome despite treatment with somatostatin analogs, with the intention of reducing tumor size and hormone output. In patients with disabling carcinoid syndrome who are still refractory, interferon is frequently used in second-line treatment; the novel somatostatin analog SOM-230 could be useful in the near future for palliation of carcinoid syndrome[101]. Perhaps the most promising avenue of research is the field of PRRT. Moreover, it is very probable that angiogenesis inhibitors and therapy targeting mTOR will become a new standard treatment for these vascular malignancies; however, as in pancreatic NETs with sunitinib, randomized phase III trials will be needed to demonstrate improvements in time to progression or overall survival.
Figure 1 Management algorithm for patients with non metastatic well-differentiated endocrine carcinoma of the small bowel (stage I-III of TNM classification).
5HIAA: 5-hydroxyindoleacetic acid.
Figure 2 Management algorithm for patients with metastatic well-differentiated endocrine carcinoma of the small bowel (stage IV of TNM classification).
TAE: Transarterial embolization; TACE: Transarterial chemoembolization; RFA: Radiofrequency ablation; mTOR: Mammalian target of rapamycin; CT: Computed tomography; MRI: Magnetic resonance imaging.
CONCLUSION
Finally, the development of centers of excellence and NET clinical teams to coordinate multicenter studies, extend clinical and tissue databases, and ultimately develop molecularly targeted therapeutics are needed to advance treatment and survival for patients with GEP NETs.
Peer reviewers: Run Yu, MD, PhD, Division of Endocrinology, Diabetes, and Metabolism, Cedars-Sinai Medical Center, 8700 Beverly Blvd, B-131, Los Angeles, CA 90048, United States; Robert Jensen, MD, Digestive Disease Branch, National Institutes of Health, Building 10, Rm 9C-103, Bethesda, MD 20892, United States
S- Editor Wang JL L- Editor Cant MR E- Editor Ma WH