Published online Apr 7, 2010. doi: 10.3748/wjg.v16.i13.1631
Revised: November 30, 2009
Accepted: December 6, 2009
Published online: April 7, 2010
AIM: To evaluate the role of thymidine phosphorylase (TP) in cholangiocarcinoma using small interfering RNA (siRNA).
METHODS: A human cholangiocarcinoma-derived cell line KKU-M139, which has a naturally high level of endogenous TP, had TP expression transiently knocked down using siRNA. Cell growth, migration, in vitro angiogenesis, apoptosis, and cytotoxicity were assayed in TP knockdown and wild-type cell lines.
RESULTS: TP mRNA and protein expression were decreased by 87.1% ± 0.49% and 72.5% ± 3.2%, respectively, compared with control cells. Inhibition of TP significantly decreased migration of KKU-M139, and suppressed migration and tube formation of human umbilical vein endothelial cells. siRNA also reduced the ability of TP to resist hypoxia-induced apoptosis, while suppression of TP reduced the sensitivity of KKU-M139 to 5-fluorouracil.
CONCLUSION: Inhibition of TP may be beneficial in decreasing angiogenesis-dependent growth and migration of cholangiocarcinoma but may diminish the response to 5-fluorouracil chemotherapy.
- Citation: Thanasai J, Limpaiboon T, Jearanaikoon P, Sripa B, Pairojkul C, Tantimavanich S, Miwa M. Effects of thymidine phosphorylase on tumor aggressiveness and 5-fluorouracil sensitivity in cholangiocarcinoma. World J Gastroenterol 2010; 16(13): 1631-1638
- URL: https://www.wjgnet.com/1007-9327/full/v16/i13/1631.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i13.1631
Cholangiocarcinoma is a common hepatobiliary malignancy among the Northeastern Thai population and it is an important public health problem because the incidence and fatality rates are high[1,2]. Liver fluke (Opisthorchis viverrini) infection is a risk factor for cholangiocarcinoma, which accounts for about 89% of all liver cancer cases in Khon Kaen province, where the liver fluke is highly endemic and the incidence of cholangiocarcinoma is the highest in the world (97.4 per 100 000 males and 39 per 10 0000 females)[1]. A number of different genes have been implicated in carcinogenesis of cholangiocarcinoma, e.g. p53[3], MDM2[3], hMLH1 and hMSH2[4,5], and TFF[6]. We previously demonstrated the high prevalence of thymidine phosphorylase (TP) gene amplification (53.8%; 35 of 65 cases) in cholangiocarcinoma tumor tissues, suggesting that TP may play an important role in carcinogenesis of liver fluke-related cholangiocarcinoma[7].
TP (EC 2.4.2.4) is located in chromosomal region 22q13.33 and encodes a protein that catalyzes the reversible phosphorolysis of thymidine, deoxyuridine, and their analogs to their respective bases and 2-deoxyribose-1-phosphate, which is then dephosphorylated to 2-deoxy-d-ribose[8]. TP has been proposed to function in DNA synthesis, cell growth, chemotaxis stimulation in endothelial cells in vitro, and to enhance angiogenesis in vivo[9,10]. TP is abnormally expressed in certain cancers of the gastrointestinal tract e.g. pancreatic cancers[11], colon carcinomas[12], gallbladder adenocarcinomas[13], and intrahepatic cholangiocarcinoma[14]. Interestingly, TP expression was found to be elevated from 10- to 260-fold in nearly all biopsies examined from carcinomas of the stomach, colon, ovary, and bladder when compared to non-neoplastic regions of these organs[15,16]. Nevertheless, how TP expression is upregulated in human tumors remains unclear.
During the past few years the utility of RNA interference (RNAi) and post-transcriptional gene silencing has significantly advanced the study of the effects of loss of individual gene functions. Therefore, we investigated the functions and roles of TP in carcinogenesis of liver fluke-related cholangiocarcinoma using small interfering RNA (siRNA) to suppress TP expression. Understanding the functions of TP involved in cholangiocarcinoma will help us elucidate the molecular mechanisms of cholangiocarcinogenesis and thus could potentially provide important strategies for prevention, early diagnosis, and early treatment to reduce the cancer incidence and increase cholangiocarcinoma patient survival.
The KKU-M139 cell line was established from the primary tumor of a 53-year-old Thai woman with squamous cell type cholangiocarcinoma. It was selected for this work after prior screening of TP expression in 5 cholangiocarcinoma-derived cell lines (data not shown). KKU-M139 was maintained in RPMI-1640 medium containing 100 IU/mL penicillin, 100 μg/mL streptomycin, and 100 mL/L heat-inactivated fetal bovine serum (FBS) as a complete medium. The human umbilical vein endothelial cells (HUVECs) isolation procedure followed the protocol of Jaffe et al[17]. HUVECs were maintained in M199 medium containing 100 U/mL penicillin, 100 μg/mL streptomycin, 50 mL/L pooled human serum, and 300 mL/L heat-inactivated FBS. Culturing was carried out at 37°C in a humidified 50 mL/L CO2 incubator. Confluent HUVEC monolayers (passages 2-4) were used in the assays.
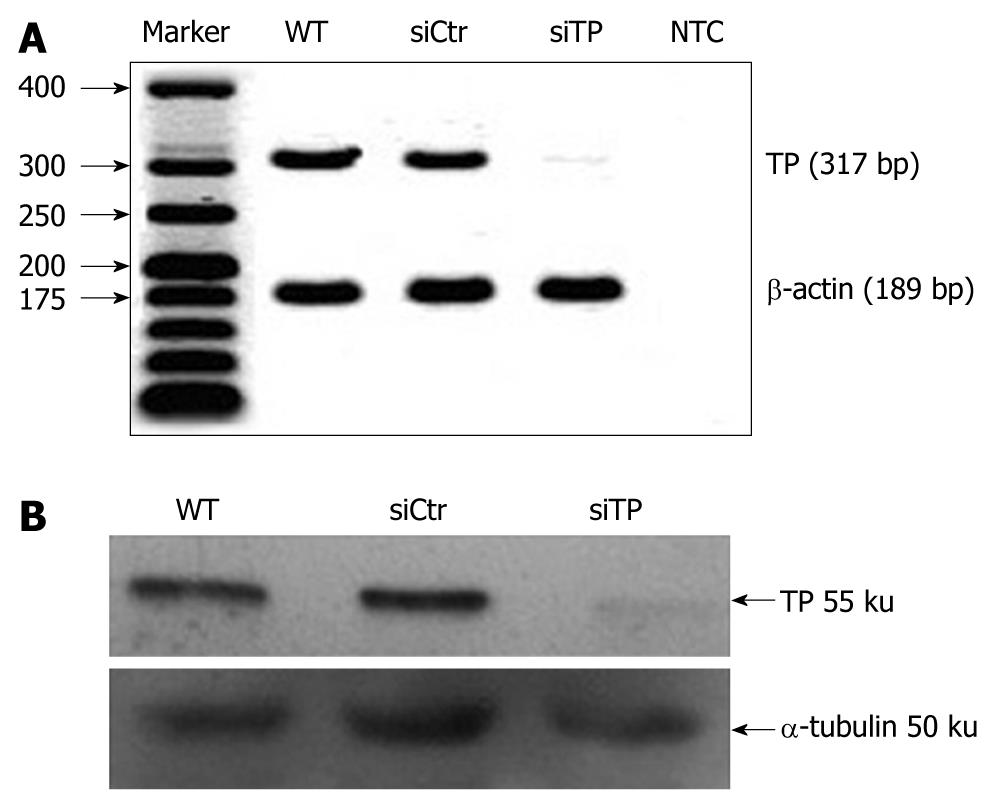
siRNA sequences against human TP were designed and synthesized by Stealth RNAi (Invitrogen, Carlsbad, CA, USA). The siRNA sequences were 5'-AUAGACUCCAGCUUAUCCAAGGUGC-3' (sense) and 5'-GCACCUUGGAUAAGCUGGAGUCUAU-3' (antisense). A non-related control siRNA that lacked identity with known gene targets was used as a control for non sequence-specific effects. TP siRNA or control siRNA (20 pmol) was transiently transfected into 1 × 105 KKU-M139 using OligofectAMINE (Invitrogen) according to the manufacturer’s instructions. Briefly, siRNA:OligofectAMINE complexes were mixed gently in Opti-MEM I reduced-serum medium and incubated for 20 min at room temperature. Then 200 μL of the complex mixture was added to cells growing in a 35-mm Petri dish containing 800 μL Opti-MEM I and mixed gently. After 24 h incubation at 37°C in a 50 mL/L CO2 incubator, the complexes were replaced by complete medium. Cells were incubated further for 24 h and gene function assays performed within 72 h post-transfection. The intensity of TP expression, either mRNA or protein, was analyzed using ImageJ software version 1.32j (NIH, Bethesda, MD, USA). To determine the knockdown efficiency, the density of TP expression was normalized with its corresponding β-actin for mRNA or α-tubulin for protein. The normalized intensity was compared between TP siRNA-transfected cells (TP-deficient cells) and control siRNA-transfected cells (control cells).
Total RNA from the KKU-M139 cell line was reverse transcribed using Omniscript reverse transcriptase (QIAgen, Hilden, Germany). The forward and reverse primer sequences of TP were 5'-GGCATGGATCTGGAGGAGAC-3' and 5'-CTCTGACCCACGATACAGCA-3', respectively. The forward and reverse primer sequences for β-actin as control were 5’-ACTGGGACGACATGGAGAAA-3' and 5'-ATAGCACAGCCTGGATAGCA-3', respectively. Polymerase chain reaction (PCR) amplification was carried out in a final volume of 20 μL containing first-strand cDNA, 10 pmol of each primer, 5 U Taq polymerase in 1 × Taq buffer (67 mmol/L Tris-HCl, 16.6 mmol/L ammonium sulfate, and 10 mL/L Tween 20), 2.5 mmol/L MgCl2, and 0.2 mmol/L dNTPs. The amplification was initiated by incubation at 94°C for 5 min followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 59°C (for TP) or 60°C (for β-actin) for 30 s, and extension at 72°C for 30 s, with a final 10-min extension at 72°C. PCR products were separated on a 20 g/L agarose gel containing 100 ng/mL ethidium bromide. Gels were visualized and photographed on a UV Transilluminator.
Total protein was separated on 10% SDS-PAGE then transferred onto a nitrocellulose membrane and blocked with TBST buffer (20 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.5 mL/L Tween 20) plus 50 g/L skim milk. The membrane was probed with anti-TP (Taiho Pharmaceutical Co., Ltd. Japan) or anti-α-tubulin (Zymed Laboratories Inc., San Francisco, CA, USA) as a control at a dilution of 1:1000 in TBST followed by a dilution of 1:2000 of a horseradish peroxidase-conjugated secondary antibody. Immunopositive bands were detected with freshly prepared ECL chemiluminescent solution (Amersham Biosciences, Buckinghamshire, England) and visualized by exposure to X-ray film.
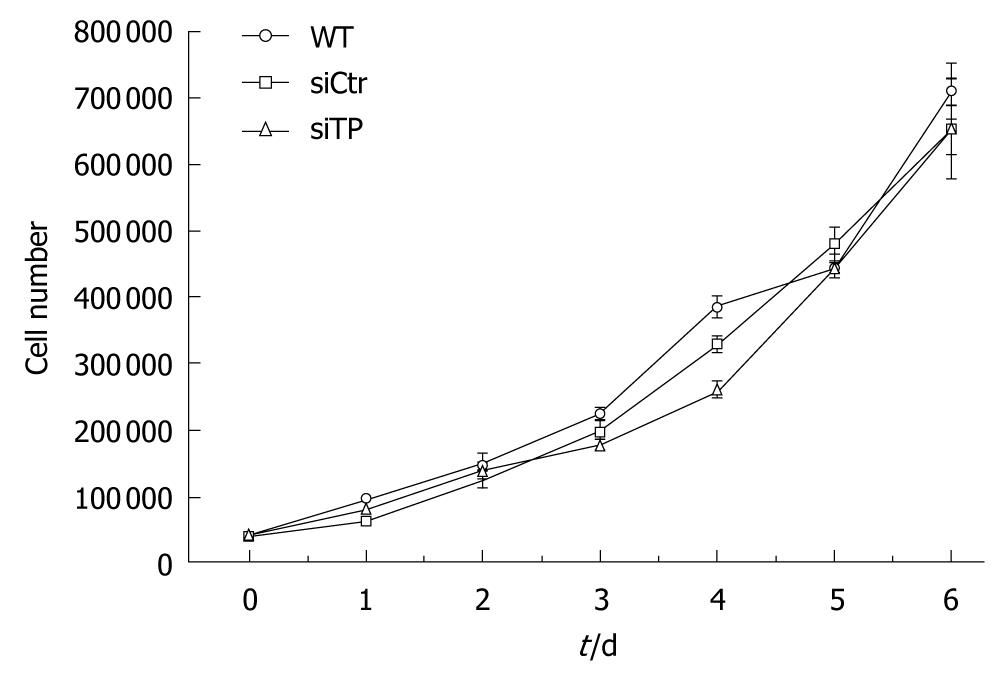
To evaluate the cellular growth rates, 1 × 104 wild-type, TP-deficient, or control cells were seeded in 6-well cell culture plates and grown in complete medium at 37°C in a 50 mL/L CO2 incubator. The cells were counted in triplicate every day for up to 6 d following a trypan blue dye exclusion assay. The population doubling time was calculated from the exponential growth phase.
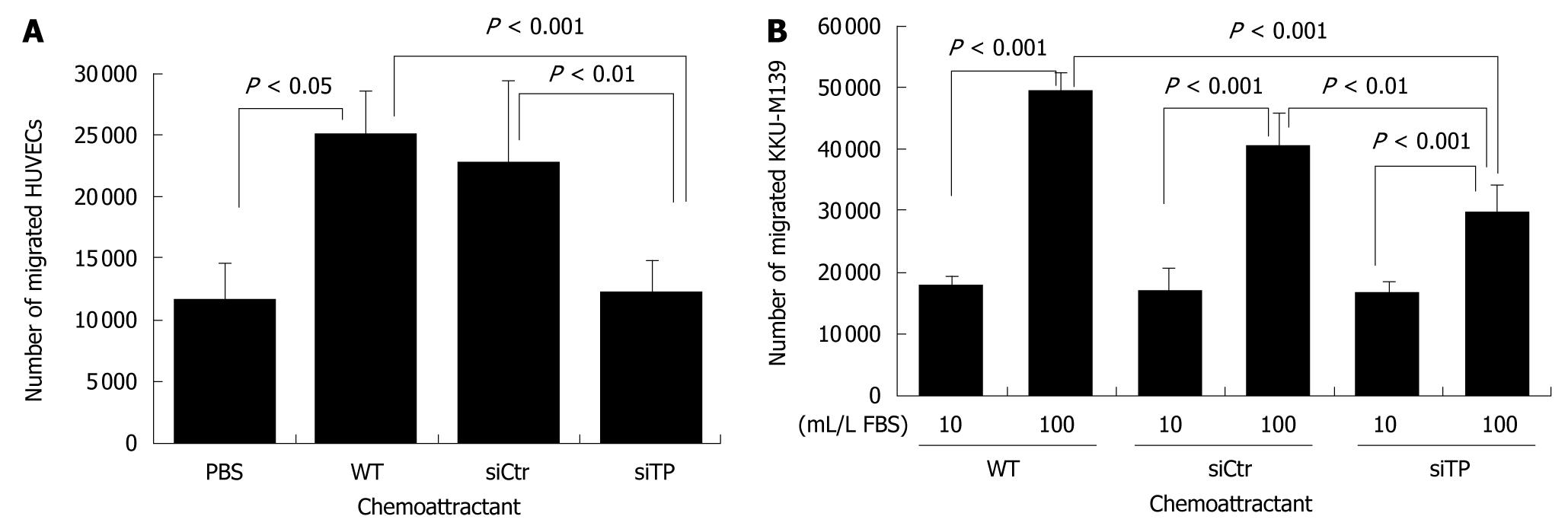
The migration of HUVECs stimulated by TP-expressing KKU-M139 cells was evaluated by a 2-chamber assay using a 96-well modified Chemotaxicell chamber (Kurabo, Japan) with an 8-μm pore size membrane. In brief, 100 μL total protein of wild-type, TP-deficient or control cells (PBS alone as a negative control) was added to the lower chamber. HUVECs (1 × 105 resuspended in 60 μL PBS) were placed in the upper chamber then incubated at 37°C in a 50 mL/L CO2 incubator for 4 h. The number of cells that moved through the pores toward the attractant-that is, TP protein produced by KKU-M139 cells-and settled on the bottom chamber was counted using trypan blue staining. The assays were performed in triplicate. The migration assay for KKU-M139 cells was carried out using the same protocol, but the lower chamber contained 100 μL of a complete medium as a chemoattractant. RPMI-1640 medium containing 10 mL/L FBS served as a negative control. Wild-type, TP-deficient or control cells (1 × 105 in suspension in 60 μL fresh RPMI-1640) were placed in the upper chamber.
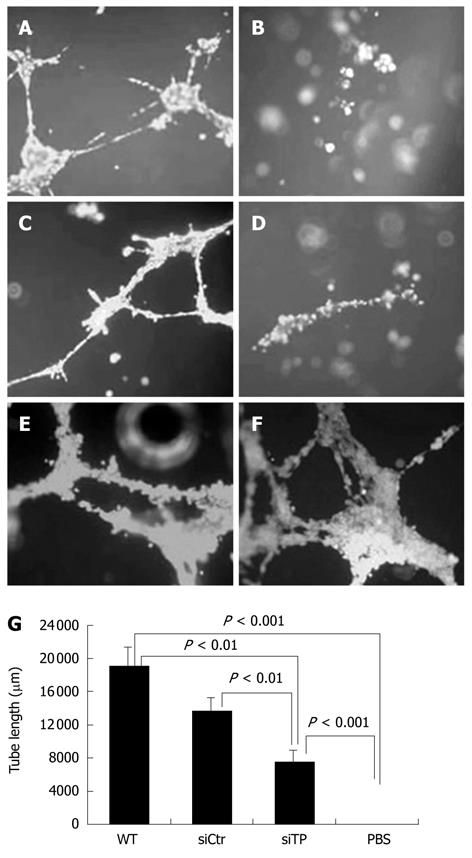
The HUVEC tube-like structure was determined using the Endothelial Tube Formation Assay (Cell Biolabs Inc., Sandiego, CA, USA) according to the manufacturer’s protocol. In brief, HUVECs were harvested and resuspended in M199 culture medium containing 100 mL/L FBS and angiogenesis mediators, i.e. serum of endometriosis patients as a positive control, total protein from KKU-M139 cells before and after treatment with TP-siRNA. Cell suspension (150 μL, 1.5 × 104 cells) was layered on the 50 μL of solidified extracellular matrix (ECM) gel in each 96-well plate, and the plate was incubated at 37°C for 18 h to allow the HUVECs to reorganize into a 3-dimensional tubular structure. The tube-like structures were stained with Calcein AM fluorescent dye, and examined and captured under a fluorescence microscope (Olympus CKX41, Tokyo, Japan). The tube length was quantified using ImageJ (NIH, Bethesda, MD, USA) and represented as total tube length (μm) for 3 photographic fields per experimental condition. Each treatment was performed in duplicate, and the set of experiments was repeated twice independently.
The DeadEnd™ Colorimetric TUNEL System (Promega, Madison, WI, USA) was used to detect apoptosis in cultured cell lines. In brief, cells grown in a 35-mm petri-dish were exposed to hypoxic conditions for 48 h. Cells were allowed to undergo apoptosis at 37°C in a humidified 50 mL/L CO2 incubator for 12 h. Treated cells were fixed with 40 g/L buffered formaldehyde, permeabilized by 2 mL/L Triton X-100, and equilibrated with Equilibration buffer (Promega). The cells were incubated with recombinant terminal deoxynucleotidyl transferase (rTdT) reaction mix, containing biotinylated nucleotides, inside a humidified chamber at 37°C for 1 h to allow end-labeling of the fragmented DNA of apoptotic cells to occur. The reactions were terminated by addition of 2 × SSC buffer, and endogenous peroxidases were blocked with 3 mL/L H2O2. To detect the incorporated biotinylated nucleotides, streptavidin-horseradish peroxidase solution (Promega) in PBS was used. A total of 100 μL diaminobenzidine chromogen was added to each plate and developed until a light brown background occurred. Cells stained brown was counted per total number of cells in the 100 × power field of inverted microscope. The assays were performed in duplicate, and the experiment was repeated at least 3 times.
To determine the concentration of 5-fluorouracil that inhibited cell proliferation by 50% (IC50), the MTS assay (Promega) was used according to the manufacturer’s instructions. Briefly, at one day post-transfection, 100 μL of cell suspension (1 × 105 cells/mL) was added to each well of a 96-well flat-bottom culture plate then incubated at 37°C in a humidified 50 mL/L CO2 incubator. After 24 h incubation, 100 μL medium containing various concentrations of 5-fluorouracil or fresh medium for untreated controls was added to each well for 48 h. The culture medium containing dead cells was then removed, and 100 μL of medium containing 20 μL MTS solution (317 μg/mL) was added to each well for 2 h to allow living cells to catalyze the MTS to a colored formazan product which was measured at 490 nm using a microplate reader (Tecan Austria GmbH, Salzburg, Austria). The assays were performed in duplicate, and the experiment was repeated twice.
Values are presented as mean ± SD. The statistical significance of the data was analyzed by one-way ANOVA using SPSS statistical software version 10.0 for Windows (SPSS Inc, Chicago, IL, USA). P < 0.05 was considered statistically significant.
After TP-siRNA transfection, expression of TP mRNA (Figure 1A) and protein (Figure 1B) was suppressed by 87.1% ± 0.49% and 72.5% ± 3.2%, respectively, compared with control cells. To evaluate TP function on the proliferation of KKU-M139, the cell growth assay was performed. The population doubling time calculated from exponential growth phase (day 1-day 4) of wild-type, TP-deficient, and control cells was 25.7 ± 4.7, 29.7 ± 2.4, and 29.6 ± 6 h (n = 4), respectively (Figure 2). Doubling time of TP-deficient cells was not significantly different from that of wild-type or control cells.
Angiogenesis, which is crucial for the development and progression of various cancers, requires migration and proliferation of endothelial cells in order to form the vasculature. Therefore, we tested the ability of TP to activate migration of endothelial cells using a 2-chamber assay. Migration of HUVECs toward total protein of TP-deficient cells was similar to that of PBS (Figure 3A). Use of total protein of TP-expressing KKU-M139 cells as a chemoattractant induced the migration of HUVECs by more than 2-fold relative to PBS (P < 0.05). Migration of cancer cells is important for invasion and metastasis. We investigated the migration of wild-type, TP-deficient, and control cells directed to 100 mL/L FBS as a chemoattractant. Migration towards 100 mL/L FBS of TP-deficient cells was significantly reduced by 2.4-fold compared with wild-type (P < 0.001) and 1.8-fold compared with control cells (P < 0.01) (Figure 3B).
To assess the effect of TP on the formation of blood vessels, we analyzed the ability of TP produced from KKU-M139 to induce HUVECs to form tube structures using an ECM gel angiogenesis assay. Tube-like structures were formed by HUVECs activated by serum of endometriosis patients as a positive control (Figure 4A) but not by the PBS as a negative control (Figure 4B). HUVECs exhibited significant tube formation when total protein from wild-type KKU-M139 cells (Figure 4E and F), which endogenously express TP, was added (P < 0.001 vs PBS). Pre-treatment of KKU-M139 cells with TP-siRNA (Figure 4D) to suppress TP expression reduced tube formation of HUVECs compared with control cells (Figure 4C) (P < 0.01) or wild-type cells (P < 0.01) as shown in Figure 4G.
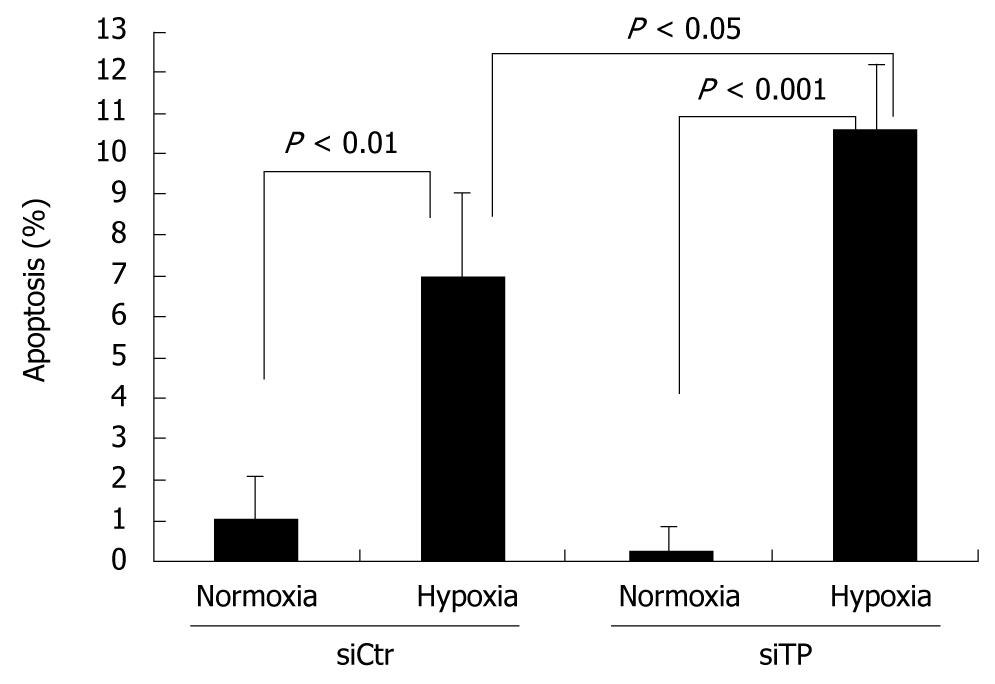
TP was reported to confer resistance to apoptosis induced by hypoxia. Under hypoxic conditions, the TP-deficient cells exhibited significantly more cell death than did the control cells (P < 0.05). Thus, TP seems to confer resistance to hypoxia-induced apoptosis in control cells. For both control and TP-deficient cells, the percent apoptosis under normoxic conditions was less than that under hypoxic conditions (P < 0.01 and P < 0.001, respectively). Anti-apoptotic activity of KKU-M139 under 48 h hypoxic conditions was suppressed by TP knockdown (Figure 5).
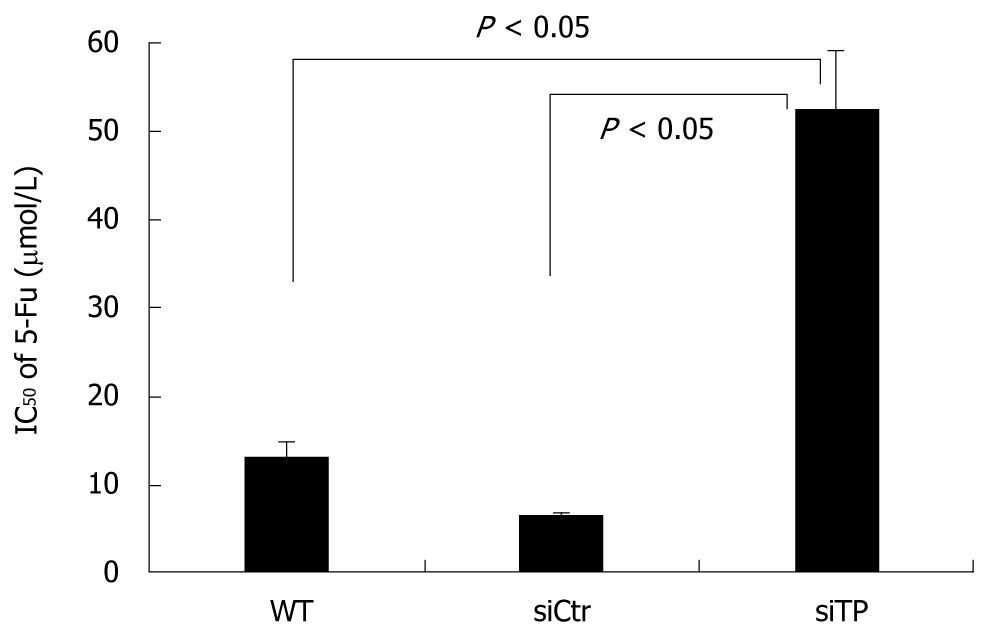
5-fluorouracil is a commonly used chemotherapeutic drug for cholangiocarcinoma, and TP is needed for 5-fluorouracil activation. To determine to what extent TP contributes to the cytotoxic effect of 5-fluorouracil, cells were exposed to a range of concentrations of 5-fluorouracil in the presence or absence of siRNA against TP. The IC50 of control and wild-type cells were 6.4 ± 0.36 and 13 ± 1.70 μmol/L 5-fluorouracil, respectively. As expected, TP-deficient cells required 52.3 ± 6.8 μmol/L 5-fluorouracil, an 8.2-fold increase over that in control cells (P < 0.05), to inhibit cell proliferation by 50% (Figure 6).
We previously reported that 35 of 65 (53.8%) liver fluke-related cholangiocarcinoma cases exhibited TP amplification, suggesting that TP may contribute to the pathogenesis of cholangiocarcinoma[7]. Here, we report that TP confers resistance to apoptosis, induces migration of a cholangiocarcinoma-derived cell line, and mediates migration and tube formation of HUVECs. However, TP is essential for activation of 5-fluorouracil, a commonly used anti-cancer drug for treatment of cholangiocarcinoma. In fact, TP amplification and overexpression have been reported for various solid tumors that exhibited invasion, metastasis, angiogenesis, and poor prognosis, including cholangiocarcinoma[13-16,18,19]. In this study, transfection with TP-siRNA suppressed TP mRNA expression by approximately 87% and protein expression by 72% (Figure 1), indicating effective gene silencing. The population doubling time of TP-deficient cells was not significantly different from that of wild-type or control cells (Figure 2), suggesting that TP is not a pivotal factor for tumor cell proliferation in cholangiocarcinoma.
Increased TP expression has been shown to increase invasiveness of KB epidermoid carcinoma cells compared with control cells[20]. Migration is an important characteristic of metastatic cancer cells. TP-expressing KKU-M139 also exhibited greater migration than TP knockdown cells (Figure 3B). Nakajima et al[21] determined the molecular basis for induction of invasive activity by TP. They suggested that TP and 2-deoxy-d-ribose induces the expression, secretion, and activity of matrix metalloprotease-9 (MMP-9) that subsequently confers invasiveness on cancer cells, but the exact mechanism by which TP and one of its degradation products, 2-deoxy-d-ribose, enhance MMP-9-mediated invasive activity is not known. The expression level of TP was reported to significantly correlate with that of the invasion-related genes MMP-1, MMP-7, and MMP-9[22]. Taken together, these data indicate that TP enhances the invasion of tumor cells through the induction of invasion-related genes in cholangiocarcinoma.
The study by Hotchkiss et al[23] demonstrated that TP-expressing cells mediated HUVEC migration via the intracellular metabolism of thymidine by TP and the subsequent extracellular release of 2-deoxy-d-ribose, which formed a chemotactic gradient. Later, they proved that both TP and 2-deoxy-d-ribose stimulated HUVEC migration by increasing cell surface expression of the integrins α5β1 and αvβ3 to form focal adhesions. Integrins bind to the ECM and directly activate focal adhesion kinase signaling pathways, mediating cell attachment and migration[24]. KKU-M139, which endogenously expresses TP, also strongly induced HUVEC migration, an early step in angiogenesis. Our study showed that TP-expressing KKU-M139 could stimulate the migration and formation of tube-like structures of HUVECs to a much greater degree than TP-silenced KKU-M139 (Figures 3A and 4). In addition, 2-deoxy-d-ribose can mediate an oxidative stress mechanism and upregulate other angiogenic factors[25]. Attracted endothelial cells proliferate upon stimulation by growth factors released from cancer cells and subsequently form new blood vessels. Suppression of TP-mediated migration and angiogenesis of HUVECs by TP-siRNA thus can be explained by the fact that TP and its product, 2-deoxy-d-ribose, could not exert their effect on cell migration and tube formation. Chemically synthesized TP inhibitor (TPI) has been demonstrated to suppress tumor growth by increasing the proportion of apoptotic cells and probably by inhibiting angiogenesis; TPI completely suppressed angiogenesis by TP-cDNA transfected KB cells[26]. 2-deoxy-L-ribose, the stereoisomer of 2-deoxy-d-ribose, is also a TPI, as shown by the ability of 2-deoxy-L-ribose to inhibit 2-deoxy-d-ribose-mediated angiogenesis and metastasis of tumor cells expressing TP[20,27]. Our study also demonstrated that TP-siRNA was able to act as TP suppressor or inhibitor.
Resistance to apoptosis is a key process in tumor formation. 2-deoxy-d-ribose was found to be involved in a hypoxia-induced apoptotic pathway. 2-deoxy-d-ribose inhibited hypoxia-induced phosphorylation of p38 mitogen-activated protein kinase but not c-jun NH2-terminal kinase/stress-activated protein kinase in human leukemia HL-60 cells[28]. In addition, 2-deoxy-d-ribose and thymine partially prevented hypoxia-induced apoptosis[29]. 2-deoxy-d-ribose may be an important energy source under hypoxic conditions[30]. The ability of TP-siRNA to increase the proportion of apoptotic KKU-M139 cells (Figure 5) was most likely attributable to an inhibition of TP pathways, however, further studies are needed to determine the exact role of TP in these tumor phenotypes.
The enzymatic activity of TP is also indispensable for the activation of prodrug 5’-deoxy-5-fluorouridine (5’-DFUR) to active 5-fluorouracil and fluorodeoxyuridine in tumors. The sensitivity of TP cDNA-transfected MCF-7 breast cancer cells to 5’-DFUR was increased approximately 20-fold compared to the parent cells or cells with control vector alone, and sensitivity to 5-fluorouracil was also somewhat increased[31]. The sensitivity of a TP-transfected SMMC-7721 hepatocellular carcinoma cell line to 5’-DFUR was also significantly enhanced; however, endothelial cell migration was also promoted at the same time[32]. We demonstrated that TP-siRNA was able to suppress TP expression in vitro and that this treatment impaired the therapeutic efficacy of 5-fluorouracil in KKU-M139 (Figure 6), indicating that TP enzyme activity is needed for 5-fluorouracil activation.
In conclusion, we found that TP plays a dual role in development and therapy of cholangiocarcinoma. TP confers apoptotic resistance and migration of a cholangiocarcinoma-derived cell line, and also induces migration and tube formation of HUVECs. On the other hand, TP is essential for the therapeutic efficacy of 5-fluorouracil chemotherapy. It may be useful to examine the expression level of TP in tumors of early stage cholangiocarcinoma patients to select those likely to respond well to 5-fluorouracil. However, most cholangiocarcinoma patients are diagnosed at a late stage, and there are several chemotherapeutic drugs of choice in addition to 5-fluorouracil for cholangiocarcinoma. Inhibition of TP activity may be helpful in decreasing tumor aggressiveness i.e. migration, angiogenesis and anti-apoptosis, and improving the poor prognosis of cancer patients who show high TP expression.
Liver fluke-related cholangiocarcinoma is the most common hepatobiliary malignancy found in Northeast Thailand. Amplification of thymidine phosphorylase (TP) copy number was found in most cholangiocarcinoma patients suggesting that it has a significant role in tumor progression.
TP catalyzes the reversible phosphorolysis of thymidine, deoxyuridine, and their analogs to their respective bases and 2-deoxyribose-1-phosphate which is then dephosphorylated to 2-deoxy-d-ribose. TP is abnormally expressed in certain cancers of the gastrointestinal tract including cholangiocarcinoma. However, how TP expression is upregulated in human tumors remains unclear. In this study, the authors investigated the roles of TP in liver fluke-related cholangiocarcinoma using RNA interference to suppress TP expression.
Suppression of TP not only reduces angiogenesis, resistance to apoptosis, and tumor migration but also diminishes chemosensitivity to 5-fluorouracil in cholangiocarcinoma.
Because of its significance in tumor aggressiveness and 5-fluorouracil sensitivity, TP expression may be used as a prognostic and predictive marker as well as targeted therapy in cholangiocarcinoma.
The TP gene is aberrantly expressed in different human malignancies including cholangiocarcinoma. In the current manuscript submitted by Thanasai et al, the authors made several interesting observations. This article reports the effects of TP expression on tumor migration and 5-fluorouracil sensitivity in cholangiocarcinoma cells, as well as tumor cell-induced angiogenesis of endothelial cells in vitro.
Peer reviewers: Gianfranco D Alpini, PhD, Professor, VA Research Scholar Award Recipient, Professor, Medicine and Systems Biology and Translation Medicine, Dr. Nicholas C Hightower Centennial Chair of Gastroenterology, Central Texas Veterans Health Care System, The Texas A & M University System Health Science Center College of Medicine, Medical Research Building, 702 SW H.K. Dodgen Loop, Temple, TX, 76504, United States; Xian-Ming Chen, MD, Associate Professor, Department of Medical Microbiology and Immunology, Creighton University, 2500 California Plaza, Omaha, NE 68178, United States
S- Editor Wang YR L- Editor Cant MR E- Editor Ma WH
| 1. | Vatanasapt V, Sriamporn S, Vatanasapt P. Cancer control in Thailand. Jpn J Clin Oncol. 2002;32 Suppl:S82-S91. |
| 2. | Uttaravichien T, Bhudhisawasdi V, Pairojkul C, Pugkhem A. Intrahepatic cholangiocarcinoma in Thailand. J Hepatobiliary Pancreat Surg. 1999;6:128-135. |
| 3. | Horie S, Endo K, Kawasaki H, Terada T. Overexpression of MDM2 protein in intrahepatic cholangiocarcinoma: relationship with p53 overexpression, Ki-67 labeling, and clinicopathological features. Virchows Arch. 2000;437:25-30. |
| 4. | Limpaiboon T, Khaenam P, Chinnasri P, Soonklang M, Jearanaikoon P, Sripa B, Pairojkul C, Bhudhisawasdi V. Promoter hypermethylation is a major event of hMLH1 gene inactivation in liver fluke related cholangiocarcinoma. Cancer Lett. 2005;217:213-219. |
| 5. | Limpaiboon T, Krissadarak K, Sripa B, Jearanaikoon P, Bhuhisawasdi V, Chau-in S, Romphruk A, Pairojkul C. Microsatellite alterations in liver fluke related cholangiocarcinoma are associated with poor prognosis. Cancer Lett. 2002;181:215-222. |
| 6. | Muenphon K, Limpaiboon T, Jearanaikoon P, Pairojkul C, Sripa B, Bhudhisawasdi V. Amplification of chromosome 21q22.3 harboring trefoil factor family genes in liver fluke related cholangiocarcinoma is associated with poor prognosis. World J Gastroenterol. 2006;12:4143-4148. |
| 7. | Thanasai J, Limpaiboon T, Jearanaikoon P, Bhudhisawasdi V, Khuntikeo N, Sripa B, Miwa M. Amplification of D22S283 as a favorable prognostic indicator in liver fluke related cholangiocarcinoma. World J Gastroenterol. 2006;12:4338-4344. |
| 8. | Brown NS, Bicknell R. Thymidine phosphorylase, 2-deoxy-D-ribose and angiogenesis. Biochem J. 1998;334:1-8. |
| 9. | Akiyama S, Furukawa T, Sumizawa T, Takebayashi Y, Nakajima Y, Shimaoka S, Haraguchi M. The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression. Cancer Sci. 2004;95:851-857. |
| 10. | Miyadera K, Sumizawa T, Haraguchi M, Yoshida H, Konstanty W, Yamada Y, Akiyama S. Role of thymidine phosphorylase activity in the angiogenic effect of platelet derived endothelial cell growth factor/thymidine phosphorylase. Cancer Res. 1995;55:1687-1690. |
| 11. | Fujimoto K, Hosotani R, Wada M, Lee JU, Koshiba T, Miyamoto Y, Tsuji S, Nakajima S, Doi R, Imamura M. Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer. 1998;34:1439-1447. |
| 12. | Saeki T, Tanada M, Takashima S, Saeki H, Takiyama W, Nishimoto N, Moriwaki S. Correlation between expression of platelet-derived endothelial cell growth factor (thymidine phosphorylase) and microvessel density in early-stage human colon carcinomas. Jpn J Clin Oncol. 1997;27:227-230. |
| 13. | Giatromanolaki A, Sivridis E, Simopoulos C, Polychronidis A, Gatter KC, Harris AL, Koukourakis MI. Thymidine phosphorylase expression in gallbladder adenocarcinomas. Int J Surg Pathol. 2002;10:181-188. |
| 14. | Aishima S, Taguchi K, Sugimachi K, Asayama Y, Nishi H, Shimada M, Sugimachi K, Tsuneyoshi M. The role of thymidine phosphorylase and thrombospondin-1 in angiogenesis and progression of intrahepatic cholangiocarcinoma. Int J Surg Pathol. 2002;10:47-56. |
| 15. | O’Brien TS, Fox SB, Dickinson AJ, Turley H, Westwood M, Moghaddam A, Gatter KC, Bicknell R, Harris AL. Expression of the angiogenic factor thymidine phosphorylase/platelet-derived endothelial cell growth factor in primary bladder cancers. Cancer Res. 1996;56:4799-4804. |
| 16. | Hotta T, Taniguchi K, Kobayashi Y, Johata K, Sahara M, Naka T, Watanabe T, Ochiai M, Tanimura H, Tsubota YT. Increased expression of thymidine phosphorylase in tumor tissue in proportion to TP-expression in primary normal tissue. Oncol Rep. 2004;12:539-541. |
| 17. | Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745-2756. |
| 18. | Sato J, Sata M, Nakamura H, Inoue S, Wada T, Takabatake N, Otake K, Tomoike H, Kubota I. Role of thymidine phosphorylase on invasiveness and metastasis in lung adenocarcinoma. Int J Cancer. 2003;106:863-870. |
| 19. | Miwa S, Soeda J, Miyagawa S. Interrelationship of platelet-derived endothelial cell growth factor, liver macrophages, and tumor microvessel density in patients with cholangiocellular carcinoma. Hepatogastroenterology. 2005;52:1398-1402. |
| 20. | Uchimiya H, Furukawa T, Okamoto M, Nakajima Y, Matsushita S, Ikeda R, Gotanda T, Haraguchi M, Sumizawa T, Ono M. Suppression of thymidine phosphorylase-mediated angiogenesis and tumor growth by 2-deoxy-L-ribose. Cancer Res. 2002;62:2834-2839. |
| 21. | Nakajima Y, Haraguchi M, Furukawa T, Yamamoto M, Nakanishi H, Tatematsu M, Akiyama S. 2-Deoxy-L-ribose inhibits the invasion of thymidine phosphorylase-overexpressing tumors by suppressing matrix metalloproteinase-9. Int J Cancer. 2006;119:1710-1716. |
| 22. | Gotanda T, Haraguchi M, Tachiwada T, Shinkura R, Koriyama C, Akiba S, Kawahara M, Nishiyama K, Sumizawa T, Furukawa T. Molecular basis for the involvement of thymidine phosphorylase in cancer invasion. Int J Mol Med. 2006;17:1085-1091. |
| 23. | Hotchkiss KA, Ashton AW, Klein RS, Lenzi ML, Zhu GH, Schwartz EL. Mechanisms by which tumor cells and monocytes expressing the angiogenic factor thymidine phosphorylase mediate human endothelial cell migration. Cancer Res. 2003;63:527-533. |
| 24. | Hotchkiss KA, Ashton AW, Schwartz EL. Thymidine phosphorylase and 2-deoxyribose stimulate human endothelial cell migration by specific activation of the integrins alpha 5 beta 1 and alpha V beta 3. J Biol Chem. 2003;278:19272-19279. |
| 25. | Sengupta S, Sellers LA, Matheson HB, Fan TP. Thymidine phosphorylase induces angiogenesis in vivo and in vitro: an evaluation of possible mechanisms. Br J Pharmacol. 2003;139:219-231. |
| 26. | Matsushita S, Nitanda T, Furukawa T, Sumizawa T, Tani A, Nishimoto K, Akiba S, Miyadera K, Fukushima M, Yamada Y. The effect of a thymidine phosphorylase inhibitor on angiogenesis and apoptosis in tumors. Cancer Res. 1999;59:1911-1916. |
| 27. | Nakajima Y, Gotanda T, Uchimiya H, Furukawa T, Haraguchi M, Ikeda R, Sumizawa T, Yoshida H, Akiyama S. Inhibition of metastasis of tumor cells overexpressing thymidine phosphorylase by 2-deoxy-L-ribose. Cancer Res. 2004;64:1794-1801. |
| 28. | Ikeda R, Che XF, Ushiyama M, Yamaguchi T, Okumura H, Nakajima Y, Takeda Y, Shibayama Y, Furukawa T, Yamamoto M. 2-Deoxy-D-ribose inhibits hypoxia-induced apoptosis by suppressing the phosphorylation of p38 MAPK. Biochem Biophys Res Commun. 2006;342:280-285. |
| 29. | Kitazono M, Takebayashi Y, Ishitsuka K, Takao S, Tani A, Furukawa T, Miyadera K, Yamada Y, Aikou T, Akiyama S. Prevention of hypoxia-induced apoptosis by the angiogenic factor thymidine phosphorylase. Biochem Biophys Res Commun. 1998;253:797-803. |
| 30. | Malhotra R, Lin Z, Vincenz C, Brosius FC 3rd. Hypoxia induces apoptosis via two independent pathways in Jurkat cells: differential regulation by glucose. Am J Physiol Cell Physiol. 2001;281:C1596-C1603. |
| 31. | Kim R, Murakami S, Toge T. Effects of introduction of dThdPase cDNA on sensitivity to 5’-deoxy-5-fluorouridine and tumor angiogenesis. Int J Oncol. 2003;22:835-841. |
| 32. | Zhou J, Xiao YS, Tang ZY, Fan J, Wu ZQ, Zhao Y, Xue Q, Shen ZZ, Liu YK, Ye SL. Transfection of thymidine phosphorylase cDNA to human hepatocellular carcinoma cells enhances sensitivity to fluoropyrimidine but augments endothelial cell migration. J Cancer Res Clin Oncol. 2005;131:547-551. |