INTRODUCTION
Gastritis is a common disorder where discontinuity of the gastric mucosa is observed. It is caused by several factors, such as alcohol, stress, infection with Helicobacter pylori (H. pylori)[1-3], resulting in an imbalance between offensive acid-pepsin secretion and defensive mucosal factors like mucin secretion and cell shedding[4]. Non-steroidal anti-inflammatory drugs (NSAIDs) such as acetyl-salicylic acid (ASA) are used worldwide as anti-inflammatory and analgesic agents in the treatment of chronic diseases such as rheumatoid arthritis and osteoarthritis[5] as well as for the prevention of cardiovascular diseases. However, gastrointestinal injury is a serious adverse effect of NSAIDs producing a broad range of toxic effects mainly in the stomach[6], the toxicity of ASA being attributed to direct damage of mucosal cells[7]. Furthermore, ASA affects various mucosal defense lines such as bicarbonate secretion, mucus synthesis, decrease of mucosal blood flow[8,9] with amplification of the inflammatory process by expression of pro-inflammatory cytokines[10].
Among the most conventional drugs employed for the treatment of gastritis are proton-pump inhibitors such as omeprazole (OM)[11]; however, most of these drugs also produce undesirable side effects and drug interactions[12].
Probiotics are “live microorganisms which when consumed in adequate numbers confer a health benefit on the host[13]”. Probiotic foods containing lactic acid bacteria (LAB) have been used in the treatment of various gastrointestinal disorders, such as gastric ulcers and inflammation related to H. pylori infection, gastrointestinal infections or antibiotic-associated diarrhea[14-16], providing beneficial effects to the host by modulating immune functions, e.g. systemic cytokine production[17]. The mucosal immune system is functionally divided into sites where foreign antigens are taken up and meet immune cells to initiate the immune response through a network of signals among different cell populations. This cell network is highly integrated by cytokine production, and finely regulated by the selective expression of cytokine receptors. The T-helper (Th) cell subsets and cytokine patterns determine the nature of the immune response[18].
Some LAB strains secrete exocellular carbohydrate polymers named exopolysaccharides (EPS). A large diversity of EPS from LAB strains exists regarding their chemical characteristics, yield, technological and functional properties[19-21]. EPS play an important role in the dairy industry mainly in yogurt production and certain kinds of cheeses such as reduced-fat cheddar and mozzarella[22], improving the textural, melting and sensory characteristics of the products. The health-promoting effects ascribed to probiotic strains or foods arise not only from the bacteria themselves but also from the metabolites produced during fermentation.
EPS from LAB have been claimed to participate in various regulatory processes such as immunomodulatory, cholesterol-lowering and anti-ulcer activities[23,24]. In previous work[25], we demonstrated that Balb/c mice fed a fermented milk with the EPS-producing S. thermophilus CRL 1190 was efficient in gastritis prevention through the modulation of the immune response and maintenance of the mucus layer. The present study addressed the potential therapeutic application of fermented milk prepared using the EPS-producing S. thermophilus CRL 1190 strain for the treatment of ASA-associated chronic gastritis.
MATERIALS AND METHODS
Strain, culture conditions and preparation of the fermented milk
S. thermophilus CRL 1190 {EPS+ and producing also capsular EPS, CPS+; [Centro de Referencia para Lactobacilos (CERELA) culture collection, Tucumán, Argentina]} was used in this study. This strain was previously selected for the physicochemical properties of its polysaccharide[20], for displaying no secondary effects such as bacterial translocation (liver and spleen), and for its effectiveness in preventing gastritis induced by ASA[25]. The strain was cultured (10 mL/L inoculum) in LAPTg broth (peptone, 15 g/L; tryptone, 10 g/L; yeast extract, 10 g/L; glucose, 10 g/L; and tween 80, 1 mL/L) and sub-cultured at least twice in reconstituted skim milk (RSM, 100 g/L) just prior to experimental use. The strain was maintained at -20°C in RSM containing 100 mL/L glycerol, 10 g/L glucose, and 5 g/L yeast extract.
Fermented milk was prepared in sterile RSM (sterilized at 115°C for 20 min and cooled down to 37°C) using a 10 mL/L inoculum of an active culture of the EPS+ strain S. thermophilus CRL 1190 (named FM 1190), incubated at 37°C for 16 h and maintained at 4°C prior to experimental use. Non-fermented milk was used as a control.
Animals
Six week-old Balb/c male mice (25-30 g) were obtained from a closed colony kept at the animal facilities of CERELA and maintained in a room with a 12-h light/dark cycle at 20 ± 2°C. Animals were individually housed in cages (20 cm × 30 cm × 15 cm) with litter tray (20 cm × 30 cm × 6 cm) and allowed to have free access to conventional balanced diet and water ad libitum. All mice received no food for 24 h before the assays but had free access to water. Animal protocols were approved by the Ethical Committee for animal care of CERELA.
Experimental protocol
Chronic gastritis was induced following the protocol previously standardized in our laboratory[25]. Oral administration of ASA (BAYER®) supplied in the drinking water given at an approximate daily dose of 400 mg/kg per day for 10 d induced chronic gastritis in Balb/c mice (gastritis group, G). The administered dose was twice the analgesic dose for mice and was applied to induce gastritis in a short experimental time period. Healthy mice (H) received drinking water without ASA during the same experimental period (negative control group).
To evaluate the therapeutic effect of FM 1190 on the chronic gastritis model, animals were randomly divided into 6 groups (n = 5 each): (1) H group: received drinking water without ASA for 10 d; (2) G group: received ASA for 10 d as described above; (3) FM 1190 group: received FM with the EPS-producing strain CRL 1190 for 7 d after gastritis induction. FM 1190 was administered ad libitum at an approximate dose of 108 cfu/mL; daily fermented milk consumption was monitored and intake was set at 5 mL/d; (4) Omeprazole (OM) group: received OM (used as positive control in ASA-induced gastric lesions) at a daily dose of 30 mg/kg per day[26] for 7 d after gastritis induction; (5) Milk group (M): received non-fermented milk for 7 d after gastritis induction; and (6) Water group (W): received water for 7 d after gastritis induction (used as negative control).
To determine whether the EPS produced by S. thermophilus CRL 1190 (EPS 1190) had an anti-gastritis effect, the polymer was isolated from 16-h milk cultures grown at 37°C by using a deproteinization/precipitation technique with 200 g/L (final concentration) trichloroacetic acid, and ethanol (ratio 1:3)[20], was further purified as described previously[19], and freeze-dried and stored at 4°C until use. The EPS 1190 was resuspended in RSM (M-EPS 1190) or in water (W-EPS 1190) and administered to mice intragastrically at a dose of 4 mg/kg per day for 7 d after gastritis induction. The administered EPS amount was calculated based on the EPS quantity received by the animals when they were fed with FM 1190.
After the experimental period (day 11 for groups 1 and 2, and day 18 for the remaining groups), mice were sacrificed by cervical dislocation and weighed. Stomachs were aseptically removed, weighed and rinsed several times with saline solution and used for the assays described below.
Histopathological evaluation of gastric samples
Stomachs were fixed in 10% paraformaldehyde in 0.1 mol/L phosphate-buffered saline (PBS) pH 7.0 and embedded in paraffin following the Sainte-Marie technique[27]. Three serial paraffin sections (4 μm) of each sample were cut from each specimen and stained with hematoxylin-eosin followed by light microscopy examination (Leica DM LS2, Wetzlar, Germany). The pathologic characteristics and degree of inflammation of the gastric mucosa were assessed according to the updated Sydney system[28] by microscopic observation without knowledge of the experimental groups and expressed as follows: normal appearance of scattered mononuclear cells in the lamina propria (same degree as healthy control mice): none = score 0; mild infiltration of mononuclear cells in the lamina propria and the submucosa, and no erosion in the epithelium: mild = score 1; moderate infiltration of mononuclear cells in the lamina propria and the submucosa, and no erosion in the epithelium: moderate = score 2; and severe infiltration of mononuclear cells in the lamina propria and the submucosa, and erosion in some parts of the epithelium: severe = score 3.
Determination of the number of IL-10, INF-γ, and TNF-α-producing cells in gastric mucosa by indirect immunofluorescence assay
Histological slices of the antral and corpus regions of the stomach, processed as described earlier, were deparaffinized and rehydrated in a graded series of ethanol. After incubation at room temperature for 30 min in 10 g/L blocking solution of bovine serum albumin (Sigma Chemical Co.), histological slices were incubated at 37°C for 60 min with rabbit anti-mouse IL-10 or INF-γ (Peprotech Inc., NJ, USA) or TNF-α (eBioscience, San Diego, CA, USA) polyclonal antibodies. Then, sections were washed twice with saline solution and treated with a 1/10 dilution of a goat anti-rabbit antibody conjugated with fluorescein isothiocyanate (FITC) (Jackson Immuno Research Inc., PA, USA) at 37°C for 45 min; washed again with saline solution and examined with a fluorescent light microscope (Leica DM LS2). Results were expressed as the number of IL-10, INF-γ and TNF-α-producing cells (fluorescent cells) per 10 fields (magnification × 1000)[29]. Data were obtained by counting 30 fields from 3 histological slices for each animal group.
Mucus layer determined by periodic acid-Schiff staining
The mucus layer was identified by periodic acid-Schiff (PAS) staining[30]. Briefly, after deparaffinization and rehydration, tissue sections were oxidized in 10 mL/L periodic acid for 5 min. Then they were rinsed in distilled water and stained with Schiff’s reagent for 10 min. After a second washing with distilled water, tissue sections were counterstained with hematoxylin and rinsed in running tap water. Finally, they were dehydrated, cleared and mounted. Sections were viewed under a microscope (Leica M LS2) and the thickness of the mucus-secreting layer in the corpus and antrum mucosa was assessed with an image analyzer (× 1000) and the ratio of the mucus gel layer thickness to that of the lamina propria mucosa was calculated as a percentage.
Statistical analysis
Experimental data were expressed as mean ± SD and statistically evaluated by analysis of variance (ANOVA) with the SPSS software. Multiple group data were analyzed using one-way ANOVA and the Tukey multiple comparison test. Differences were considered statistically significant at P < 0.05.
RESULTS
Body weight and stomach weight
No significant changes in body weight or stomach weight were found during the entire experimental period in animals of any group other than those of the FM 1190 and M-EPS 1190 groups, which showed an increase (between 30%-40% with respect to H and G, respectively) in the stomach weight at the end of the experimental period (data not shown). No correlation between stomach weight and induced gastritis was found.
Histopathological evaluation of gastric samples
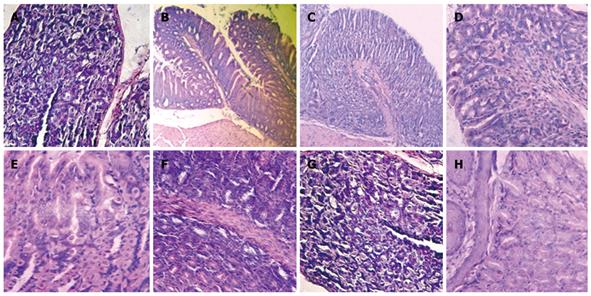
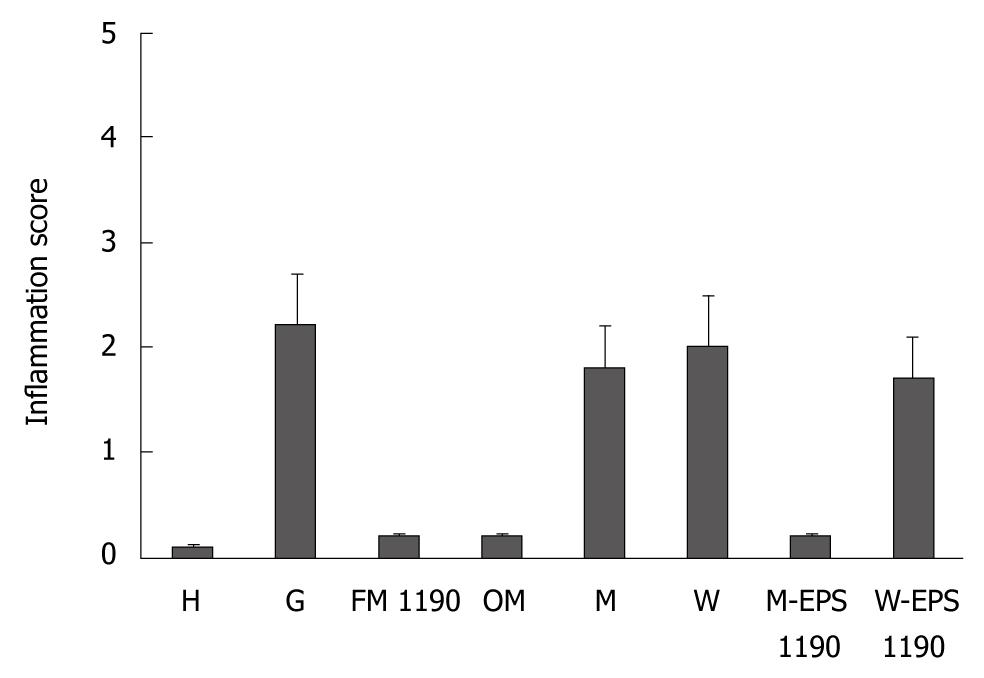
Mice subjected to oral administration of ASA at doses of 400 mg/kg per day for 10 d (gastritis group, G) showed moderate infiltration with scattered lymphocytes and macrophages in the surface of the mucosa and folds of mucosa, in the direction of the submucosa, without formation of lymphoid follicles, and predominance in the gastric corpus region (Figure 1B). The induced lesions were classified as superficial chronic gastritis with inflammation score = 2 (Figure 2). No significant increase in polymorphonuclear infiltration in the normal gastric mucosa was observed. Healthy animals (group H) showed an absence of gastritis (Figure 1A). Interestingly, the stomachs from animals treated with FM 1190 and M-EPS 1190 displayed no leukocyte infiltration in the gastric mucosa immediately after treatment (day 18) (Figure 1C and G). Similar stomach structures of mice therapeutically treated with OM (Figure 1D) were observed, showing lower inflammatory scores than the gastritis group (0.2 ± 0.02 vs 2.2 ± 0.4, P < 0.05, Figure 2). In contrast, the animals treated with milk, water and W-EPS 1190 showed high inflammation scores (1.8 ± 0.5, 2.0 ± 0.5, 1.7 ± 0.4, respectively, P < 0.05), compared to those of the gastritis group.
Figure 1 Histological micrographs of mice stomachs.
A: H (Healthy) group; B: G (Gastritis) group; C: FM 1190 group (fermented milk with Streptococcus thermophilus CRL 1190); D: OM (omeprazole) group; E: M (Milk) group; F: W (Water) group; G: M-EPS 1190 group (EPS produced by S. thermophilus CRL 1190 resuspended in milk) showed conserved histological structures; and H: W-EPS 1190 group (EPS produced by Strep. thermophilus CRL 1190 resuspended in water) displayed chronic inflammatory infiltrations in the mucosa surface (Hematoxylin & eosin, light microscope, × 100).
Figure 2 Inflammation score of stomachs in different groups (mean ± SD).
Determination of the number of regulatory and pro-inflammatory cytokine-producing cells in the gastric mucosa
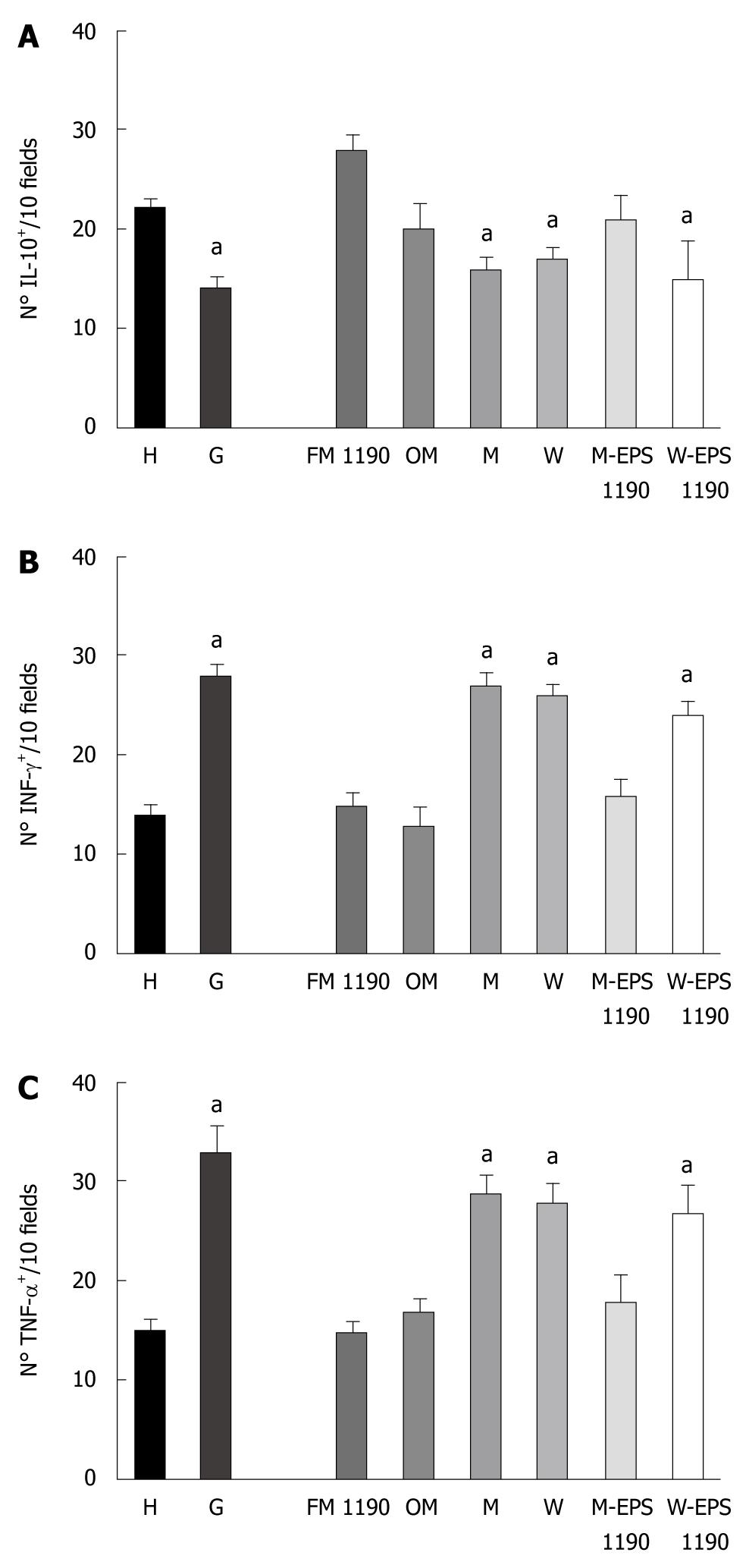
The stomachs from group G mice showed a significant decrease in the regulatory cytokine-producing cells (IL-10+: 14 ± 1.3 vs 22 ± 1.7, P < 0.05) and an increase in the pro-inflammatory cytokine-producing cells (INFγ+: 28 ± 1.2 vs 14 ± 1.0 and TNF-α+: 33 ± 3.0 vs 15 ± 2.0, P < 0.05) with respect to healthy animals (Figure 3A-C).
Figure 3 Number of (A) IL-10-, (B) INF-γ- and (C) TNF-α-producing cells on histological slices of stomachs of mice of different groups.
Results are presented as means of 3 determinations and are expressed as the number of IL-10-, INF-γ- and TNF-α-producing cells per 10 fields (magnification × 100). aP < 0.05 compared to the controls.
The therapeutic administration of FM 1190 to mice regulated the gastric inflammatory process, significantly decreasing the number of pro-inflammatory cytokine-producing cells (INFγ+: 15 ± 1.0 vs 28 ± 1.2, P < 0.05 and TNF-α+ 16 ± 3.0 vs 33 ± 3.0, P < 0.05) and increasing the regulatory cytokine-producing cells (IL-10+: 28 ± 1.5 vs 14 ± 1.3, P < 0.05), as compared to the G group. Furthermore, the number of cytokine-producing cells were similar to those of OM and H groups showing IL-10+ values slightly higher than these 2 groups (28 ± 1.5 vs 20 ± 2.9; 28 ± 1.5 vs 22 ± 1.7, respectively, P < 0.05). In contrast, a significant decrease in the number of IL-10+ cells and an increase in the number of both pro-inflammatory cytokine-producing cells (INFγ+- and TNF-α+) similar to those of the G group (Figure 3) were found in the M and W groups, used as controls.
M-EPS 1190 but not W-EPS 1190 was able to modulate the induced gastritis in a similar way to FM 1190, showing a decrease in the number of pro-inflammatory cytokine-producing cells (INFγ+: 15 ± 2.0 vs 28 ± 1.2, P < 0.05 and TNF- α+: 20 ± 2.6 vs 33 ± 3.0, P < 0.05) and an increase in the regulatory cytokine-producing cells (IL-10+: 21 ± 2.1 vs 14 ± 1.3, P < 0.05), as compared to the G group (Figure 3).
A correlation between the histopathological structure and the number of regulatory and pro-inflammatory cytokine producing-cells was observed for all groups.
Mucus layer determined by PAS staining
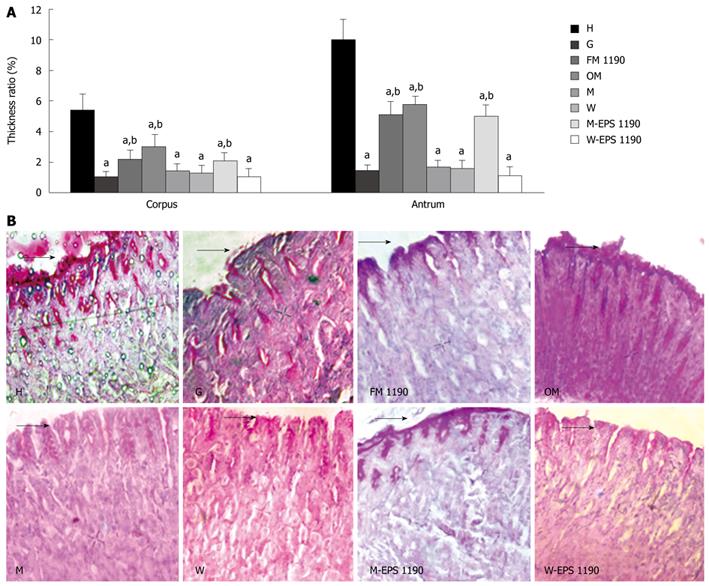
The thickness ratio in the corpus and antrum mucosa, and representative photomicrographs of PAS-stained corpus mucosa from control (H), gastritis (G) and different therapeutic groups (FM 1190, OM, M and W) are shown in Figure 4A and B. The animals treated with ASA (G group) displayed a disruption in the protective mucus layer and exhibited a significant decrease of 80%-85% in the thickness of the mucus gel layer in both the corpus and antrum gastric regions as compared to healthy animals (corpus: 1.07 ± 0.3 vs 5.4 ± 1.0; antrum: 1.47 ± 0.4 vs 10.0 ± 1.3, P < 0.05).
Figure 4 Periodic acid-Schiff (PAS)-positive mucus on the mice gastric mucosa.
A: Thickness ratio of the mucus gel layer to the lamina propria mucosa in corpus and antrum mucosa. B: Representative photomicrographs of corpus mucosa from the various groups. Sections were stained using PAS reagent. Values represent mean ± SE of the mean for 5 mice. aP < 0.05 compared to the healthy group (H), bP < 0.05 compared to the gastritis group (G). Original magnification: × 400. Arrows indicate thickness of mucus layer.
In general, the different treatments for gastritis revealed a marked depletion (approximately 80%) in the mucus layer with concomitant reduction in the volume of PAS-positive intramucosal mucus in either the corpus or antrum mucosa of animals of most groups compared to the H group. A different behavior was observed in animals of group FM 1190, which showed a decrease of 49%-59% in the mucus layer of the antrum and corpus mucosa, compared with healthy animals (2.2 ± 0.6 vs 5.4 ± 1.0; 5.1 ± 0.8 vs 10.0 ± 1.3, respectively, P < 0.05). However, an increase in the thickness of the mucus gel layer as compared to animals displaying gastritis (G group) was observed (2.2 ± 0.6 vs 1.0 ± 0.3; 5.1 ± 0.8 vs 1.5 ± 0.4, in corpus and antrum mucosa respectively, P < 0.05). The volume of intramucosal mucus was maintained compared to the G group, suggesting that FM 1190 was able to protect the stomach mucosal barrier.
Mice treated with M-EPS 1190 showed an increase in the thickness of the mucus gel layer compared to animals with gastritis (2.1 ± 0.5 vs 1.0 ± 0.3; 5.0 ± 0.75 vs 1.5 ± 0.4, in corpus and antrum mucosa, respectively, P < 0.05) and was similar to the FM 1190 group.
DISCUSSION
It has been demonstrated previously that ASA administration to mice caused chronic inflammation of the gastric mucosa[25]. Mononuclear cell infiltration, an increase in the number of pro-inflammatory cytokine-producing cells, a decrease in the regulatory cytokine-producing cells, and depletion of both the mucus gel layer and volume of intramucosal mucus were observed. The use of probiotics has been proposed to ameliorate different gastrointestinal tract disorders including inflammatory bowel diseases, diarrhea, pancreatitis, irritable bowel syndrome and colorectal cancer[31,32]; however, little attention has been paid to gastric disease. Uchida and Kurakazu[33] reported that yogurt LG21 significantly inhibited the formation of acute gastric lesions caused by HCl in rats; the beneficial effect being dose-dependent. Recently, Liu et al[34] reported that continuously feeding LAB-fermented soy-skim milk to rats for 28 d inhibited acute gastric lesions induced by ethanol and pylorus ligation in a dose-dependent manner, and improved prostaglandin E2 and superoxide dismutase activities. In addition, some authors have demonstrated the anti-ulcer properties of LAB-fermented milks on H. pylori-induced gastric lesions[14,35]. Based on the multiple functional effects of probiotics on the gastrointestinal tract, we have previously studied the capability of 2 EPS-producing LAB strains to protect the gastric mucosa from challenges produced by ASA; only the strain S. thermophilus CRL 1190 successfully prevented gastric damage by modulation of inflammation with significant preservation of the mucus gel layer[25]. S. thermophilus CRL 1190 produces a slime heteropolysaccharide of high molecular mass (MM) composed of D-glucose and D-galactose (molar ratio 1.0:1.5) as well as CPS in milk cultures[20]. In this work, we evaluated the therapeutic effect of milk fermented with S. thermophilus CRL 1190 on ASA-induced chronic gastritis. The therapeutic administration of FM 1190 showed a marked (P < 0.05) immunomodulatory effect when comparing the pro-inflammatory cytokine-producing cells (TNF-α+ and INF-γ+) of healthy animals, as a result of inhibition of IL-10 on Th1[36]. Similar immunological modulation of the preventive effect of S. thermophilus CRL 1190 on chronic gastritis was observed in our previous work. Bibiloni et al[37] demonstrated that the Th1 cell response could be modulated by probiotic bacteria in pathological processes such as inflammatory bowel disease or colon cancer[38]. Th1 cytokines such as INF-γ and TNF-α, released by lymphocytes and macrophages that infiltrate the gastric mucosa are associated with immune activation and tissue injury. TNF-α has been shown to be a crucial mediator of NSAID-induced gastric mucosal damage[39]. In contrast, IL-10 (Th2 type cytokine) suppresses the differentiation and effector functions of Th1 cells and the production of pro-inflammatory cytokines by dendritic cells and macrophages, thus maintaining immune homeostasis[40]. The proton pump inhibitor drugs used clinically, such as omeprazole, exert an anti-inflammatory action beyond strong acid suppression[41]. It has been reported that omeprazole regulates the cytokine profile in H. pylori-infected patients with duodenal ulcer disease by suppressing cytokine synthesis of the Th1 cells[42]. In our study, this drug was used as a positive control in the treatment of ASA-induced gastritis, and it displayed similar therapeutic effectiveness to FM 1190, though the fermented milk displayed slightly higher numbers of regulatory cytokine-producing cells (IL-10+) than did omeprazole. On the other hand, this drug has diverse adverse effects such as diarrhea, abdominal pain, cutaneous reactions, decreased bone density and microscopic colitis[43,44] as well as drug interactions[45]. Thus, the use of FM 1190 as a therapeutic agent constitutes a safe and nutritional alternative for gastritis treatment.
Myeloperoxidase activity as a neutrophil infiltration marker in gastric tissue was assayed but it could not be used as an inflammatory parameter in the chronic gastritis model as the values obtained were similar to that of the healthy groups (data not shown).
Milk alone, water, omeprazole or the prepared fermented milk did not cause any damage per se on the gastric mucosa of healthy animals.
To determine if the therapeutic effect obtained after administration of FM 1190 resulted from the presence of its EPS, the produced biopolymer was isolated, purified and resuspended in milk or water, and assayed for its potential therapeutic effect. As previously observed[25], only the EPS dissolved in milk (M-EPS 1190) displayed a similar behavior to the fermented milk with respect to the cytokine profile and the histological structures, suggesting that EPS-milk protein interactions play a major role in the immune response modulation and consequently, in the therapeutic effect observed. Whey proteins have been reported to possess biological functions including immunomodulatory activities[46] in addition to their nutritional value. Rosaneli et al[47] reported the protective effect of bovine milk whey protein concentrate on the ulcerative lesions caused by administration of indomethacin. Moreover, the gastro-protective effect of α-lactoalbumin, one of the major whey proteins, against gastric injury induced by ethanol was demonstrated by enhancing the gastric defense mechanisms such as mucin synthesis and secretion in mucus-producing cells[48-50].
The mucus gel layer is an important defense barrier, covering gastric epithelial cells and holding bicarbonate ions to neutralize hydrogen ions that diffuse back into the gastric mucosa. However, this layer is frequently disrupted by acid, pepsin, alcohol, and other injurious agents in the gastric lumen resulting in damage to gastric epithelial cells[51]. The mucus gel layer thickness in the mouse gastric mucosa as well as the volume of intramucosal PAS-positive mucus were evaluated, as a continuous supply of mucus from the intramucosa is important to preserve the surface gel layer. Therapeutic administration of FM 1190 and M-EPS 1190 increased the thickness of the mucus gel layer in both corpus and antrum mucosa without reducing intramucosal mucus. Thus, the activation of mucin synthesis by FM 1190 and M-EPS 1190 led to the increase in the mucus gel layer and a stable mucus supply from the intramucosa. The stimulation of mucus metabolism contributes to the gastroprotective action. L. rhamnosus GG, a probiotic EPS-producing strain widely used in dairy products, is able to increase the mucus layer thickness in the gastric glandular mucosa[29]. Also, it has been observed that the fungal polysaccharide from Ganoderma lucidum reinstated the gastric mucus levels[52].
Nagaoka et al[53] reported anti-ulcer effects of EPS produced by bifidobacteria, Lactobacilli and Streptococci strains, which were attributed to the high rhamnose content (> 60%) of the polysaccharides. Conversely, the fermented milk with the EPS-producing strain S. thermophilus CRL 804, which produced an EPS with rhamnose and galactose in its monomer composition[20], did not show any anti-gastritis effect in contrast to the EPS 1190 that contained galactose and glucose and displayed a gastroprotective effect. Sengül et al[54] showed that a high MM EPS produced by the probiotic strain L. delbrueckii subsp. bulgaricus B3 significantly ameliorated experimental colitis in rats. Gao et al[52,55] found that treatment of acetic acid-induced ulcers in rats with high MM polysaccharide from Ganoderma lucidum suppressed or restored the decreased gastric mucus levels, increased gastric prostaglandin concentrations and partly suppressed the TNF-α gene. In addition, a high MM pectin polysaccharide from Chinese herbs has shown to be a potent anti-ulcer compound in experimental HCl-ethanol induced ulcers[56]. Moreover, a high MM-homopolysaccharide from marine microalga Gyrodinium impudicum strain KG03 presented immunostimulatory effects, enhancing the tumoricidal activities of macrophages and natural killer cells in vivo[57]. In this case, the beneficial effect was attributed to the sulfate groups present in the polymer, which also contained galactose and uronic acids. In our work, the anti-gastritis effect observed for FM 1190 may be ascribed to the large polymer size of EPS 1190, independently of its monomer composition. In addition, it was recently demonstrated[58] that EPS 1190 was partially degraded when the polymer was submitted to the harsh conditions of an in vitro gastric system, indicating that this polymer may still exert its beneficial properties in vivo.
The present findings indicate that the milk fermented with S. thermophilus CRL 1190 and/or its EPS was effective in the therapeutic treatment of chronic gastritis by modulating the immune response of the mice and by increasing the thickness of the gastric mucus gel layer. Thus, the application of this fermented milk and/or its EPS constitutes a potential natural alternative for the prevention and treatment of ASA-associated gastric damage.
COMMENTS
Background
Gastritis is a common disorder where there is discontinuity in the gastric mucosa. It is caused by several factors including the intensive consumption of anti-inflammatory drugs such as acetyl-salicylic acid (ASA), commonly used in the treatment of chronic diseases and prevention of cardiovascular pathologies. The conventional drugs employed as therapies against gastritis often produce undesirable side effects. The administration of specific probiotics provides a new therapy against gastric disease.
Research frontiers
Gastritis affects 80% of the worldwide population according to data of the Worldwide Health Organization. The use of probiotics has been proposed to ameliorate different gastrointestinal tract disorders such as inflammatory bowel disease, diarrhea, irritable bowel syndrome, etc.; however, little attention has been paid to gastric disease. Thus, the authors decided to investigate the therapeutic effect of milk fermented with exopolysaccharide (EPS)-producing S. thermophilus CRL 1190 and its polymer on chronic gastritis induced by aspirin in mice.
Innovations and breakthroughs
This research demonstrates for the first time the therapeutic effect of the fermented milk with the polymer-producing strain S. thermophilus CRL 1190 and/or its EPS on chronic gastritis induced by ASA in mice. Both the fermented milk and the EPS were able to modulate the immune response in mice and increased the thickness of the gastric mucus gel layer. Furthermore, the therapeutic effectiveness observed was similar to omeprazole®, a commercial drug commonly employed in the treatment of gastritis.
Applications
The fermented milk with the EPS-producing strain S. thermophilus CRL 1190 and/or its EPS constitutes a potential natural alternative for the prevention and treatment of ASA-associated gastric damage.
Terminology
EPS are carbohydrate polymers naturally produced by certain bacteria, alga, yeasts and fungi. These polymers are extensively used in several industries mainly due to their thickening, texturizing, and gelifying properties.
Peer review
The research is a well carried out study.