Published online Apr 7, 2010. doi: 10.3748/wjg.v16.i13.1577
Revised: March 22, 2010
Accepted: March 29, 2010
Published online: April 7, 2010
This review provides an overview of the current state of the art of magnetic resonance spectroscopy (MRS) in in vivo investigations of diffuse liver disease. So far, MRS of the human liver in vivo has mainly been used as a research tool rather than a clinical tool. The liver is particularly suitable for static and dynamic metabolic studies due to its high metabolic activity. Furthermore, its relatively superficial position allows excellent MRS localization, while its large volume allows detection of signals with relatively low intensity. This review describes the application of MRS to study the metabolic consequences of different conditions including diffuse and chronic liver diseases, congenital diseases, diabetes, and the presence of a distant malignancy on hepatic metabolism. In addition, future prospects of MRS are discussed. It is anticipated that future technical developments such as clinical MRS magnets with higher field strength (3 T) and improved delineation of multi-component signals such as phosphomonoester and phosphodiester using proton decoupling, especially if combined with price reductions for stable isotope tracers, will lead to intensified research into metabolic syndrome, cardiovascular disease, hepato-biliary diseases, as well as non-metastatic liver metabolism in patients with a distant malignant tumor.
- Citation: Dagnelie PC, Leij-Halfwerk S. Magnetic resonance spectroscopy to study hepatic metabolism in diffuse liver diseases, diabetes and cancer. World J Gastroenterol 2010; 16(13): 1577-1586
- URL: https://www.wjgnet.com/1007-9327/full/v16/i13/1577.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i13.1577
The liver is a particularly suitable and interesting organ for metabolic studies as it plays a central role in intermediary metabolism, has a high metabolic activity with rapid response to metabolic insults, and is profoundly altered in acute and chronic diseases. Magnetic resonance spectroscopy (MRS) has been established as a non-invasive technique to study cellular biochemistry and metabolism, both at high magnetic strengths in vitro and in the whole body in vivo at field strengths of up to 3 T.
MRS has a number of important advantages over conventional approaches when studying metabolism in diffuse liver disease in humans. The non-invasive character of the technique allows valid assessment of the metabolic profile of many hepatic metabolites in vivo which cannot be accurately measured using invasive biopsy techniques, due to the instability of these compounds and the invasiveness of biopsies. In addition, the non-destructive character of MRS, combined with the large hepatic blood flow, allows repeated measurements over time in the same subject, facilitating long-term longitudinal (observational/intervention) studies as well as short-term dynamic metabolic intervention studies with real-time monitoring of biochemical or metabolic alterations after a meal or an oral or intravenous metabolic challenge by nutrients (sugars, amino acids, lipids), hormones etc. Moreover, the technique can be complemented by dynamic stable isotope tracer studies, allowing the simultaneous assessment of hepatic metabolite concentrations by MRS and turnover measurements in plasma. Of note, although challenge techniques are also used in clinical practice for diagnostic purposes, for instance, to diagnose inherited metabolic errors of metabolism, MRS of the human liver in vivo has so far mainly been used as a research tool rather than a clinical tool.
However, one limitation of MRS studies is that only compounds present at mmol/L concentrations can be detected, due to the low inherent sensitivity of the MR signal in vivo.
The liver is a particularly suitable and interesting organ for metabolic studies for a number of reasons: first, it plays a central role in intermediary metabolism, has a high metabolic activity with a rapid response to metabolic insults, and is profoundly altered in acute and chronic diseases. Second, its superficial location in the right upper abdomen, covered only by a thin layer of skin, adipose tissue and muscle, allows excellent MRS localization using a double-tuned surface coil, by different localization techniques such as chemical shift imaging (spectroscopic imaging, CSI/SI)[1-3] and image-selected in vivo spectroscopy (ISIS)[4,5]. Third, the relatively large volume of the liver allows the detection of signals with relatively low intensity in volumes of up to 1 L; of note, for the study of diffuse liver disease, the inherent low spatial resolution of MRS does not play a restrictive role.
Different nuclei such as 31P, 1H, 13C and 19F have been applied in studies of diffuse liver disease. So far, 1H MRS has mainly been applied to detect hepatic lipid levels, 31P MRS to investigate intracellular energy metabolism, phospholipid metabolism and gluconeogenesis, and 13C MRS to study liver lipid and glycogen metabolism. 1H and 31P are naturally abundant isotopes, whereas 13C represents only 1% of total carbon (the majority being 12C). Therefore, in many 13C studies, investigators have infused 13C-labeled compounds to increase the MR signal in dynamic metabolic studies.
The present review provides an overview of the current state of the art of MRS in in vivo investigations of diffuse liver disease. First, we discuss publications regarding the application of MRS to study diffuse and chronic liver diseases including cirrhosis, fibrosis and alcoholic liver disease, followed by the application of MRS in congenital diseases and pediatrics. We then discuss the application of MRS to study the metabolic involvement of the liver in diseases, such as diabetes or the presence of a malignant tumor elsewhere in the body. Finally, future prospects including potential clinical applications of MRS are discussed.
From the present review, we excluded T2 and MRS studies on liver iron and fat content as well as in vitro applications of MRS in hepato-biliary disease[6,7], as well as MRS studies of focal liver diseases such as secondary liver tumors, lymphomas and adenomas, and transplantation. We also excluded 19F MRS studies of metabolites of fluorinated chemotherapeutic drugs such as 5-FU and capecitabine[8].
Over the last two decades, the usefulness of 31P MRS for the diagnosis of liver disease has been investigated as a non-invasive alternative to liver biopsy, which is still the gold standard and carries significant morbidity[9]. The in vivo spectra of the human liver reflect metabolic and biochemical alterations in disease. Several publications on liver functionality have addressed spectral changes related to the underlying liver disease. However, in order to be helpful as a diagnostic tool, MR spectra should provide both sensitive and specific information for different types of liver disease, such as hepatitis, steatosis, fibrosis or cirrhosis.
The 31P MRS spectrum provides information on phosphorylated compounds of hepatic metabolism: alpha-, beta- and gamma peaks of nucleotide triphosphates (NTP), inorganic phosphate (Pi), phosphomonoesters (PME) and phosphodiesters (PDE). The NTP, PME and PDE peaks are multicomponent and individual resonances of their components cannot be distinguished by the majority of techniques currently used. PME reflects components from glucose metabolism (gluconeogenesis and glycolysis) and cell membrane precursors such as phosphoethanolamine (PE) and phosphocholine (PC)[10]. The majority of the NTP resonance contains ATP components; below, we will use ATP and NTP as synonyms, following the nomenclature of the cited publications. The PDE peak contains information on cell membrane breakdown products, such as glycerophosphorylethanolamine (GPE) and glycerophosphorylcholine (GPC), and endoplasmic reticulum[11].
The diagnostic value of 31P MRS for various types of diffuse liver disease has been investigated in several studies. Overall, diffuse liver disease was associated with increasing levels of PME and decreasing levels of PDE. These changes have been attributed to hepatocyte damage, increased phospholipid turnover in hepatocyte membranes, and/or altered glucose metabolism. In general, the magnitude of MRS changes increased significantly with increased disease severity and increased functional impairment.
In one of the earliest reports[12], liver metabolite concentrations were studied in 24 patients with various types of diffuse liver disease as compared to healthy control subjects. The authors reported high PME and low PDE levels in patients with acute viral hepatitis, high PME levels in patients with alcoholic hepatitis, and decreased Pi and Pi/ATP ratios in primary biliary cirrhosis and in some patients with hepatitis[12]. However, they noted that these changes were not present in all patients.
Cox et al[1] studied 49 patients with liver disease of varying etiology, including 25 patients with diffuse liver disease such as cirrhosis and non-hepatic malignancies. A non-specific elevation in PME/PDE was observed in the 31P MR spectra of 10 (40%) out of these 25 patients with mixed diffuse liver disease. Even though the spectral pattern did not distinguish between diseases of varying etiologies, there was a linear correlation between increasing PME/PDE and a reduction in plasma albumin concentrations (P = 0.03).
Subsequent MRS studies showed elevated PME/NTP levels compared to healthy controls in patient populations with primary biliary cirrhosis[13] and compensated/decompensated cirrhosis of various etiology[14]. Jalan et al[13] studied 23 patients with primary biliary cirrhosis of varying functional severity and healthy subjects using 31P MRS. PME/NTP, Pi/NTP, PME/PDE, and PME/Pi ratios were higher and PDE/NTP ratios significantly lower in patients compared with healthy volunteers. Significant correlations were seen between PME/Pi ratios and prognostic indicators such as the Christensen index, the Mayo R value, and the Pugh score.
Taylor-Robinson et al[14] compared 14 patients with compensated cirrhosis (Pugh’s score ≤ 7) and 17 with decompensated cirrhosis (Pugh’s score ≥ 8) of various etiology with healthy subjects. Worsening liver function was associated with increased PME/NTP and decreased PDE/NTP ratios. In freeze-clamped tissue, elevated PE and PC, and reduced GPE and GPC mirrored these in vivo changes, but no distinction was noted between compensated and decompensated cirrhosis. In contrast, electron microscopy showed that functional decompensation was associated with reduced endoplasmic reticulum (ER) in parenchymal liver disease, but elevated ER in biliary cirrhosis. In a more recent study in only 14 cirrhotic subjects[15], reduced ATP and elevated PME/PDE levels were detected in patients with decompensated cirrhosis only.
The capability of 31P MRS to detect pathological processes was further investigated by comparing in vivo MRS results with histological samples. In 38 patients with various types of diffuse liver disease, van Wassenaer-van Hall et al[5] showed that the degree of elevation of PME/Ptotal in individual patients was significantly correlated with necrosis, intralobular degeneration, and portal inflammation scorings in liver biopsies[5], but not with fibrosis. However, 31P MRS was not able to classify patients into diagnostic categories, such as fibrosis vs cirrhosis, and no diagnostic value of MRS was found with respect to steatosis and cholangitis.
Dezortova et al[16] studied 80 patients with liver cirrhosis of different etiology and functional status, described by Child-Pugh score, and a control group of healthy subjects. Patients with both alcoholic and viral etiology had lower absolute PDE and ATP levels than healthy subjects. Patients with alcoholic etiology, but not viral etiology, also had lower Pi levels than controls. Patients with cholestatic disease had elevated PDE levels. Thus, these authors were able to distinguish alcoholic, viral and cholestatic etiologies of liver cirrhosis based on MR spectra.
Noren et al[17] studied patients with non-alcoholic fatty liver disease (NAFLD) and none to moderate inflammation (n = 13), patients with severe fibrosis or cirrhosis (n = 16), and healthy controls. All patients underwent liver biopsy and extensive biochemical evaluation. Absolute concentrations and the anabolic charge (AC), defined as (PME)/[(PME)+(PDE)], were calculated. AC was increased and PDE reduced in the cirrhotic group relative to healthy subjects, whereas NAFLD patients showed values similar to controls. Using a PDE concentration of 10.5 mmol/L as a cut-off value to discriminate between mild, (F0-2) and advanced (F3-4) fibrosis, the sensitivity and specificity of PDE were 81% and 69%, respectively. AC, using a cut-off value of 0.27, showed a sensitivity of 93% and a specificity of 54%. The authors concluded that PDE is a potential marker of liver fibrosis, whereas AC is a potentially clinically useful parameter discriminating mild from advanced fibrosis.
Interestingly, few authors have performed dynamic 31P MRS studies with a metabolic challenge, in contrast with diabetes and cancer (see below). In healthy subjects, it is well known that fructose infusion induces a rapid rise in PME and marked reductions in ATP and Pi[3,18,19]. An early Japanese study[20] evaluated changes in the metabolic state of the liver after an intravenous fructose load (250 mg/kg) in six patients with liver cirrhosis and eight healthy volunteers. The cirrhotic livers did not show the usual increase in PME after the fructose load, suggesting that fructose metabolism in the cirrhotic livers had impediments before the fructose-1-phosphate stage. Furthermore, the spectra of the cirrhotic livers showed a significant drop in Pi, PDE and ATP peak after the fructose challenge. Dufour and colleagues[21] studied nine patients with nonalcoholic cirrhosis. Fructose (250 mg/kg) was injected intravenously, and further spectra were collected sequentially every 6 min for 1 h. PME formation and utilization of Pi were markedly attenuated in cirrhotic patients; these measures correlated with the impairment of liver function as measured by galactose-elimination capacity[21].
In summary, the general observation in cirrhosis and fibrosis is an increase in PME, often combined with reductions in PDE and ATP. Of note, the observed changes were correlated with classical markers such as Pugh score and plasma albumin. However, 31P MRS was not able to classify patients into diagnostic categories, such as fibrosis vs cirrhosis, and no diagnostic value of MRS was found with respect to steatosis and cholangitis. An intravenous fructose load induced a rapid rise in PME and reductions in ATP and Pi.
A study of 26 patients with an acute viral hepatitis A infection showed increased PME/PDE ratios[22]. After 6 wk of recovery, these abnormalities in liver metabolites were restored to normal levels, as reflected by decreasing PME and increasing PDE[22].
Lim and colleagues[9] used 31P MRS to assess disease severity in 48 patients with hepatitis C virus (HCV)-related liver disease. Worse liver function was correlated with an in vivo elevation in PME and decrease in PDE. PME/PDE ratios showed an increase from control (0.15), via mild disease (0.18) and moderate disease (0.25), to the cirrhosis group (0.38). An 80% sensitivity and specificity was achieved when using a PME/PDE ratio less than or equal to 0.2 to denote mild hepatitis and a corresponding ratio greater than or equal to 0.3 to denote cirrhosis[9]. In a subsequent study[23], the same authors applied 31P MRS to prospectively study 47 patients with biopsy-proven hepatitis C undergoing viral eradication treatment with interferon and ribavirin at 6-mo intervals over a total period of 6-18 mo. In 25 out of 32 patients with virological response to HCV treatment, this was accompanied by decreasing PME/PDE ratios over time, whereas in 15 patients without virological response, PME/PDE ratios increased[23], suggesting that PME and PDE can be used to monitor treatment response to HCV.
Elevated levels of PME were also observed in a study of 75 chronic hepatitis B and C infected patients using 1H-MRS[24]. In addition, compared to healthy control subjects, glutamine/glutamate and glycogen/glucose resonances were increased, whereas lipids were decreased[24]. As in studies using 31P MRS, increases in metabolite levels were correlated with disease severity[24]. In a study in patients with hepatitis C virus infection (HCV, n = 14) and 20 HIV/HCV co-infected individuals[25], a significant increase in glutamine/glutamate and PME, both measured relative to hepatic lipid levels, was observed in both groups when compared with healthy individuals. These changes in metabolite ratios were attributed to an increase in the particular metabolite contents and a decrease in lipid levels. HIV/HCV-infected patients treated with anti-retroviral therapy showed elevated PME and glutamine/glutamate levels and decreased total lipid levels compared to patients not undergoing anti-retroviral treatment[25]. The authors concluded that 1H-MRS could be used to detect even slight alterations in hepatic metabolite ratios in this type of patient.
Orlacchio et al[26] also applied 1H-MRS but used the water signal as a reference instead of the lipid signal. These authors studied 23 patients with biopsy-proven precirrhotic HCV-related liver disease, graded by the Ishak fibrosis (F) scoring system. Similar to the previous studies, increasing disease severity correlated with a significant increase in choline-containing compounds and glutamine/glutamate. In contrast, lipid levels in this study were found to be increased in patients relative to healthy subjects[26].
In summary, 31P MRS studies have demonstrated that PME/PDE ratios in viral hepatitis are increased, and normalization of PME/PDE over time correlates with treatment effectiveness. 1H MRS studies showed increased glutamine/glutamate and glycogen/glucose resonances. The observation of either increased or decreased lipid resonances in different studies requires further investigation.
Although the above data show that different 31P MRS studies have demonstrated elevated PME/NTP levels in patient populations with alcoholic liver disease compared to healthy subjects, there are few reports on the effects of alcohol abuse and alcohol abstinence per se. Menon et al[27] studied 26 chronic alcohol abusers by 31P MRS 6-12 h after their last alcoholic drink and following abstinence from alcohol. Results showed that in patients with minimal liver injury, recent drinking was associated with a significant elevation in PDE/ATP and a non-significant rise in PME/ATP, and abstinence with normalization of both metabolite ratios. In contrast, in patients with alcoholic cirrhosis, recent drinking was associated with a significant elevation in mean PME/ATP and a non-significant increase in PDE/ATP, whereas abstinence was associated with no significant change in PME/ATP but with a reduction in PDE/ATP. The authors suggested that the changes in PDE/ATP most likely reflected the induction of hepatocyte ER.
In summary, recent alcohol consumption is associated with elevated PME and PDE. In patients with minimal liver injury, both metabolites are normalized during abstinence; in contrast, in patients with cirrhosis, only PDE is normalized during alcohol abstinence.
Several studies have been performed on acute poisoning, inherited diseases and pediatrics using 31P MRS. A study in 18 patients after acetaminophen overdose showed that liver metabolites including PME, PDE and ATP, were all dramatically decreased with increasing liver damage expressed as the international normalized ratio (INR) of prothrombin[28]. Repeated MRS measurements in the same patients would be particularly valuable to see whether improvement in liver damage could also be confirmed by 31P MRS.
The presence of hemolysis, elevated liver enzymes and low platelets in pregnant women (HELLP syndrome) can be associated with disturbed hepatic metabolism. To investigate whether or not women with HELLP syndrome have detectable abnormalities of hepatic energetics, Magee et al[29] studied seven patients with HELLP syndrome. One pregnancy was later terminated but the other women gave birth to healthy infants. One patient with the most clinically severe HELLP syndrome by laboratory criteria exhibited MR spectra which showed a relative increase in phosphomonoester and an absolute decrease in hepatic ATP (to 62% of control)[29]. Most patients with HELLP syndrome had normal liver metabolism as assessed by MRS, although these results show that clinically severe HELLP syndrome can be associated with disturbed hepatic metabolism consistent with that seen in hepatic ischemia and/or granulocytic infiltration of the liver.
In ten patients with severe hypothyroidism, prospective 31P MRS measurements before and after thyroid hormone treatment were performed. In contrast with striated muscle, which showed a marked normalization of phosphocreatine/Pi ratios within several weeks of thyroid treatment, no changes in hepatic metabolism during treatment were detected by 31P MRS[30].
In a single patient with amyloid light chain (AL) amyloidosis, hepatic 1H MR spectra were characterized by small line widths, a striking increase in trimethylammonium compounds, and the presence of a further resonance at 3.8 ppm[31]. None of the healthy control subjects showed trimethylammonium levels of comparable intensity[31].
Changes in liver metabolites in inherited liver diseases have been reported using 31P MRS. In a report by Dixon et al[32] on an infant with galactosemia, these authors showed increased levels of galactose-1-phosphate in the liver which decreased during diet therapy, paralleling the falling level of galactose-1-phosphate in red blood cells.
Elevated plasma uric acid concentrations (hyperuricemia), which are a characteristic feature in gout patients, may be caused by altered liver fructose metabolism. As hereditary fructose intolerance is known to be associated with hyperuricemia, this concept has been used in 31P MRS studies[33-35]. The effect of a fructose challenge on liver metabolism was studied by 31P MRS in five patients with hereditary fructose intolerance (HFI) and eight heterozygotes for HFI[33]. In patients with HFI, ingestion of small amounts of fructose was followed by an increase in sugar phosphates (PME) and a decrease in Pi in hepatic 31P MR spectra, combined with a rise in plasma uric acid. 31P MRS could be used to diagnose fructose intolerance in heterozygotes. Oral fructose (50 g) also led to sugar phosphate accumulation and Pi depletion in the liver, which was associated with a larger increase in plasma uric acid than in control subjects. The effect of fructose on liver Pi and plasma uric acid was most pronounced in heterozygotes with gout (n = 3). In a subsequent study in 18 additional subjects from different families with familial gout[34], the authors demonstrated a positive association between response in 31P MR spectra (i.e. increase in PME, decrease in Pi) and response in serum uric acid after oral glucose, suggesting 31P MRS as a method for initial screening of this defect.
Limited MRS data are available in the pediatric population. Nevertheless, 31P MR spectra of infants have been compared with adult spectra in an attempt to investigate changes in hepatic metabolism with age[36]. Spectra from three infants showed that PME/ATP levels were markedly higher than in adults, whereas PDE/ATP levels were decreased when compared with adults. Spectral hepatic concentrations of a single adolescent studied were intermediary between the neonates and the adults. The authors hypothesized that this indicated an increased rate of membrane synthesis in the infant livers, and concluded that these differences should be taken into account when comparing studies using varying ages[36].
In summary, a considerable amount of data on the use of MRS for the study of liver metabolism in disease is currently available, although most studies were performed in relatively small groups. Static measurements mainly reveal alterations in relative PME and/or PDE concentrations as compared to healthy controls. In contrast, dynamic applications of the MRS technique to obtain information during a challenge have remained limited to date. Challenge tests will be discussed in more detail in the following sections.
Impairment of hypoglycemic counterregulation, which occurs even in intensively treated type diabetes, has traditionally been attributed to deficits in counterregulatory hormone secretion in type I diabetes, and to impaired hormone sensitivity of target organs in type II diabetes. In the normal fed state, the liver takes up glucose for energy production (via glycolysis and TCA cycle) and stores excess energy as glycogen, whereas in the fasted state, the liver releases glucose produced from glycogen and via gluconeogenesis; in fact, the liver is almost exclusively responsible for endogenous glucose production (EGP). After a glucose load, liver glycogen can be synthesized both directly from glucose (via glucose-6P, glucose-1P and UDP-glucose) and indirectly from 3 carbon units (via phosphoenolpyruvate-glucose-6P etc.). 13C MRS, either at natural abundance or combined with 13C tracer infusion, provides a tool to study glycogen content in the liver and thus, in dynamic studies, net glycogen synthesis and breakdown over time. Moreover, combined with turnover measurements using deuterated glucose tracer infusions and measurement of isotopic enrichment of UDP-glucose using acetaminophen (measured as acetaminophen glucuronide in plasma/urine) allows an estimation of relative contributions of the direct and indirect pathways of glycogen synthesis. Noninvasive sampling of hepatic glutamine pools by oral administration of phenylacetate allows simultaneous estimation of the contribution of pyruvate oxidation to the TCA cycle flux[37].
Over the past decades, 13C MRS studies have significantly contributed to the notion that the liver plays a critical role in the derangements of plasma glucose regulation in both type I and type II diabetes. Most MRS studies to date have been performed in diabetes type I. To determine alterations in the direct and indirect pathways of glycogen synthesis in diabetes, Cline and coworkers[37] studied subjects with poorly controlled diabetes type I using a 5 h hyperglycemic-hyperinsulinemic clamp (plasma glucose: 9 mmol/L, insulin: 400 pmol/L) and [1-13C]-glucose. Hepatic pools of UDP-glucose and glutamine were noninvasively sampled by oral administration of acetaminophen and phenylacetate, respectively. Although total hepatic glycogen synthesis was similar in both groups, the flux through the indirect (gluconeogenic) pathway was found to be proportionately about twice as active in the diabetic subjects compared to the control subjects. Moreover, the relative contribution of pyruvate oxidation to the TCA cycle flux in diabetic subjects was decreased by circa 30%, indicating reduced glycolysis. The abnormalities were not immediately reversed by normalizing intraportal concentrations of glucose, insulin and glucagon, and might contribute to postprandial hyperglycemia.
Hwang et al[38] studied poorly controlled diabetes type I patients and weight-matched control subjects during a day in which three isocaloric mixed meals were ingested. Although fasting hepatic glycogen levels were identical in the two groups, the diabetic subjects synthesized less glycogen over the day than healthy subjects. Again, the flux through the gluconeogenic pathway relative to the direct pathway of glycogen synthesis was markedly reduced in diabetic subjects.
Bischof et al[39] studied poorly controlled diabetes type I patients (HbA1C: 8.8% and matched non-diabetic subjects (HbA1C: 5.5%) in an experiment over 24 h with 3 isocaloric mixed meals. Over 24 h, the mean plasma glucose concentration was 2.4-fold higher in diabetic subjects. Net liver glycogen synthesis and breakdown were calculated from linear regression of the glycogen concentration time curves from 19:30-22:30, and 22:30-8:00, respectively. Glycogen synthesis was reduced by 74% and glycogen breakdown by 47%, and both were partly but not completely normalized by intensified insulin treatment.
In a subsequent study in long-term well-controlled diabetes type I patients, Bischof et al[40], showed that tight glycemic control in diabetes type I patients normalized overall glycogen synthesis and breakdown as well as glucose production. However, the relative contribution of the indirect pathway of glycogen synthesis remained elevated in diabetic subjects, a finding which indicated augmented gluconeogenesis in type I diabetic patients, which would be consistent with experimental models showing increased PEP-carboxykinase and/or reduced glucokinase activity in the liver of diabetic animals.
Also, in 6 to 12-year-old children with diabetes type I[41], the capacity to replenish hepatic glycogen after an overnight fast was at least as good as in age-matched healthy children.
Kishore et al[42] compared the hepatic response to hypoglycemia in well-controlled type I diabetes patients and healthy subjects. In the overnight fasted state, diabetes type I patients had decreased hepatic glycogen levels compared to controls. In insulin-induced hypoglycemia, the normal response of glycogenolysis observed in healthy subjects was virtually absent in diabetic subjects.
Type II diabetes patients were studied by Magnusson et al[43], who compared seven diabetic (mean duration 13 years) and five healthy subjects matched for age and body mass index (BMI) during 23 h of fasting after an initial liquid meal. Oral medication with sulfonylurea agents (n = 5) was discontinued 3 d before the study and insulin treatment (n = 2) was discontinued the evening before the study. Blood glucose levels in the diabetic subjects were consistently higher throughout the experiment. Four hours after the meal, hepatic glycogen levels in diabetic subjects were < 50% of those in control subjects. During the subsequent fasting period, glycogen breakdown was reduced in diabetic subjects; instead, their rate of gluconeogenesis was markedly increased, resulting in a circa 20% overall increase in glucose output compared to healthy subjects.
Krssak and colleagues[44] studied hepatic glycogen synthesis, glycogen breakdown and gluconeogenesis in patients with type II diabetes (mean duration 6 years) and age- and weight-matched healthy subjects before and after a mixed meal. Hepatic glycogen concentrations were lower in diabetic patients before and after dinner, and post-meal glycogen synthesis was reduced by circa 44%. Overnight, rates of glycogenolysis were also circa 50% lower in diabetic patients than controls. Endogenous glucose production was elevated in diabetic patients before dinner and remained so for 3 h after dinner; also, the nadir of glucose production was delayed in diabetic patients (240 min vs 60-90 min); thereafter, glucose production in patients and controls was similar. At 6-9 h after dinner, gluconeogenesis amounted to 67% of EGP in diabetic patients vs 43% in controls.
During a subsequent hyperglycemic-hyperinsulinemic clamp, glycogen synthesis was circa 46% lower in diabetic patients, with a similar contribution from the direct and indirect pathways. Glycogen breakdown was similar, resulting in circa 54% lower net glycogen synthesis. EGP was circa 30% elevated in diabetic patients both before and during the clamp. Hepatocellular lipid content was three times higher in type II diabetic patients and negatively correlated with rates of net glycogen synthesis and whole-body glucose uptake during the clamp test (which was circa 37% reduced in diabetic patients)[44]. Petersen et al[45] demonstrated that an average weight loss of 8 kg in type II diabetic patients over a period of 7 wk led to normalization of plasma glucose concentrations as well as hepatic glycogenolysis, glucose production and gluconeogenesis, indicating marked restoration of hepatic insulin sensitivity. These changes were associated with a reduction in hepatic lipid content from 12% to 3%, whereas muscle lipid content and muscle insulin resistance remained unchanged[45].
In summary, the presence of marked alterations in hepatic glucose and glycogen metabolism in type I and type II diabetes has been substantiated by 13C MRS in combination with isotope tracer studies. When compared with non-diabetic humans, both patients with type Iand type II diabetes exhibit elevated endogenous glucose production by increased gluconeogenesis, combined with reduced glycogen synthesis and breakdown. In type I diabetes, this defect can be partly restored by combined long- and short-term optimized treatment with insulin. In contrast, in type II diabetes, increased gluconeogenesis appears to be the main cause of elevated glucose production and fasting hyperglycemia, and it is normalized by weight reduction.
The cancer-bearing state is generally associated with profound alterations in host metabolism. Many patients lose weight due to decreasing fat and muscle mass, a condition known as cancer cachexia, which not only leads to functional decline but is also an important predictor of poor prognosis. Although cancer cachexia is partly caused by decreased appetite, altered metabolism in host organs such as liver and muscle are now thought to play a major role in the pathogenesis of the condition. So far, most studies have concentrated on experimental animal models of cancer cachexia. To further explore the role of altered liver metabolism in the etiology of cancer cachexia in humans, Dagnelie et al[46] and Leij-Halfwerk et al[47-51] performed a series of studies in patients with advanced lung and breast cancer. Importantly, patients were only included if they were free of liver metastases, as confirmed by CT and/or ultrasound. In all studies, weight-stable and weight-losing cancer patients were compared with healthy subjects. Dietary energy intake was similar in all subjects, and all spectra were acquired in the overnight-fasted state. Since ATP peak areas differed between patients and controls, total phosphorous content of the liver (which was demonstrated to be stable) was used as a reference.
In one study, ATP peak areas were significantly reduced in both weight-stable (WS) and weight-losing (WL) cancer patients[46]; in the second study, the reduction was only statistically significant in WL but not WS cancer patients[47]. During a 90-min infusion of 2.8 mmol/kg per minute of the gluconeogenic amino acid alanine, liver ATP levels decreased in healthy subjects and patients to a similar extent for 60 min; however, from 60-90 min, ATP levels in WS cancer patients and healthy subjects recovered to baseline values despite continued infusion, but further decreased in WL patients, indicating impaired ATP recovery[47]. Intravenous infusion of ATP at 75 μg/kg per minute over 24 h induced complete normalization of liver ATP levels both in WL and WS patients to levels similar to healthy subjects[51].
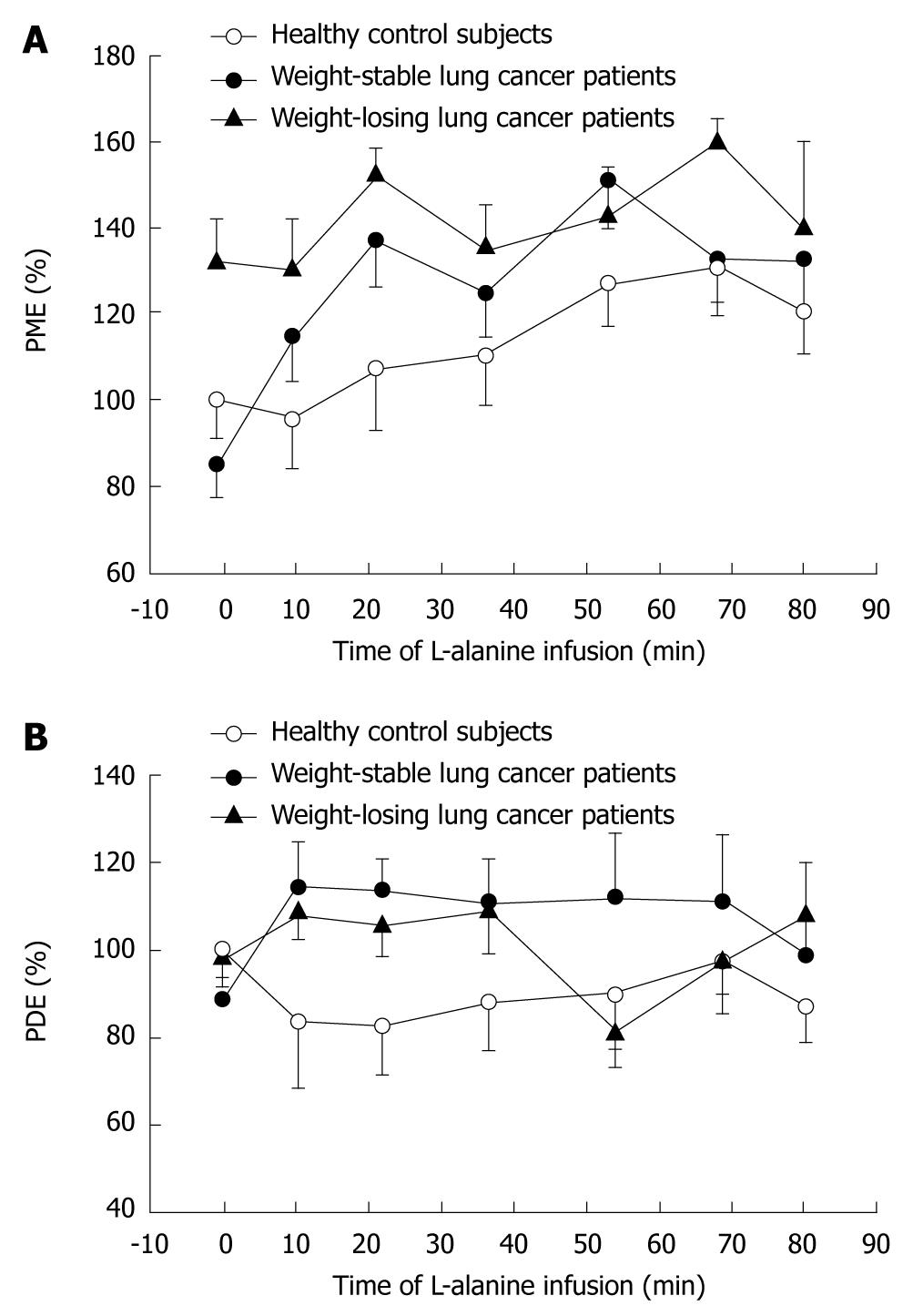
As shown in Figure 1, baseline PME values in the liver were markedly increased in WL cancer patients relative to WS patients and healthy controls, due to increased glucose cycling and gluconeogenesis as indicated by both the observed downfield alteration of the PME chemical shift[46] and the significant correlation of PME levels with glucose turnover and gluconeogenesis from alanine[49]. Baseline alanine turnover as measured by tracer techniques was elevated in WL cancer patients, but not in WS patients[48]. L-alanine infusion induced a steady 33% rise in PME in healthy subjects (Figure 1). In WS cancer patients, a markedly faster and higher (i.e. 69%) response of PME to alanine infusion was observed, whereas WL patients showed virtually no further rise in PME (7%) relative to their already elevated baseline PME levels (Figure 1)[48]. In cancer patients, but not in healthy subjects, the rise in PME levels during alanine infusion was strongly inversely correlated with baseline PME (r = -0.82). Liver intracellular pH increased in the order: healthy < cancer-WS < cancer-WL[46], suggesting that the host liver may have a similar intra-extracellular pH gradient as reported for cancerous tissue[52-55].
In summary, using 31P MRS, marked alterations in hepatic glucose metabolism were demonstrated in the non-metastatic liver of cancer patients, as shown by elevated hepatic concentrations of gluconeogenic intermediates in the overnight-fasted state, as well as during infusion of a gluconeogenic challenge. Of note, the rapid response of the PME peak to alanine infusion demonstrated rapid induction of the gluconeogenic pathway in WS cancer patients, corroborating the power of MRS as a tool to detect enzymatic alterations within the liver.
So far, MRS has primarily been used as a research tool. MRS in vivo allows rapid assessment of the metabolic profile and function, including alterations in liver metabolism and physiology under different conditions. Dynamic studies have so far mainly been performed in diabetes and cancer. The limitation of these studies is the relatively high cost of the equipment and, even more, the cost of 13C enriched compounds which are infused to increase hepatic 13C content and to directly monitor the signal increase of 13C in different metabolites above the 1% natural abundance of 13C. As high-field machines become more available in the next decades, it is expected that the application of 13C MRS studies in the liver may continue. However, the possibilities of 31P MRS have by no means been exhausted, as is shown by the dynamic studies demonstrating altered host liver metabolism in patients with a malignant tumor elsewhere in the body. As the studies in cancer and diabetes demonstrate, metabolic challenges such as carbohydrates (like as glucose and fructose), amino acids (such as alanine) and lipids are likely to yield substantial new knowledge in the near future.
Comparing individual papers investigating disease states is difficult as there is no overall standard for representation of data or methods. Interpretation of part of the studies in diffuse liver disease is hampered by the notion that healthy controls were not always matched for age, weight/body mass index (BMI), gender, etc.; often, healthy subjects were younger than patients. As it has been shown that these factors may impact the quality of the spectra[56] and concentrations of metabolites[36], attempts should be made to minimize their bias. Many studies showed substantial between-subject variability within patient and control groups, e.g. with regard to PME.
Another important issue is the difference in magnetic field strengths and techniques used in different studies with regard to spatial resolution and differences in repetition times (TR), which lead to T1 weighting. T1 weighting in quantification of metabolites in liver MRS studies has so far been relatively neglected[2]. Each data acquisition step in MRS comprises a brief excitation of nuclei of interest by irradiation with non-ionizing radiofrequency energy, followed by measurement of the signal derived from relaxing nuclei in the tissue of interest. TR is defined as the time between two subsequent radiofrequency pulses. The full information is only derived when the nuclei of interest are allowed sufficient time to fully relax. T1 weighting occurs when a new radiofrequency pulse is administered before full relaxation of the nuclei of interest. Although depending on the pulse angle, a rule of thumb is that the applied TR must be circa 5 times T1 or longer in order to obtain fully relaxed spectra. Sijens et al[2], using chemical shift imaging with 1 D phase encoding for localized measurement of 31P metabolites to measure a large liver voxel, showed that, with a pulse angle of 70o at TR 1 s, concentrations of liver metabolites are only 54%-84% of those at full relaxation (TR 50 s). Importantly, T1 relaxation times may change in disease: Dagnelie et al[46] showed ATP concentrations in WL-cancer patients to be reduced relative to healthy subjects at TR 5 s and TR 20 s, but not at TR 1 s. In a later study, Leij-Halfwerk et al[51] showed that ATP infusion induced an increase in hepatic ATP levels at TR 15 s; however, this was not observed at TR 1 s (Leij-Halfwerk et al, 2002, unpublished data). Thus, accurate assessment of changes in metabolite concentrations in different conditions is only possible if TR is chosen to be sufficiently long to allow a large degree of relaxation. As the measured MR signal becomes larger as TR increases, signal to noise ratios will improve, allowing a smaller number of acquisitions per phase encoding step, thereby relatively reducing measurement time. Thus, using 4 phase encoding steps, Sijens et al[2] showed the following number of acquisitions and measurement time at TR 1, 5 and 20 s, respectively: 60 (4:08 min), 20 (7:20 min), and 8 acquisitions (13:20 min).
What are the prospects for the clinical application of MRS in diffuse liver disease? For use as a diagnostic tool, not only high sensitivity and specificity at an individual patient levels are essential, but also practicality of application including patient burden and costing. These requests are most likely to be fulfilled in inherited diseases, where marked metabolic alterations are typical, however, for the majority of diseases, other, less invasive tools will remain methods of first choice. However, MRS may be an excellent tool for assessing disease severity and for monitoring disease progress or recuperation, as shown e.g. in diabetes[45] and patients with primary biliary cirrhosis[13].
Finally, altered findings in MRS may predict disease progression, weight loss or survival, and thus assist in the estimation of patients’ prognosis. For instance, preliminary analyses (Leij-Halfwerk & Dagnelie, unpublished observations) demonstrated that elevated baseline PME levels in WS cancer patients predicted subsequent weight loss and shorter survival.
MRS of diffuse liver disease has given important new insight in a number of diseases, including inherited diseases, hepatitis, steatosis and cirrhosis. Importantly, MRS has also allowed new insights in metabolic derangements in non-liver diseases such as diabetes and non-liver cancer. Based on the progress achieved so far by MRS studies in diabetes, it is anticipated that future technical developments such as clinical MRS magnets with higher field strength (3 T) and improved delineation of multi-component signals such as PME and PDE using proton decoupling, especially if combined with price reductions for stable isotope tracers, will lead to intensified research into metabolic syndrome, cardiovascular disease, hepato-biliary diseases, as well as non-metastatic liver metabolism in patients with a distant malignant tumor.
Of note, there are also potential drawbacks: thus, tightening regulations related to preparing and administering compounds by intravenous infusion or orally threaten to make metabolic research extremely costly (or perhaps even impossible) in the near future: a development which could have dramatic negative consequences for scientific and clinical progress in the field.
Emphasis should be placed on the development of standards of the techniques used in order to be able to compare data and to obtain valid estimates of absolute metabolite concentrations. In order to allow MRS to compete with standards of care, efforts should be made to validate results in larger patient cohorts and to minimize bias in distinguishing diseased states from healthy states.
Peer reviewer: Paul E Sijens, PhD, Associate Professor, Radiology, UMCG, Hanzeplein 1, 9713GZ Groningen, The Netherlands
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH
| 1. | Cox IJ, Menon DK, Sargentoni J, Bryant DJ, Collins AG, Coutts GA, Iles RA, Bell JD, Benjamin IS, Gilbey S. Phosphorus-31 magnetic resonance spectroscopy of the human liver using chemical shift imaging techniques. J Hepatol. 1992;14:265-275. |
| 2. | Sijens PE, Van Dijk P, Dagnelie PC, Oudkerk M. Non-T1-weighted 31P chemical shift imaging of the human liver. Magn Reson Imaging. 1995;13:621-628. |
| 3. | Terrier F, Vock P, Cotting J, Ladebeck R, Reichen J, Hentschel D. Effect of intravenous fructose on the P-31 MR spectrum of the liver: dose response in healthy volunteers. Radiology. 1989;171:557-563. |
| 4. | Matson GB, Twieg DB, Karczmar GS, Lawry TJ, Gober JR, Valenza M, Boska MD, Weiner MW. Application of image-guided surface coil P-31 MR spectroscopy to human liver, heart, and kidney. Radiology. 1988;169:541-547. |
| 5. | van Wassenaer-van Hall HN, van der Grond J, van Hattum J, Kooijman C, Hoogenraad TU, Mali WP. 31P magnetic resonance spectroscopy of the liver: correlation with standardized serum, clinical, and histological changes in diffuse liver disease. Hepatology. 1995;21:443-449. |
| 6. | Sijens PE. Parametric exploration of the liver by magnetic resonance methods. Eur Radiol. 2009;19:2594-2607. |
| 7. | Cox IJ, Sharif A, Cobbold JF, Thomas HC, Taylor-Robinson SD. Current and future applications of in vitro magnetic resonance spectroscopy in hepatobiliary disease. World J Gastroenterol. 2006;12:4773-4783. |
| 8. | Klomp D, van Laarhoven H, Scheenen T, Kamm Y, Heerschap A. Quantitative 19F MR spectroscopy at 3 T to detect heterogeneous capecitabine metabolism in human liver. NMR Biomed. 2007;20:485-492. |
| 9. | Lim AK, Patel N, Hamilton G, Hajnal JV, Goldin RD, Taylor-Robinson SD. The relationship of in vivo 31P MR spectroscopy to histology in chronic hepatitis C. Hepatology. 2003;37:788-794. |
| 10. | Bell JD, Cox IJ, Sargentoni J, Peden CJ, Menon DK, Foster CS, Watanapa P, Iles RA, Urenjak J. A 31P and 1H-NMR investigation in vitro of normal and abnormal human liver. Biochim Biophys Acta. 1993;1225:71-77. |
| 11. | Bailes DR, Bryant DJ, Bydder GM, Case HA, Collins AG, Cox IJ, Evans PR, Harman RR, Hall AS, Rose MR. Localised phosphorus-31 NMR spectroscopy of normal and pathological human organs in vivo using phase encoding techniques. J Magn Reson. 1987;74:158-170. |
| 12. | Oberhaensli R, Rajagopalan B, Galloway GJ, Taylor DJ, Radda GK. Study of human liver disease with P-31 magnetic resonance spectroscopy. Gut. 1990;31:463-467. |
| 13. | Jalan R, Sargentoni J, Coutts GA, Bell JD, Rolles K, Burroughs AK, Taylor Robinson SD. Hepatic phosphorus-31 magnetic resonance spectroscopy in primary biliary cirrhosis and its relation to prognostic models. Gut. 1996;39:141-146. |
| 14. | Taylor-Robinson SD, Sargentoni J, Bell JD, Saeed N, Changani KK, Davidson BR, Rolles K, Burroughs AK, Hodgson HJ, Foster CS. In vivo and in vitro hepatic 31P magnetic resonance spectroscopy and electron microscopy of the cirrhotic liver. Liver. 1997;17:198-209. |
| 15. | Corbin IR, Ryner LN, Singh H, Minuk GY. Quantitative hepatic phosphorus-31 magnetic resonance spectroscopy in compensated and decompensated cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G379-G384. |
| 16. | Dezortova M, Taimr P, Skoch A, Spicak J, Hajek M. Etiology and functional status of liver cirrhosis by 31P MR spectroscopy. World J Gastroenterol. 2005;11:6926-6931. |
| 17. | Noren B, Dahlqvist O, Lundberg P, Almer S, Kechagias S, Ekstedt M, Franzén L, Wirell S, Smedby O. Separation of advanced from mild fibrosis in diffuse liver disease using 31P magnetic resonance spectroscopy. Eur J Radiol. 2008;66:313-320. |
| 18. | Oberhaensli RD, Galloway GJ, Taylor DJ, Bore PJ, Radda GK. Assessment of human liver metabolism by phosphorus-31 magnetic resonance spectroscopy. Br J Radiol. 1986;59:695-699. |
| 19. | Boesch C, Elsing C, Wegmüller H, Felblinger J, Vock P, Reichen J. Effect of ethanol and fructose on liver metabolism: a dynamic 31Phosphorus magnetic resonance spectroscopy study in normal volunteers. Magn Reson Imaging. 1997;15:1067-1077. |
| 20. | Kachi K, Araki T, Uchiyama G. [Effect of intravenous fructose load on the P-31 MR spectrum of the cirrhotic liver]. Nippon Igaku Hoshasen Gakkai Zasshi. 1991;51:127-132. |
| 21. | Dufour JF, Stoupis C, Lazeyras F, Vock P, Terrier F, Reichen J. Alterations in hepatic fructose metabolism in cirrhotic patients demonstrated by dynamic 31phosphorus spectroscopy. Hepatology. 1992;15:835-842. |
| 22. | Yamane Y, Umeda M, O’uchi T, Mitsushima T, Nakata K, Nagataki S. Phosphorus-31 nuclear magnetic resonance in vivo spectroscopy of human liver during hepatitis A virus infection. Dig Dis Sci. 1994;39:33-38. |
| 23. | Lim AK, Patel N, Hamilton G, Mylvahan K, Kuo YT, Goldin RD, Taylor-Robinson SD. 31P MR spectroscopy in assessment of response to antiviral therapy for hepatitis C virus-related liver disease. AJR Am J Roentgenol. 2007;189:819-823. |
| 24. | Cho SG, Kim MY, Kim HJ, Kim YS, Choi W, Shin SH, Hong KC, Kim YB, Lee JH, Suh CH. Chronic hepatitis: in vivo proton MR spectroscopic evaluation of the liver and correlation with histopathologic findings. Radiology. 2001;221:740-746. |
| 25. | Tarasów E, Wiercińska-Drapało A, Jaroszewicz J, Siergiejczyk L, Orzechowska-Bobkiewicz A, Prokopowicz D, Walecki J. Metabolic disturbances in liver 1H MR spectroscopy in HIV and HCV co-infected patients as a potential marker of hepatocyte activation. Acta Radiol. 2004;45:803-809. |
| 26. | Orlacchio A, Bolacchi F, Angelico M, Mancini A, Cozzolino V, Cadioli M, Simonetti G. In vivo, high-field, 3-Tesla 1H MR spectroscopic assessment of liver fibrosis in HCV-correlated chronic liver disease. Radiol Med. 2008;113:289-299. |
| 27. | Menon DK, Harris M, Sargentoni J, Taylor-Robinson SD, Cox IJ, Morgan MY. In vivo hepatic 31P magnetic resonance spectroscopy in chronic alcohol abusers. Gastroenterology. 1995;108:776-788. |
| 28. | Dixon RM, Angus PW, Rajagopalan B, Radda GK. 31P magnetic resonance spectroscopy detects a functional abnormality in liver metabolism after acetaminophen poisoning. Hepatology. 1992;16:943-948. |
| 29. | Magee LA, Dixon RM, Kemp GJ, Redman CW, Styles P. 31P magnetic resonance spectroscopy of the liver in HELLP syndrome. Br J Obstet Gynaecol. 1999;106:582-588. |
| 30. | Hagspiel KD, von Weymarn C, McKinnon G, Haldemann R, Marincek B, von Schulthess GK. Effect of hypothyroidism on phosphorus metabolism in muscle and liver: in vivo P-31 MR spectroscopy study. J Magn Reson Imaging. 1992;2:527-532. |
| 31. | Roser W, Stock KW. 1H MRS of liver and brain in a patient with AL amyloidosis. Magn Reson Imaging. 1997;15:993-996. |
| 32. | Dixon RM, Ouwerkerk R, Rajagopalan B, Radda GK. 31P magnetic resonance spectroscopy of the liver in an infant with galactosaemia. MAGMA. 1993;1:119-121. |
| 33. | Oberhaensli RD, Rajagopalan B, Taylor DJ, Radda GK, Collins JE, Leonard JV, Schwarz H, Herschkowitz N. Study of hereditary fructose intolerance by use of 31P magnetic resonance spectroscopy. Lancet. 1987;2:931-934. |
| 34. | Seegmiller JE, Dixon RM, Kemp GJ, Angus PW, McAlindon TE, Dieppe P, Rajagopalan B, Radda GK. Fructose-induced aberration of metabolism in familial gout identified by 31P magnetic resonance spectroscopy. Proc Natl Acad Sci USA. 1990;87:8326-8330. |
| 35. | Seegmiller JE, Dixon RM, Kemp GJ, Angus PW, McAlindon TE, Dieppe P, Rajagopalan B, Radda GK. An aberration of fructose metabolism in familial gout identified by 31P magnetic resonance spectroscopy of the liver. Trans Assoc Am Physicians. 1990;103:298-306. |
| 36. | Iles RA, Cox IJ, Bell JD, Dubowitz LM, Cowan F, Bryant DJ. 31P magnetic resonance spectroscopy of the human paediatric liver. NMR Biomed. 1990;3:90-94. |
| 37. | Cline GW, Rothman DL, Magnusson I, Katz LD, Shulman GI. 13C-nuclear magnetic resonance spectroscopy studies of hepatic glucose metabolism in normal subjects and subjects with insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2369-2376. |
| 38. | Hwang JH, Perseghin G, Rothman DL, Cline GW, Magnusson I, Petersen KF, Shulman GI. Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. J Clin Invest. 1995;95:783-787. |
| 39. | Bischof MG, Krssak M, Krebs M, Bernroider E, Stingl H, Waldhäusl W, Roden M. Effects of short-term improvement of insulin treatment and glycemia on hepatic glycogen metabolism in type 1 diabetes. Diabetes. 2001;50:392-398. |
| 40. | Bischof MG, Bernroider E, Krssak M, Krebs M, Stingl H, Nowotny P, Yu C, Shulman GI, Waldhäusl W, Roden M. Hepatic glycogen metabolism in type 1 diabetes after long-term near normoglycemia. Diabetes. 2002;51:49-54. |
| 41. | Matyka K, Dixon RM, Mohn A, Rajagopalan B, Shmueli E, Styles P, Dunger DB. Daytime liver glycogen accumulation, measured by 13C magnetic resonance spectroscopy, in young children with Type 1 diabetes mellitus. Diabet Med. 2001;18:659-662. |
| 42. | Kishore P, Gabriely I, Cui MH, Di Vito J, Gajavelli S, Hwang JH, Shamoon H. Role of hepatic glycogen breakdown in defective counterregulation of hypoglycemia in intensively treated type 1 diabetes. Diabetes. 2006;55:659-666. |
| 43. | Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90:1323-1327. |
| 44. | Krssak M, Brehm A, Bernroider E, Anderwald C, Nowotny P, Dalla Man C, Cobelli C, Cline GW, Shulman GI, Waldhäusl W. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes. 2004;53:3048-3056. |
| 45. | Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603-608. |
| 46. | Dagnelie PC, Sijens PE, Kraus DJ, Planting AS, van Dijk P. Abnormal liver metabolism in cancer patients detected by (31)P MR spectroscopy. NMR Biomed. 1999;12:535-544. |
| 47. | Leij-Halfwerk S, Dagneli PC, Kappert P, Oudkerk M, Sijens PE. Decreased energy and phosphorylation status in the liver of lung cancer patients with weight loss. J Hepatol. 2000;32:887-892. |
| 48. | Leij-Halfwerk S, Dagnelie PC, van Den Berg JW, Wattimena JD, Hordijk-Luijk CH, Wilson JP. Weight loss and elevated gluconeogenesis from alanine in lung cancer patients. Am J Clin Nutr. 2000;71:583-589. |
| 49. | Leij-Halfwerk S, Dagnelie PC, Van Den Berg JW, Wilson JH, Sijens PE. Hepatic sugar phosphate levels reflect gluconeogenesis in lung cancer: simultaneous turnover measurements and 31P magnetic resonance spectroscopy in vivo. Clin Sci (Lond). 2000;98:167-174. |
| 50. | Leij-Halfwerk S, van den Berg JW, Sijens PE, Wilson JH, Oudkerk M, Dagnelie PC. Altered hepatic gluconeogenesis during L-alanine infusion in weight-losing lung cancer patients as observed by phosphorus magnetic resonance spectroscopy and turnover measurements. Cancer Res. 2000;60:618-623. |
| 51. | Leij-Halfwerk S, Agteresch HJ, Sijens PE, Dagnelie PC. Adenosine triphosphate infusion increases liver energy status in advanced lung cancer patients: an in vivo 31P magnetic resonance spectroscopy study. Hepatology. 2002;35:421-424. |
| 52. | Stubbs M, Bhujwalla ZM, Tozer GM, Rodrigues LM, Maxwell RJ, Morgan R, Howe FA, Griffiths JR. An assessment of 31P MRS as a method of measuring pH in rat tumours. NMR Biomed. 1992;5:351-359. |
| 53. | Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194-1198. |
| 54. | Raghunand N, Gillies RJ. pH and chemotherapy. Novartis Found Symp. 2001;240:199-211; discussion 265-268. |
| 55. | Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5:1275-1279. |