Published online Apr 7, 2010. doi: 10.3748/wjg.v16.i13.1560
Revised: February 25, 2010
Accepted: March 4, 2010
Published online: April 7, 2010
Hepatic steatosis as the most prevalent liver disorder can either be related to alcoholic liver disease (ALD) or non-alcoholic fatty liver disease (NAFLD). In both conditions, hepatocytes excessively accumulate fat-containing vacuoles within their cytoplasm, which is the key histological feature. In contrast to ALD, NAFLD is commonly associated with metabolic syndrome, obesity and insulin resistance. To determine increased liver fat content, liver biopsy is currently considered the gold standard. Besides the invasive technique, various other non-invasive techniques have been developed, such as ultrasound, computed tomography (CT), magnetic resonance spectroscopy (MRS) and magnetic resonance imaging (MRI) based methods. Among these techniques, ultrasound and CT provide only qualitative information about hepatic steatosis, whereas MRS- or MRI-based methods are able to determine even small amounts of fat accurately. These non-invasive magnetic resonance techniques have already proven their great potential, especially in longitudinal and cross-sectional studies regarding various metabolic conditions and medical treatment regimens. In this review, the most common, non-invasive MRS/MRI techniques for assessment of intrahepatic lipid content are described with their inherent advantages and limitations.
- Citation: Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol 2010; 16(13): 1560-1566
- URL: https://www.wjgnet.com/1007-9327/full/v16/i13/1560.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i13.1560
Hepatic steatosis is a common finding during liver examination and is found in a broad spectrum of diseases. It is related to an increased deposition of triglycerides within the cytoplasm of hepatocytes. Besides alcoholic liver disease (ALD), intrahepatic accumulation of lipids can also be associated with obesity, insulin resistance and metabolic syndrome, and is then termed non-alcoholic fatty liver disease (NAFLD). NAFLD is constantly gaining prevalence throughout the western world and is related to obesity as an increasing problem in recent decades[1,2]. Nevertheless, NAFLD can also be found in non-obese subjects with a body mass index within the normal range. Those patients often suffer from insulin resistance. Thus, intrahepatic fat fraction denotes an interesting metabolic parameter for longitudinal or cross-sectional studies regarding various metabolic conditions. Moreover, it is considered an independent risk factor for insulin resistance and atherosclerosis[3-7]. The current gold standard for quantification of intrahepatic lipid content is based on invasive liver biopsies and subsequent histological analysis. However, due to its invasive character, it is not useful for longitudinal studies or metabolic studies on otherwise healthy subjects.
Magnetic resonance spectroscopy (MRS) and magnetic resonance imaging (MRI) provide non-invasive means to accurately quantify intrahepatic lipid content[8-10]. In contrast to other modalities such as ultrasound and computed tomography (CT), MRI/MRS are capable of detecting even small amounts of intrahepatic lipid accumulation[10]. Therefore, MRI/MRS are especially useful to measure changes in hepatic steatosis during various treatment regimens. During recent years, clinical and research investigations have been performed on this subject.
This review gives an overview of various magnetic-resonance-based methods that are capable of quantifying intrahepatic lipid content non-invasively. Different strategies of 1H-MRS, as well as phase-sensitive and frequency-selective MRI methods are described.
In 1993, Longo et al[11,12] first published their results of 1H-MRS of liver parenchyma and correlated the data with CT studies and biopsies. In these studies, they found an excellent agreement between the different investigated methods. Since then, several studies have been performed that have further verified these results by means of whole-body MR scanning[13-15].
However, various strategies have been developed to obtain volume-selective 1H-MR spectra from liver parenchyma in vivo. Spectra are usually recorded from volumes ranging from 1 to 27 cm3, which are small enough to be positioned well in the liver parenchyma. To record reliable spectra from pure liver parenchyma, voxels have to be carefully placed in order to avoid artificial signal contributions from surrounding adipose tissue or intrahepatic blood vessels.
Two main strategies are used for single-voxel spectroscopy (SVS): point resolved spectroscopy (PRESS) or stimulated-echo acquisition mode (STEAM)[16,17]. The PRESS acquisition scheme (multi-echo single-shot technique) uses a 90°-180°-180° pulse sequence with long echo time (TE) and allows for better visualization of metabolites with long T1 relaxation times. In contrast, the STEAM sequence applies a 90°-90°-90° pulse sequence and is less sensitive to J-coupling effects. The STEAM sequence provides shorter TE and lower signal yield compared to PRESS, which is usually not a limitation for fat quantification in the liver. However, both techniques can be applied for intrahepatic fat quantification in clinical examinations.
Since both techniques only provide spectra of a small sub-region of the liver parenchyma, so-called spectroscopic imaging techniques with 2D or even 3D matrices of spectra have been developed to obtain detailed information on lipid distribution[18,19]. Compared to SVS, these techniques are rarely used clinically for routine investigation of liver parenchyma, due to their rather long acquisition and post-processing times[20,21]. In most cases of NAFLD, hepatic lipid distribution has been shown to be relatively homogeneous, which allows one to quantify intrahepatic fat fraction by only one single representative voxel[22-24]. However, it should be noted that significant differences in sub-regions of both liver lobes have also been reported[13].
The above-mentioned 1H-MRS techniques have been applied in studies investigating NAFLD in the general adult population[25]. Moreover, an increasing number of longitudinal clinical studies have been performed evaluating intrahepatic fat fraction in the obese population or patients at risk for developing type 2 diabetes[26-31]. Intrahepatic fat fraction has also been evaluated in morbidly obese patients undergoing bariatric surgery[32-35]. Moreover, additional cross-sectional studies have revealed different intrahepatic fat fractions depending on genetic background or hormonal status of the examined subjects[36-42].
All of these MRS fat quantification techniques have been shown to be safe and non-invasive alternatives to the current invasive gold standard (liver biopsy). They have been tested regarding their accuracy and have shown high intra-individual reproducibility in repeated measurements[13,23,25]. However, one has to consider that MR spectroscopic fat quantification relies on determination of overall volume fraction of lipids in the liver parenchyma. In contrast, in histological examinations, the percentage of hepatocytes that show distinct fat droplets is used for quantification. Thus, the reported percentage values that characterize steatosis from MR examinations might differ from those in histological analysis. On the other hand, data from MRI and histology correlate with each other and both techniques allow, nevertheless, for reliable quantification of intrahepatic lipid content.
It should be also mentioned that spectroscopic examinations are especially recommended for assessment of small lipid fractions in the liver, because sensitivity to low signal intensities from fat is higher than for imaging-based strategies. Furthermore, water and fat signals can be well distinguished.
1H-MRS capabilities are still not available on all standard clinical scanners and require dedicated prerequisites including spectroscopic sequences and post-processing software. Therefore, 1H-MRS still remains a research tool for clinical studies and is usually not used in daily routine liver examinations. There are, nevertheless, MRI sequences that allow for reliable and accurate quantification of intrahepatic lipid content.
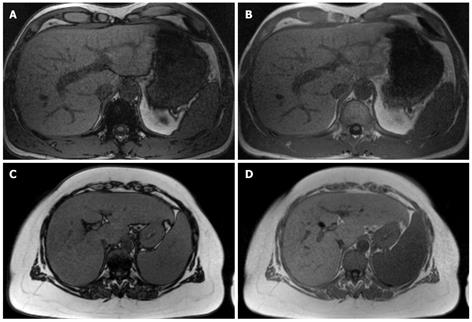
So-called in-phase/opposed-phase (IP/OP) techniques are available on most MR units and can be performed easily in routine examinations. Using this technique, T1-weighted images can be acquired extremely fast, with the use of multi-segment phased array coils and parallel imaging techniques. Moreover, T1-weighted gradient echo sequences can cover most of the liver parenchyma within a single breath-hold[43-46]. The IP/OP technique is based upon the fact that, during TE, transverse magnetization vectors of fat and water develop a phase difference that results in decreased overall length of the magnetization vector under OP conditions. At a main magnetic field strength of 1.5 T, the frequency shift between fat and water is approximately 220 Hz, which results in OP conditions at a TE of about 2.4 ms and in-phase conditions at a TE of about 4.8 ms[47-49]. The hepatic fat fraction can then be quantified by calculating the loss of signal intensity in OP images compared to IP images[50-52], as shown in Figure 1. From congruent sets of IP and OP images, acquired within the same breath-hold, the fat fraction can be calculated pixel-wise and misregistration errors can be avoided. Thus, maps of intrahepatic fat fraction can be obtained to estimate liver fat content and show differences in regional fat distribution.
However, not only the phase difference between water and fat protons contribute to the observed signal loss in OP images, but also additional transverse and longitudinal relaxation effects may play a major role. Recent studies have shown that especially transverse relaxation time can vary largely between different individuals, as well as intra-individually in the time-course of longitudinal studies[46,53,54]. These changes in transverse relaxation time are mainly due to increased iron deposition in the liver parenchyma; either artificially acquired or, for example, hemochromatosis-associated[55]. It has been shown that transverse relaxivity correlates well with serum ferritin levels[53,56]. Thus, transverse relaxation time of liver parenchyma has to be measured additionally using a multi-echo gradient echo sequence. The data necessary for estimation of T2* can than be obtained within a single additional breath-hold. Integration of individual T2* values in the calculation of the fat fraction requires a somewhat more sophisticated approach[57].
In contrast, longitudinal relaxation times are relatively stable throughout the population and individual calculation requires additional time-consuming sequences. Therefore, it seems legitimate for the general population to account for longitudinal relaxivity using constant values for longitudinal relaxation time of liver parenchyma.
Compared to the above-described gradient-echo-based IP/OP technique, Dixon et al[47] described in 1984 the use of a spin-echo technique with a small timing-offset of the 180° refocusing pulse, which is used to create a so-called OP image. The IP image is then acquired using a conventional spin-echo sequence. From these two images, fat- and water-selective images can be subsequently obtained. However, sensitivity to magnetic field inhomogeneity cannot be neglected and has prevented the widespread routine clinical usage of the Dixon technique. Since its introduction more than 20 years ago, several modifications have been reported that have aimed at overcoming its inherent limitations[45,48,58,59]. Three-point Dixon methods have been developed that additionally acquire a third image with a phase shift of -180° or 360°.
Then, using three different images and sophisticated phase-correction algorithms, true fat- and water-selective images are derived from the recorded data. This technique allows one to distinguish which constituent (water or fat) is predominant in each voxel[60-66]. Acquiring all three images in a single breath-hold is often not possible, whereas recording in multiple breath-holds poses the problem of misregistration artefacts due to variable positions of the liver parenchyma.
Another approach was first described by Reeder et al[67-69] and is termed the IDEAL technique (iterative decomposition of water and fat with echo asymmetry and least squares estimation). Using optimized echo shifts and gradient echo imaging, it provides robust quantification of the intrahepatic fat fraction. This technique allows for fat quantification even in the presence of moderate inhomogeneities of the static magnetic field, which are often encountered in examinations of extremely obese patients on wide-bore MRI scanners. However, it is not free of limitations. Liu et al[70] have reported techniques for reduction of noise bias and longitudinal relaxation effects that affect quantification of the hepatic fat fraction in the IDEAL technique. These drawbacks can be partially overcome by small- or dual-flip angle approaches, magnitude discrimination and phase-constrained methods. Besides its capabilities in measuring parenchymal fat content, the IDEAL method has also been used for fat suppression in clinical studies of various body regions[71-75].
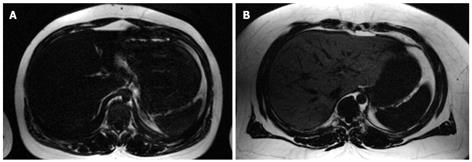
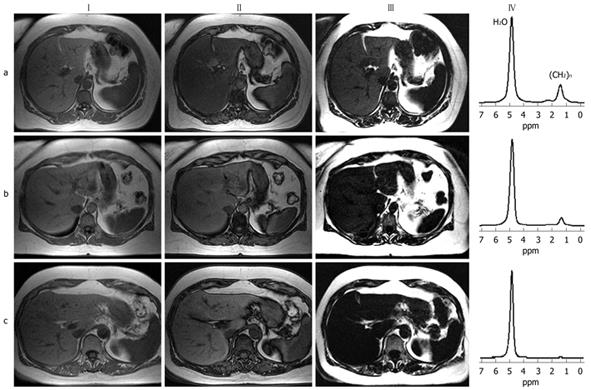
Previous studies have described a so-called spectral-spatial excitation technique to quantify fat content accurately in parenchymal organs and muscles[57,76]. A combination of chemical shift selectivity and slice-selective excitation in gradient echo or spin echo imaging sequences provides a high sensitivity to detect even small amounts of fat[23,49,77,78]. Furthermore, spatial information about parenchymal lipid distribution is also obtained. Slice-selectivity is implemented using six equidistant radio frequency pulses (time increment between pulses, 2.38 ms at 1.5 T) with nearly binomial amplitude ratios. These radio frequency pulses excite the methylene and methyl signal of fatty acids (0.8-2.0 ppm) selectively, as shown in Figure 2. Thus, signal contributions from water protons are below the noise level. To achieve this optimal spectral-spatial excitation, relatively homogeneous static magnetic fields are required, which makes adequate shimming procedures necessary. However, especially in wide-bore MR scanners that are designed to examine extremely obese patients, the inhomogeneity of the static magnetic field is often problematic. Even time-consuming shimming procedures might fail. For quantitative assessment of intrahepatic fat, adjacent subcutaneous or visceral fat is used as an internal reference because it contains almost 100% fat. The spectral-spatial excitation method is capable of detecting even small amounts of lipids (starting at 1%-2% volume fraction of fat in the liver), with additional spatial information about its distribution[23]. However, some advantages and disadvantages of this technique should be noted. As a result of highly selective visualization of fat, the technique offers relatively low soft tissue contrast compared to conventional gradient echo sequences (Figure 3). Moreover, only a small number of representative slices can be acquired during a single breath-hold. Since only a reference region-of-interest in subcutaneous adipose tissue adjacent to liver parenchyma is needed for quantification of fat fraction, the calculation of intrahepatic lipid content can easily be done. Furthermore, there is no need for additional time-consuming sequences that are necessary to correct for transverse and longitudinal relaxation effects.
Several non-invasive methods have been developed for quantification of intrahepatic fat content using whole-body MRI scanners. Being aware of the inherent advantages and disadvantages of each technique, one has to choose carefully the appropriate method for specific examination circumstances, as well as for hard- and software capabilities. Correctly applied, each technique (MRS/MRI) provides accurate data on intrahepatic fat fraction, correlating well with findings in liver biopsies, which is often considered as the current gold standard. The methods described above provide non-invasive quantification of the intrahepatic fat fraction, and give a reliable basis for longitudinal clinical and research studies. Thus, the influence of various medical treatments and diseases on intrahepatic lipid storage can be easily investigated in a non-invasive way.
Peer reviewer: Paul E Sijens, PhD, Associate Professor, Radiology, UMCG, Hanzeplein 1, 9713GZ Groningen, The Netherlands
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 2. | Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388-1393. |
| 3. | Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939-960. |
| 4. | Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med. 2007;24:1-6. |
| 5. | Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609-1616. |
| 6. | Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3490-3497. |
| 7. | Roden M. Mechanisms of Disease: hepatic steatosis in type 2 diabetes--pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab. 2006;2:335-348. |
| 8. | Sijens PE. Parametric exploration of the liver by magnetic resonance methods. Eur Radiol. 2009;19:2594-2607. |
| 9. | Mehta SR, Thomas EL, Bell JD, Johnston DG, Taylor-Robinson SD. Non-invasive means of measuring hepatic fat content. World J Gastroenterol. 2008;14:3476-3483. |
| 10. | Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433-445. |
| 11. | Longo R, Ricci C, Masutti F, Vidimari R, Crocé LS, Bercich L, Tiribelli C, Dalla Palma L. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol. 1993;28:297-302. |
| 12. | Longo R, Pollesello P, Ricci C, Masutti F, Kvam BJ, Bercich L, Crocè LS, Grigolato P, Paoletti S, de Bernard B. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281-285. |
| 13. | Cowin GJ, Jonsson JR, Bauer JD, Ash S, Ali A, Osland EJ, Purdie DM, Clouston AD, Powell EE, Galloway GJ. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging. 2008;28:937-945. |
| 14. | Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, Stein DT. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977-E989. |
| 15. | Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487-495. |
| 16. | Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333-348. |
| 17. | Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hänicke W, Sauter R. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med. 1989;9:79-93. |
| 18. | Pykett IL, Rosen BR. Nuclear magnetic resonance: in vivo proton chemical shift imaging. Work in progress. Radiology. 1983;149:197-201. |
| 19. | Skoch A, Jiru F, Bunke J. Spectroscopic imaging: basic principles. Eur J Radiol. 2008;67:230-239. |
| 20. | Sijens PE, Smit GP, Borgdorff MA, Kappert P, Oudkerk M. Multiple voxel 1H MR spectroscopy of phosphorylase-b kinase deficient patients (GSD IXa) showing an accumulation of fat in the liver that resolves with aging. J Hepatol. 2006;45:851-855. |
| 21. | Irwan R, Edens MA, Sijens PE. Assessment of the variations in fat content in normal liver using a fast MR imaging method in comparison with results obtained by spectroscopic imaging. Eur Radiol. 2008;18:806-813. |
| 22. | Machann J, Stefan N, Schick F. (1)H MR spectroscopy of skeletal muscle, liver and bone marrow. Eur J Radiol. 2008;67:275-284. |
| 23. | Machann J, Thamer C, Schnoedt B, Stefan N, Haring HU, Claussen CD, Fritsche A, Schick F. Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn Reson Med. 2006;55:913-917. |
| 24. | Thomas EL, Hamilton G, Patel N, O’Dwyer R, Doré CJ, Goldin RD, Bell JD, Taylor-Robinson SD. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122-127. |
| 25. | Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462-E468. |
| 26. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. |
| 27. | Thamer C, Machann J, Stefan N, Schäfer SA, Machicao F, Staiger H, Laakso M, Böttcher M, Claussen C, Schick F. Variations in PPARD determine the change in body composition during lifestyle intervention: a whole-body magnetic resonance study. J Clin Endocrinol Metab. 2008;93:1497-1500. |
| 28. | Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD, Frost G, Bell JD, Taylor-Robinson SD. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:5813-5819. |
| 29. | Thomas EL, Potter E, Tosi I, Fitzpatrick J, Hamilton G, Amber V, Hughes R, North C, Holvoet P, Seed M. Pioglitazone added to conventional lipid-lowering treatment in familial combined hyperlipidaemia improves parameters of metabolic control: relation to liver, muscle and regional body fat content. Atherosclerosis. 2007;195:e181-e190. |
| 30. | Westerbacka J, Lammi K, Häkkinen AM, Rissanen A, Salminen I, Aro A, Yki-Järvinen H. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 2005;90:2804-2809. |
| 31. | Borra R, Lautamäki R, Parkkola R, Komu M, Sijens PE, Hällsten K, Bergman J, Iozzo P, Nuutila P. Inverse association between liver fat content and hepatic glucose uptake in patients with type 2 diabetes mellitus. Metabolism. 2008;57:1445-1451. |
| 32. | Heath ML, Kow L, Slavotinek JP, Valentine R, Toouli J, Thompson CH. Abdominal adiposity and liver fat content 3 and 12 months after gastric banding surgery. Metabolism. 2009;58:753-758. |
| 33. | Verna EC, Berk PD. Role of fatty acids in the pathogenesis of obesity and fatty liver: impact of bariatric surgery. Semin Liver Dis. 2008;28:407-426. |
| 34. | Wolf AM, Beisiegel U. The effect of loss of excess weight on the metabolic risk factors after bariatric surgery in morbidly and super-obese patients. Obes Surg. 2007;17:910-919. |
| 35. | Phillips ML, Boase S, Wahlroos S, Dugar M, Kow L, Stahl J, Slavotinek JP, Valentine R, Toouli J, Thompson CH. Associates of change in liver fat content in the morbidly obese after laparoscopic gastric banding surgery. Diabetes Obes Metab. 2008;10:661-667. |
| 36. | Kantartzis K, Peter A, Machicao F, Machann J, Wagner S, Königsrainer I, Königsrainer A, Schick F, Fritsche A, Häring HU. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. 2009;58:2616-2623. |
| 37. | Kantartzis K, Machicao F, Machann J, Schick F, Fritsche A, Häring HU, Stefan N. The DGAT2 gene is a candidate for the dissociation between fatty liver and insulin resistance in humans. Clin Sci (Lond). 2009;116:531-537. |
| 38. | Kantartzis K, Rittig K, Cegan A, Machann J, Schick F, Balletshofer B, Fritsche A, Schleicher E, Häring HU, Stefan N. Fatty liver is independently associated with alterations in circulating HDL2 and HDL3 subfractions. Diabetes Care. 2008;31:366-368. |
| 39. | Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Schleicher E, Fritsche A, Häring HU. High circulating retinol-binding protein 4 is associated with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans. Diabetes Care. 2007;30:1173-1178. |
| 40. | Stefan N, Peter A, Cegan A, Staiger H, Machann J, Schick F, Claussen CD, Fritsche A, Häring HU, Schleicher E. Low hepatic stearoyl-CoA desaturase 1 activity is associated with fatty liver and insulin resistance in obese humans. Diabetologia. 2008;51:648-656. |
| 41. | Silbernagel G, Stefan N, Hoffmann MM, Machicao-Arano F, Machann J, Schick F, Winkelmann BR, Boehm BO, Häring HU, Fritsche A. The L162V polymorphism of the peroxisome proliferator activated receptor alpha gene (PPARA) is not associated with type 2 diabetes, BMI or body fat composition. Exp Clin Endocrinol Diabetes. 2009;117:113-118. |
| 42. | Haupt A, Thamer C, Heni M, Tschritter O, Machann J, Schick F, Machicao F, Häring HU, Staiger H, Fritsche A. Impact of variation near MC4R on whole-body fat distribution, liver fat, and weight loss. Obesity (Silver Spring). 2009;17:1942-1945. |
| 43. | Park HW, Kim YH, Cho ZH. Fast gradient-echo chemical-shift imaging. Magn Reson Med. 1988;7:340-345. |
| 44. | Chen Q, Stock KW, Prasad PV, Hatabu H. Fast magnetic resonance imaging techniques. Eur J Radiol. 1999;29:90-100. |
| 45. | Hussain HK, Chenevert TL, Londy FJ, Gulani V, Swanson SD, McKenna BJ, Appelman HD, Adusumilli S, Greenson JK, Conjeevaram HS. Hepatic fat fraction: MR imaging for quantitative measurement and display--early experience. Radiology. 2005;237:1048-1055. |
| 46. | Alústiza JM, Castiella A. Liver fat and iron at in-phase and opposed-phase MR imaging. Radiology. 2008;246:641. |
| 48. | Fishbein MH, Gardner KG, Potter CJ, Schmalbrock P, Smith MA. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn Reson Imaging. 1997;15:287-293. |
| 49. | Machann J, Bachmann OP, Brechtel K, Dahl DB, Wietek B, Klumpp B, Häring HU, Claussen CD, Jacob S, Schick F. Lipid content in the musculature of the lower leg assessed by fat selective MRI: intra- and interindividual differences and correlation with anthropometric and metabolic data. J Magn Reson Imaging. 2003;17:350-357. |
| 50. | Fishbein MH, Stevens WR. Rapid MRI using a modified Dixon technique: a non-invasive and effective method for detection and monitoring of fatty metamorphosis of the liver. Pediatr Radiol. 2001;31:806-809. |
| 51. | Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Makita O, Kadota M, Takahashi M. Adrenal masses: quantification of fat content with double-echo chemical shift in-phase and opposed-phase FLASH MR images for differentiation of adrenal adenomas. Radiology. 2001;218:642-646. |
| 52. | Ma X, Holalkere NS, Kambadakone R A, Mino-Kenudson M, Hahn PF, Sahani DV. Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics. 2009;29:1253-1277. |
| 53. | Schwenzer NF, Machann J, Haap MM, Martirosian P, Schraml C, Liebig G, Stefan N, Häring HU, Claussen CD, Fritsche A. T2* relaxometry in liver, pancreas, and spleen in a healthy cohort of one hundred twenty-nine subjects-correlation with age, gender, and serum ferritin. Invest Radiol. 2008;43:854-860. |
| 54. | Westphalen AC, Qayyum A, Yeh BM, Merriman RB, Lee JA, Lamba A, Lu Y, Coakley FV. Liver fat: effect of hepatic iron deposition on evaluation with opposed-phase MR imaging. Radiology. 2007;242:450-455. |
| 55. | Olthof AW, Sijens PE, Kreeftenberg HG, Kappert P, van der Jagt EJ, Oudkerk M. Non-invasive liver iron concentration measurement by MRI: comparison of two validated protocols. Eur J Radiol. 2009;71:116-121. |
| 56. | Olthof AW, Sijens PE, Kreeftenberg HG, Kappert P, Irwan R, van der Jagt EJ, Oudkerk M. Correlation between serum ferritin levels and liver iron concentration determined by MR imaging: impact of hematologic disease and inflammation. Magn Reson Imaging. 2007;25:228-231. |
| 57. | Schwenzer NF, Machann J, Martirosian P, Stefan N, Schraml C, Fritsche A, Claussen CD, Schick F. Quantification of pancreatic lipomatosis and liver steatosis by MRI: comparison of in/opposed-phase and spectral-spatial excitation techniques. Invest Radiol. 2008;43:330-337. |
| 58. | Levenson H, Greensite F, Hoefs J, Friloux L, Applegate G, Silva E, Kanel G, Buxton R. Fatty infiltration of the liver: quantification with phase-contrast MR imaging at 1.5 T vs biopsy. AJR Am J Roentgenol. 1991;156:307-312. |
| 59. | Zhang X, Tengowski M, Fasulo L, Botts S, Suddarth SA, Johnson GA. Measurement of fat/water ratios in rat liver using 3D three-point dixon MRI. Magn Reson Med. 2004;51:697-702. |
| 60. | Glover GH, Schneider E. Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magn Reson Med. 1991;18:371-383. |
| 61. | Glover GH. Multipoint Dixon technique for water and fat proton and susceptibility imaging. J Magn Reson Imaging. 1991;1:521-530. |
| 62. | Borrello JA, Chenevert TL, Meyer CR, Aisen AM, Glazer GM. Chemical shift-based true water and fat images: regional phase correction of modified spin-echo MR images. Radiology. 1987;164:531-537. |
| 63. | Lodes CC, Felmlee JP, Ehman RL, Sehgal CM, Greenleaf JF, Glover GH, Gray JE. Proton MR chemical shift imaging using double and triple phase contrast acquisition methods. J Comput Assist Tomogr. 1989;13:855-861. |
| 64. | Szumowski J, Coshow WR, Li F, Quinn SF. Phase unwrapping in the three-point Dixon method for fat suppression MR imaging. Radiology. 1994;192:555-561. |
| 65. | Szumowski J, Coshow W, Li F, Coombs B, Quinn SF. Double-echo three-point-Dixon method for fat suppression MRI. Magn Reson Med. 1995;34:120-124. |
| 66. | Coombs BD, Szumowski J, Coshow W. Two-point Dixon technique for water-fat signal decomposition with B0 inhomogeneity correction. Magn Reson Med. 1997;38:884-889. |
| 67. | Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, Gold GE, Beaulieu CH, Pelc NJ. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54:636-644. |
| 68. | Reeder SB, McKenzie CA, Pineda AR, Yu H, Shimakawa A, Brau AC, Hargreaves BA, Gold GE, Brittain JH. Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging. 2007;25:644-652. |
| 69. | Reeder SB, Hargreaves BA, Yu H, Brittain JH. Homodyne reconstruction and IDEAL water-fat decomposition. Magn Reson Med. 2005;54:586-593. |
| 70. | Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354-364. |
| 71. | Chen CA, Lu W, John CT, Hargreaves BA, Reeder SB, Delp SL, Siston RA, Gold GE. Multiecho IDEAL gradient-echo water-fat separation for rapid assessment of cartilage volume at 1.5 T: initial experience. Radiology. 2009;252:561-567. |
| 72. | Grayev A, Shimakawa A, Cousins J, Turski P, Brittain J, Reeder S. Improved time-of-flight magnetic resonance angiography with IDEAL water-fat separation. J Magn Reson Imaging. 2009;29:1367-1374. |
| 73. | Kijowski R, Tuite M, Passov L, Shimakawa A, Yu H, Reeder SB. Cartilage imaging at 3.0T with gradient refocused acquisition in the steady-state (GRASS) and IDEAL fat-water separation. J Magn Reson Imaging. 2008;28:167-174. |
| 74. | Costa DN, Pedrosa I, McKenzie C, Reeder SB, Rofsky NM. Body MRI using IDEAL. AJR Am J Roentgenol. 2008;190:1076-1084. |
| 75. | Reeder SB, Markl M, Yu H, Hellinger JC, Herfkens RJ, Pelc NJ. Cardiac CINE imaging with IDEAL water-fat separation and steady-state free precession. J Magn Reson Imaging. 2005;22:44-52. |
| 76. | Schick F, Machann J, Brechtel K, Strempfer A, Klumpp B, Stein DT, Jacob S. MRI of muscular fat. Magn Reson Med. 2002;47:720-727. |
| 77. | Schick F. Simultaneous highly selective MR water and fat imaging using a simple new type of spectral-spatial excitation. Magn Reson Med. 1998;40:194-202. |
| 78. | Schick F, Forster J, Machann J, Huppert P, Claussen CD. Highly selective water and fat imaging applying multislice sequences without sensitivity to B1 field inhomogeneities. Magn Reson Med. 1997;38:269-274. |