INTRODUCTION
Gallstones are a common problem and is likely to have existed since the appearance of mankind on Earth. Interestingly, gallstones have been identified in autopsy studies of Egyptian mummies[1]. Gallstones and associated complications cost approximately 6.5 billion dollars annually in the USA. The incidence of gallstones is more frequent in females than males and increase with age. The role of ethnicity and genetics is known in gallstone formation. Importantly, the distribution of gallstones in different populations appears to be related to high dietary intake of cholesterol and fats (Western diets). Furthermore, gallstones are primarily cholesterol or black pigment stones (bilirubin polymers) or brown pigment stones (calcium bilirubinate). The only established dietary risk is a high caloric intake. Other modifiable risk factors for gallstones are obesity, the metabolic syndrome, rapid weight loss, certain diseases (cirrhosis, Crohn’s disease) and gallbladder stasis (from spinal cord injury or drugs such as somatostatin). Therefore, it is anticipated that diets containing fibre, vegetable protein, nuts, calcium, vitamin C, coffee, plus physical activity are all regarded as protective factors. In developed countries, cholesterol gallstones predominate; 15% are black pigment stones. It is thought that gallstones affect around 15% of white adults in developed countries. East Asians develop brown pigment stones in bile ducts, associated with biliary infection or parasites, or in intrahepatic ducts (hepatolithiasis). In view of the high prevalence of obesity and metabolic syndrome, the burden of disease is epidemic in American Indians at around 60%-70%. The frequency of gallstones is reduced in Black Americans, Hispanics of mixed Indian origin, East Asians and sub-Saharan Africans[2,3]. Most asymptomatic gallstone carriers require no therapy. Laparoscopic cholecystectomy is the best definitive therapy for symptomatic gallstone disease. Primary prevention is unproven but focuses on early identification and risk alteration to decrease the possibility of developing gallstones. Ursodeoxycholic acid has a limited role in stone dissolution but can prevent stone development in severe obesity during rapid weight reduction with diet therapy or after bariatric surgery[4].
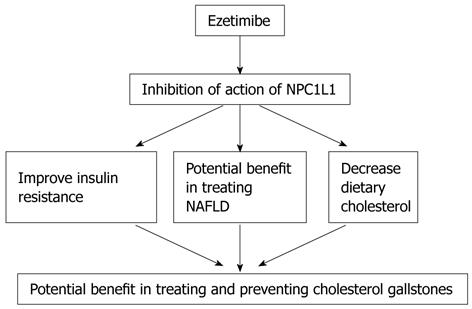
EZETIMIBE AND CHOLESTEROL GALLSTONES
Recently, it was shown both in animal models and humans that ezetimibe may prevent and treat cholesterol gallstones[5]. Ezetimibe inhibits intestinal uptake of cholesterol with a half life of approximately of 22 h. The major metabolic pathway for ezetimibe consists of glucuronidation of the 4-hydroxyphenyl group by uridine 5’-diphosphate-glucuronosyltransferase isoenzymes to form ezetimibe-glucuronide in the intestine and liver. Approximately 78% of the dose is excreted in the faeces predominantly as ezetimibe, with the balance found in the urine mainly as ezetimibe-glucuronide[6]. Niemann-Pick C1 like1 (NPC1L1), highly expressed in the jejunum of different species and only in human liver, is the main transporter of intestinal cholesterol. Mice deficient in NPC1L1 showed a significant > 70% reduction in cholesterol absorption, and a further reduction in cholesterol level with ezetimibe administration was not achievable. It was concluded that eztimibe reduces intestinal absorption by inhibiting the action of NPC1L1. Ezetimibe significantly reduces low-density lipoprotein (LDL) cholesterol and is used as monotherapy or in combination with statins to treat hyperlipidaemia[7].
Wang et al[5] showed that ezetimibe treatment for 8 to 12 wk in male gallstone-susceptible C57L mice and in 7 patients (treated with 20 mg ezetimibe for 30 d) resulted in a decrease in intestinal cholesterol absorption and biliary cholesterol secretion. Ezetimibe also protected gallbladder motility function by desaturating bile. Furthermore, ezetimibe treatment promoted the dissolution of gallstones by forming an abundance of unsaturated micelles and reduced biliary cholesterol saturation. However, Tamel et al[8] found that mice transgenic for NPC1L1 gene, displayed an increase in biliary cholesterol concentration, suggesting that ezetimibe treatment may reduce biliary cholesterol secretion and increase the cholesterol saturation index. This is not in agreement with previous studies which showed the potential benefit of ezetimibe in treating gallstones, and may lead to speculation that it is actually intestinal cholesterol absorption which is largely responsible for the formation of cholesterol gallstones.
Furthermore, administration of ezetimibe in Golden Syrian hamsters fed a diet high in cholesterol and sunflower oil resulted in a significant reduction in absolute and relative cholesterol levels in bile[9]. Ezetimibe treatment in C5BL female mice, prevented biliary crystals and normalized gallbladder wall fat and function[10]. Importantly, gallstone-susceptible C57BL/6 inbred mice were fed control and lithogenic diets with or without simultaneous ezetimibe administration. Lithogenic diets increased biliary cholesterol content and secretion, and induced sludge or gallstone formation in 100% of the animals. Ezetimibe administration reduced intestinal cholesterol absorption by 90% in control animals and by 35% in mice receiving the lithogenic diets. Ezetimibe prevented the appearance of cholesterol crystals and gallstones. In addition, mice fed the lithogenic diets plus ezetimibe exhibited a 60% reduction in the biliary cholesterol saturation index. Of note, ezetimibe treatment caused a significant increase in bile flow (+50%, P < 0.01) as well as bile salt, phospholipid and glutathione secretion rates (+60%, +44% and +100%, respectively, P < 0.01), which was associated with a moderately increased expression of hepatic bile salt transporters[11]. From the above discussion it is possible to suggest that ezetimibe acts by decreasing intestinal cholesterol absorption and biliary cholesterol secretion, preserving gallbladder motility function by de-saturating bile in mice, promoting the dissolution of gallstones by forming an abundance of unsaturated micelles and significantly reducing biliary cholesterol saturation and retarding cholesterol crystallization in the bile of patients with gallstones.
Another important precipitating factor for gallstones and a therapeutic target for ezetimibe is insulin resistance. Interestingly, Chang et al[12] showed in 19 503 Korean men, that the prevalence of obesity, abdominal obesity, and metabolic syndromes in the subjects with gallstones were higher than in those without gallstones. The prevalence of elevated homeostatic model assessment (HOMA) (> 75 percentile) in subjects with gallstones was significantly higher than in those without gallstones, and this association remained even after the obesity stratification was applied. In multiple logistic regression analyses, only age and HOMA proved to be independent predictors of gallstones. Insulin resistance was positively associated with gallstones in non-diabetic Korean men, and this occurred regardless of obesity. Importantly, gallstones appear to be a marker for insulin resistance, even in non-diabetic, nonobese men. Furthermore, hepatic insulin resistance directly promotes the formation of cholesterol gallstones in mice[13]. Nakeeb et al[14] showed that in lean, non-diabetic volunteers without gallstones, gallbladder dysmotility is associated with an elevated fasting glucose as well as a high index of insulin resistance, and their conclusion was that insulin resistance alone may be responsible for gallbladder dysmotility which may result in acalculous cholecystitis or gallstone formation. Insulin resistance is also associated with non-alcoholic fatty liver disease (NAFLD)[15]. Loria et al[16] showed a higher prevalence of gallstones in association with NAFLD compared with a normal population. In liver biopsy screening for NAFLD, it was documented that 55% of subjects have gallstones[17]. Recent studies showed the potential benefit of ezetimibe as treatment for NAFLD and associated hyperlipidaemia and insulin resistance. Interestingly, Zheng et al[18] showed that ezetimibe treatment for 4 wk reduced alanine transaminase (ALT), hepatic triglyceride, hepatomegaly, cholesterol ester and free cholesterol in diet-induced obese mice fed a high fat/cholesterol diet for 7 mo with proof of NAFLD. Importantly, administration of ezetimibe in obese Zucker rats (a model of NAFLD and metabolic syndrome) resulted in a significant improvement in both cholesterol and triglyceride levels, hepatic steatosis and improved insulin resistance[19]. This is in accordance with a recent study by Nomura et al[20] who showed that ezetimibe improved hepatic insulin sensitivity.