Published online Mar 21, 2010. doi: 10.3748/wjg.v16.i11.1409
Revised: January 17, 2010
Accepted: January 24, 2010
Published online: March 21, 2010
AIM: To investigate the relation between gastric cancer and microsatellite instability (MSI), loss of heterozygosity (LOH) and promoter region methylation.
METHODS: Fifty primary gastric carcinoma specimens were collected from patients with no family history of cancer. In addition, normal tissues were also collected from patients as controls. DNA was extracted by polymerase chain reaction for single-strand conformation polymorphism, bisulfite DNA sequencing, and methylation-specific band analysis.
RESULTS: The positive rate for MSI and LOH in gastric carcinoma was 16% and 20%, respectively. According to the tumor, node and metastasis staging system, the LOH frequency was higher in gastric carcinoma at stages III and IV than in gastric carcinoma at stages I and II (P = 0.01), which was also significantly correlated with lymph node metastasis and clinico- pathological characteristics of gastric carcinoma. Methylation of bone morphogenetic protein 3 (BMP3) gene promoter was detected in 64.44% of gastric carcinoma tissue samples. However, no statistical significance was observed between promoter region methylation and carcinoma differentiation. Interestingly, the BMP3 gene methylation rate was 71.05% and 28.58%, respectively, in MSI positive and negative cases (P = 0.031), suggesting that BMP3 genetic instability and promoter methylation are initiated during gastric carcinogenesis. LOH was detected mostly in the late stages of gastric carcinoma, indicating that gastric carcinoma at late stages has a higher infiltration and a poorer prognosis.
CONCLUSION: Promotor region methylation of the BMP3 gene may cause gastric carcinoma in Chinese people.
-
Citation: Chen XR, Wang JW, Li X, Zhang H, Ye ZY. Role of
BMP3 in progression of gastric carcinoma in Chinese people. World J Gastroenterol 2010; 16(11): 1409-1413 - URL: https://www.wjgnet.com/1007-9327/full/v16/i11/1409.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i11.1409
Gastric carcinoma is one of the leading causes of cancer-related death in China. Its incidence and mortality are increasing in Chinese people. Research has been focused on the development of cancer and its progression, by studying epigenetics and tumor suppressor genes. It was reported that microsatellite instability (MSI) and loss of heterozygosity (LOH) are associated with gastric cancer[1-5]. In addition, gene promoter region hypermethylation can activate certain tumor suppressor genes. Most of the MSI and LOH studies have been focused on p53[6], p16 and fragile histidine triade genes[7] with less efforts made on the role of bone morphogenetic protein (BMP) 3 epigenetics in tumor suppressor genes.
BMP3, belonging to the transforming growth factor β superfamily proteins, plays an important role in embryonic development by inducing and patterning early skeletal formation. It has been recently reported that BMP3 is associated with tumor development and progression[8]. However, no report is available on the role of BMP3 in gastric carcinoma development. The present study was to investigate the D4S2922 and D4S2964 loci in 4q21 region of the BMP3 gene by examining their MSI, LOH as well as the promoter region methylation and to reveal the relation between BMP3 gene and gastric carcinogenesis.
Fifty gastric carcinoma samples were obtained from patients with no family history of cancer and normal tissue samples were also collected from patients as controls. The study was approved by the Ethics Committee and patient families before chemotherapy and radiotherapy.
DNA was extracted from gastric carcinoma and normal tissue samples using a QIAGEN kit according to its manufacturer’s instructions. PCR for D4S2922 was performed using the primers (sense: 5'-TGCTTATGCAAGAGGTTGTTC-3' and antisense: 5'-AAAGGGCAGTTAGGGATGCT-3') and for D4S2964 using the primers of (sense: 5'-CTTCCTTCCCAACTCACACA-3' and antisense: 5'-GGTCATTCATGCAATCCACA-3'). Thirty-five PCR amplification cycles were performed at 94°C for 1 min (denaturation), at 59°C for 1 min (annealing), and at 72°C for 1 min (extension) in a 25 μL reaction buffer containing 1.0 mmol/L MgCl2 and PCR buffer (QIAGEN PCR kit). The PCR products were resolved in 2% agarose gels. The fragment size of D4S2922 and D4S2964 was 230 bp and 164 bp, respectively.
For SSCP analysis, 3 μL of each amplification product was added to 3 μL denaturing buffer containing 98% formamide and 0.09% bromophenol blue. The samples were heat-denatured at 98°C for 10 min and then placed on ice for 10 min. The denatured DNA was electrophoresed on 8% non-denaturing polyacrylamide gels (140 V for 2 h, 4°C). SSCP patterns on the gels were visualized with silver staining.
Using a CpGenome™ fast DNA modification kit (Chemicon International, Inc.), genomic DNA (1 μg) in a volume of 100 μL was denatured with 7 μL of 3 mol/L NaOH freshly prepared at 37°C for 10 min, 550 μL of freshly prepared DNA modification reagent was then added and the reaction was continued at 55°C for 16-20 h. The modified DNA was cooled on ice for 5 min before 750 μL binding buffer was added. After centrifugation, the products were washed with 750 μL 1 × washing buffer and denatured with 50 μL 20 mmol/L NaOH/90% EtOH for 10 min, followed by an additional 750 μL 1 × washing buffer. The eluted products were stored at -20°C for use.
Primers (sense: 5'-GGAGTTTAATTTTTGGTTTTGTTGTT-3' and antisense: 5'-ATCAACTCCCAACATCACTACA-3') were used for the nonmethylated specific BMP3 gene, yielding a PCR product of 73 bp. Primers (sense: 5'-GTTTAATTTTCGGTTTCGTCGTCGT-3' and antisense: 5'-GTCGACTCCCGACGTCG TACG-3') were used for the methylated specific BMP3 gene, yielding a PCR product of 70 bp. After an initial preheating step at 94°C for 10 min, 40 cycles of PCR were performed at 94°C for 30 s, at 64°C for 45 s, at 72°C for 75 s, and a final extension at 72°C for 10 min. The amplified PCR products were examined on 8% agarose gels.
PCR-SSCP showed one main band of the D17S396 locus in normal lymph node genomic DNA. The presence of two main bands indicated that a sample could be further evaluated in LOH analysis. LOH was considered when less than 50% bands or a lower band density was found in tumor tissue samples than in normal tissue samples, while MSI was considered when more than 50% bands or a band migration was observed in tumor tissue samples.
All data were analyzed with SPSS version 16.0 by one-way ANOVA and t-test. P < 0.05 was considered statistically significant.
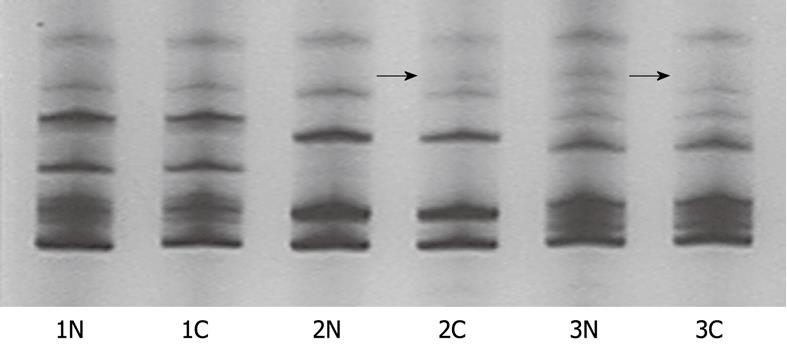
Both D4S2922 and D4S2964 from tumor and normal tissue samples were successfully amplified and the bands on gel images were heterozygous for the allele. Compared with the normal tissue samples, MSI was considered when more bands were found in gastric carcinoma tissue samples and LOH was considered when less bands were observed in gastric carcinoma tissue samples (Figure 1). A BMP3 was considered positive when the two loci showed at least one MSI or one LOH.
In the present study, 16.00% of the gastric carcinoma tissue samples were positive for MSI of the BMP3 gene (Table 1). However, MSI was not correlated with tumor differentiation, infiltration, lymphatic metastasis and tumor, node and metastasis (TNM) staging. The frequency of LOH was 20.00% (Table 1). Interestingly, LOH was significantly related with lymphatic metastasis and TNM staging of gastric carcinoma. The frequency of LOH was not correlated with differentiation and infiltration of gastric carcinoma and much higher in gastric carcinoma with lymph node metastasis than in gastric carcinoma without lymph node metastasis (30.30% vs 0.00%, P < 0.05). The LOH detection rate was higher in tumors at stages III and IV than in tumors at stages I and II (47.05% vs 6.06%, P < 0.01).
| Clinicopathological features | MSI | LOH | ||
| % | (+/n) | % | (+/n) | |
| Differentiation degree | 16.00 | (8/50) | 20.00 | (10/50) |
| High differentiation | 25.00 | (2/8) | 0.00 | (0/8) |
| Middle differentiation | 8.69 | (2/23) | 21.74 | (5/23) |
| Low differentiation | 21.05 | (4/19) | 26.32 | (5/19) |
| Serosa infiltration | ||||
| Positive | 17.15 | (6/35) | 22.86 | (8/35) |
| Negative | 13.33 | (2/15) | 13.33 | (2/15) |
| Lymph node metastasis | ||||
| Positive | 12.12 | (4/33) | 30.30 | (10/33) |
| Negative | 23.53 | (4/17) | 0.00b | (0/17) |
| TNM stage | ||||
| I + II | 15.16 | (5/33) | 6.06 | (2/33) |
| III + IV | 17.65 | (3/17) | 47.05c | (8/17) |
The methylation status in promoter region of BMP3 gene was analyzed by methylation-specific PCR (Table 1). Positively methylated BMP3 promoter region was found in 45 of the 50 gastric carcinoma tissue samples, with a methylation rate of 64.44%. The frequency of methylation in normal tissue was 53.49% (43/50) with no statistical difference between gastric carcinoma and normal tissue samples (P > 0.05). In addition, BMP3 gene promoter region methylation was not correlated with tumor differentiation, infiltration, lymphatic metastasis and clinical TNM staging.
The frequency of BMP3 gene promoter region methylation was much lower in MSI negative than in MSI positive cases (28.58% vs 71.05%, P < 0.05, Table 2). However, the frequency of BMP3 gene promoter region methylation was 68.75% and 50.00%, respectively, in LOH negative and positive cases with no statistical difference.
| Clinicopathological features | Methylation frequency | P value | |
| % | (+/n) | ||
| Differentiation degree | 64.44 | (29/45) | |
| High | 75.00 | (6/8) | |
| Middle | 68.18 | (15/22) | 0.273 |
| Low | 53.33 | (8/15) | |
| Serosa infiltration | |||
| Positive | 59.38 | (19/32) | 0.275 |
| Negative | 76.92 | (10/13) | |
| Lymph node metastasis | |||
| Positive | 62.07 | (18/29) | 0.663 |
| Negative | 68.75 | (11/16) | |
| TNM stage | |||
| I + II | 62.50 | (10/16) | 0.844 |
| III + IV | 65.52 | (19/29) | |
| MSI | |||
| Negative | 71.05 | (27/38) | 0.031a |
| Positive | 28.58 | (2/7) | |
| LOH | |||
| Negative | 68.57 | (24/35) | 0.290 |
| Positive | 50.00 | (5/10) | |
Microsatellites are the short sequences of DNA, usually 2-6 base pairs in a row along a DNA molecule. MSI is mutations in genes, whereby repair of damaged DNA causes microsatellite-associated regions to become longer or shorter. LOH in a cell represents the loss of normal function in one allele of a gene in which the other alleles have been inactivated. MSI and LOH play an important role in tumor development and progression. LOH occurs when the remaining functional allele in a somatic cell of the offspring becomes inactivated due to mutation. No normal tumor suppressor is produced, thus resulting in tumorigenesis[9].
Microsatellites were first reported in 1981 by Miesfeld et al[10] and further elucidated in 1993 by Aaltonen et al[11] who revealed a higher MSI frequency in hereditary nonpolyposis colorectal cancer cells. MSI has been reported in colon, gastric, cervical, breast, prostate and pancreatic carcinomas[12-17].
Alexander et al[18] investigated the sporadic colon carcinoma cases and found that MSI occurs in early stages with a better prognosis, suggesting that MSI can serve as an early diagnostic index for gastric and colon carcinomas. Our previous report also demonstrated that the frequency of MSI is higher in D17S396 loci of the nm23H1 gene in gastric and colon carcinomas at stages I and II with a better prognosis than in those at stages III and IV with a worse prognosis, suggesting that MSI can serve as an early diagnostic index for gastric and colon carcinomas[19]. In this study, however, no statistical significance was found between MSI of BMP3 gene and tumor differentiation, infiltration, lymphatic metastasis and tumor TNM staging.
Berney et al[20] showed that the LOH frequency of nm23H1 gene is significantly correlated with tumor infiltration and metastasis. Candusso et al[21] reported that LOH is more frequently found in late stage tumors, often with lymphatic metastasis. In our study, the LOH frequency of BMP3 gene was much higher in gastric carcinomas at stages III and IV with lymphatic metastasis than in those at stages I and II without lymphatic metastasis, suggesting that LOH occurs more often in late tumor stages with lymphatic metastasis and can thus serve as an index for the evaluation of malignancy, metastasis and prognosis of gastric carcinomas.
DNA methylation is believed to be closely associated with tumor development and progression. Reduced methylation and hypermethylation in gene promoter regions can inactivate tumor suppressor genes, leading to tumor development. In the present study, the methylation level in promoter region of BMP3 gene in gastric carcinomas was high, leading to the occurrence of gastric carcinoma. Loh et al[8] reported that BMP3 gene methylation is associated with MSI in colon cancer, indicating that the higher the methylation is, the higher the MSI frequency is. In this study, the level of methylation was higher in MSI negative than in MSI positive gastric carcinoma cases, which is not consistent with the findings of Loh et al[8]. Further study is needed to verify the differences.
In conclusion, genetic instability of the BMP3 gene and promoter region methylation occur in gastric carcinomas, thus affecting tumor characteristics through different pathways. LOH is observed more frequently in late stage tumors with infiltration. Hypermethylation in promoter region of BMP3 gene may result in gastric carcinoma in Chinese people.
Gastric carcinoma is one of the leading causes of cancer-related death in China. Its incidence and mortality are increasing in Chinese people. Microsatellite instability (MSI) and loss of heterozygosity (LOH) are known to be associated with gastric cancer. Moreover, gene promoter region hypermethylation can inactivate certain tumor suppressor genes.
Genetic instability and gene promoter region methylation play an important role in gene mutation, gene inactivation, and carcinogenesis. However, the role and mechanism of bone morphogenetic protein 3 (BMP3) in cancer development are poorly characterized. The relation between the development of gastric cancer and the number of altered microsatellite loci (D4S2922 and D4S2964), LOH, and promoter region methylation was elucidated in the present study.
In the present study, the methylation levels in promoter region of BMP3 gene in gastric carcinoma was high, which may lead to gastric carcinoma and is not consistent with the reported findings. The methylation level was higher in MSI negative than in MSI positive gastric carcinoma.
MSI and LOH of the BMP3 gene can serve as an index for the evaluation of malignancy, metastasis and prognosis of gastric carcinoma.
MSI is a condition manifested as damaged DNA due to defects in normal DNA repair process. LOH in a cell represents the loss of normal function in one allele of a gene in which the other allele has been inactivated.
The paper is well-written and interesting. BMP3 gene, MIS, LOH and promoter region methylation were evaluated in patients with gastric adenocarcinoma, thus making an additional contribution to studies on the role of MIS and LOH in carcinogenesis.
Peer reviewers: Tamara Vorobjova, Senior Researcher in Immunology, Department of Immunology, Institute of General and Molecular Pathology, University of Tartu, Ravila, 19, Tartu 51014, Estonia; Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City, Cuba
S- Editor Wang JL L- Editor Wang XL E- Editor Zheng XM
| 1. | Cai YC, So CK, Nie AY, Song Y, Yang GY, Wang LD, Zhao X, Kinzy TG, Yang CS. Characterization of genetic alteration patterns in human esophageal squamous cell carcinoma using selected microsatellite markers spanning multiple loci. Int J Oncol. 2007;30:1059-1067. |
| 2. | Chakrabarti S, Sengupta S, Sengupta A, Basak SN, Roy A, Panda C, Roychoudhury S. Genomic instabilities in squamous cell carcinoma of head and neck from the Indian population. Mol Carcinog. 2006;45:270-277. |
| 3. | Inoue Y, Miki C, Watanabe H, Ojima E, Kusunoki M. Genomic instability and tissue expression of angiogenic growth factors in sporadic colorectal cancer. Surgery. 2006;139:305-311. |
| 4. | Nowacka-Zawisza M, Bryś M, Hanna RM, Zadrozny M, Kulig A, Krajewska WM. Loss of heterozygosity and microsatellite instability at RAD52 and RAD54 loci in breast cancer. Pol J Pathol. 2006;57:83-89. |
| 5. | Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95:866-871. |
| 6. | Juvan R, Hudler P, Gazvoda B, Repse S, Bracko M, Komel R. Significance of genetic abnormalities of p53 protein in Slovenian patients with gastric carcinoma. Croat Med J. 2007;48:207-217. |
| 7. | Xiao YP, Wu DY, Xu L, Xin Y. Loss of heterozygosity and microsatellite instabilities of fragile histidine triad gene in gastric carcinoma. World J Gastroenterol. 2006;12:3766-3769. |
| 8. | Loh K, Chia JA, Greco S, Cozzi SJ, Buttenshaw RL, Bond CE, Simms LA, Pike T, Young JP, Jass JR. Bone morphogenic protein 3 inactivation is an early and frequent event in colorectal cancer development. Genes Chromosomes Cancer. 2008;47:449-460. |
| 9. | Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5:45-54. |
| 10. | Miesfeld R, Krystal M, Arnheim N. A member of a new repeated sequence family which is conserved throughout eucaryotic evolution is found between the human delta and beta globin genes. Nucleic Acids Res. 1981;9:5931-5947. |
| 11. | Aaltonen LA, Peltomäki P, Leach FS, Sistonen P, Pylkkänen L, Mecklin JP, Järvinen H, Powell SM, Jen J, Hamilton SR. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812-816. |
| 12. | Svrcek M, El-Bchiri J, Chalastanis A, Capel E, Dumont S, Buhard O, Oliveira C, Seruca R, Bossard C, Mosnier JF. Specific clinical and biological features characterize inflammatory bowel disease associated colorectal cancers showing microsatellite instability. J Clin Oncol. 2007;25:4231-4238. |
| 13. | Sakurai M, Zhao Y, Oki E, Kakeji Y, Oda S, Maehara Y. High-resolution fluorescent analysis of microsatellite instability in gastric cancer. Eur J Gastroenterol Hepatol. 2007;19:701-709. |
| 14. | An HJ, Kim KI, Kim JY, Shim JY, Kang H, Kim TH, Kim JK, Jeong JK, Lee SY, Kim SJ. Microsatellite instability in endometrioid type endometrial adenocarcinoma is associated with poor prognostic indicators. Am J Surg Pathol. 2007;31:846-853. |
| 15. | Pizzi C, Di Maio M, Daniele S, Mastranzo P, Spagnoletti I, Limite G, Pettinato G, Monticelli A, Cocozza S, Contegiacomo A. Triplet repeat instability correlates with dinucleotide instability in primary breast cancer. Oncol Rep. 2007;17:193-199. |
| 16. | Burger M, Denzinger S, Hammerschmied CG, Tannapfel A, Obermann EC, Wieland WF, Hartmann A, Stoehr R. Elevated microsatellite alterations at selected tetranucleotides (EMAST) and mismatch repair gene expression in prostate cancer. J Mol Med. 2006;84:833-841. |
| 17. | House MG, Herman JG, Guo MZ, Hooker CM, Schulick RD, Cameron JL, Hruban RH, Maitra A, Yeo CJ. Prognostic value of hMLH1 methylation and microsatellite instability in pancreatic endocrine neoplasms. Surgery. 2003;134:902-908; discussion 909. |
| 18. | Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527-535. |
| 19. | Yang YQ, Wu L, Chen JX, Sun JZ, Li M, Li DM, Lu HY, Su ZH, Lin XQ, Li JC. Relationship between nm23H1 genetic instability and clinical pathological characteristics in Chinese digestive system cancer patients. World J Gastroenterol. 2008;14:5549-5556; discussion 5555. |
| 20. | Berney CR, Fisher RJ, Yang J, Russell PJ, Crowe PJ. Genomic alterations (LOH, MI) on chromosome 17q21-23 and prognosis of sporadic colorectal cancer. Int J Cancer. 2000;89:1-7. |
| 21. | Candusso ME, Luinetti O, Villani L, Alberizzi P, Klersy C, Fiocca R, Ranzani GN, Solcia E. Loss of heterozygosity at 18q21 region in gastric cancer involves a number of cancer-related genes and correlates with stage and histology, but lacks independent prognostic value. J Pathol. 2002;197:44-50. |