Published online Jan 7, 2010. doi: 10.3748/wjg.v16.i1.119
Revised: November 4, 2009
Accepted: November 11, 2009
Published online: January 7, 2010
Pancreatic schwannoma is a very uncommon tumor of the pancreas, with only 27 cases reported. Most pancreatic schwannomas are benign, with only four malignant tumors reported. We describe a case of giant malignant schwannoma of the pancreatic body and tail, which involved the transverse colon. The tumor was treated successfully with en bloc distal splenopancreatectomy and colon resection. This is believed to be the first reported radical operation for malignant schwannoma of the pancreatic body, with infiltration of the transverse colon, with excellent long-term results. The patient is alive and well 28 mo after the operation. The authors conclude that pancreatic schwannomas should be considered in the differential diagnosis of cystic neoplasms of the pancreas, although the diagnosis can only be confirmed by microscopic examination. In the case of the benign tumors, local excision is adequate, but in the case of malignant schwannoma, oncological standards must be fulfilled.
-
Citation: Stojanovic MP, Radojkovic M, Jeremic LM, Zlatic AV, Stanojevic GZ, Jovanovic MA, Kostov MS, Katic VP. Malignant schwannoma of the pancreas involving transversal colon treated with
en-bloc resection. World J Gastroenterol 2010; 16(1): 119-122 - URL: https://www.wjgnet.com/1007-9327/full/v16/i1/119.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i1.119
Mesenchymal tumors derived from Schwann cells that envelope the peripheral nerves (schwannoma/neurilemmoma) can be found throughout the body, with the most common localization in the extremities, trunk, head and neck, retroperitoneum, mediastinum and pelvis[1]. Visceral schwannomas, which arise from sympathetic and parasympathetic nerve fibers, are very rare[2]. Pancreatic schwannoma is notably uncommon, with only 27 cases reported in the English and Japanese literature to date[3-7]. Most pancreatic schwannomas are benign, with only four malignant tumors reported.
We describe a case of giant malignant schwannoma of the pancreatic body and tail, which involved the transverse colon. The tumor was treated successfully with en bloc distal splenopancreatectomy and colon resection.
A 24-year-old woman was hospitalized because of unclear abdominal symptoms, dyspepsia, weight loss and palpable tumor in the left hypochondrium. She had no anamnesis of pancreatitis or trauma, and there was no sign of von Recklinghausen’s disease. The laboratory data (complete blood count, hepatic and pancreatic function tests) were within normal limits. Tumor marker levels also were within normal limits [carcinoembryonic antigen (CEA), 2.3 U/mL and carbohydrate antigen 19-9 (CA 19-9), 16.8 U/mL].
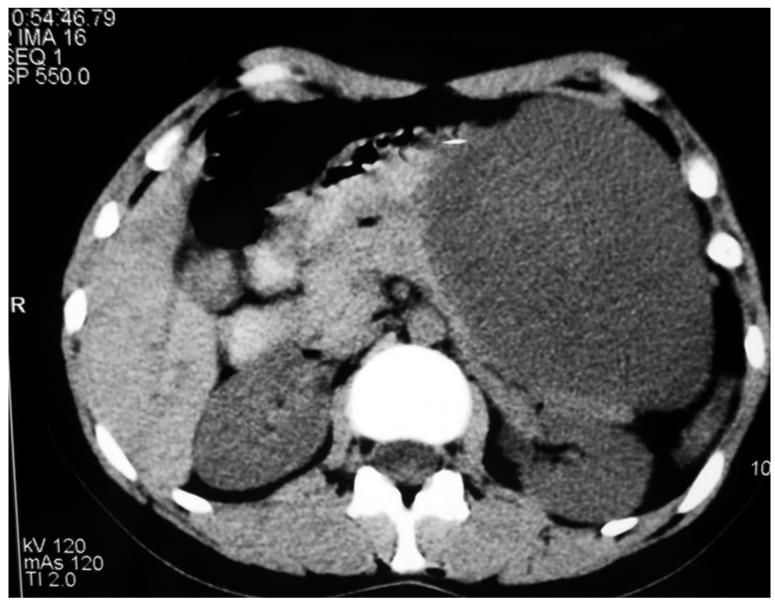
Ultrasonography of the abdomen revealed a well-demarcated, large, predominantly hyperechogenic mass, with hypoechogenic components in the body and tail of the pancreas, and compression of the posterior gastric wall. Computed tomography (CT) showed a well-circumscribed round hypodense mass (32 HU), 18 cm in diameter, which occupied the body and tail of the pancreas and displaced the splenic vein. There was suspect infiltration of the stomach and transverse colon (Figure 1).
Percutaneous needle aspiration was performed and a very small amount of the fluid was aspirated. Biochemical analysis showed normal amylase (48 U/L), CEA (1.9 ng/mL) and CA 19-9 (13 U/L) levels. Cytology revealed rare large cells with high nuclear-cytoplasmic ratios, prominent nucleoli, and cytoplasmic vacuoles, which were suggestive of malignancy. The cell block contained fragments of connective tissue and stroma with some spindle cells and hemosiderin-laden histiocytes.
We decided on operative treatment under the tentative diagnosis of a cystic neoplasm of the pancreas. Laparotomy revealed an encapsulated solid tumor in the pancreatic body and tail, which involved the transverse colon. There was no macroscopic regional lymphadenopathy. The patient underwent en bloc resection that consisted of hemipancreatectomy, splenectomy, omentectomy and transverse colon resection. Systematic lymphadenectomy was performed with removal of the 7, 9, 10 and 11 groups of lymph nodes, according to the Japanese Gastric Cancer Association Classification. Overall number of lymph nodes was 12, with micrometastases founded in two. The postoperative course was uneventful and the patient was discharged on postoperative day 11. No chemo or radiotherapy was added. She is free of symptoms 28 mo after surgery.
Surgical biopsies were fixed in 10% formaldehyde overnight, embedded in paraffin wax, and cut at a thickness of 4 μm. The sections were stained with hematoxylin and eosin, Alcian blue/periodic acid-Schiff, van Gieson and immunohistochemical avidin biotin complex techniques, by using S-100 antibody for detection of schwannoma, and Ki 67 antibody to evaluate mitotic activity in tumor cells.
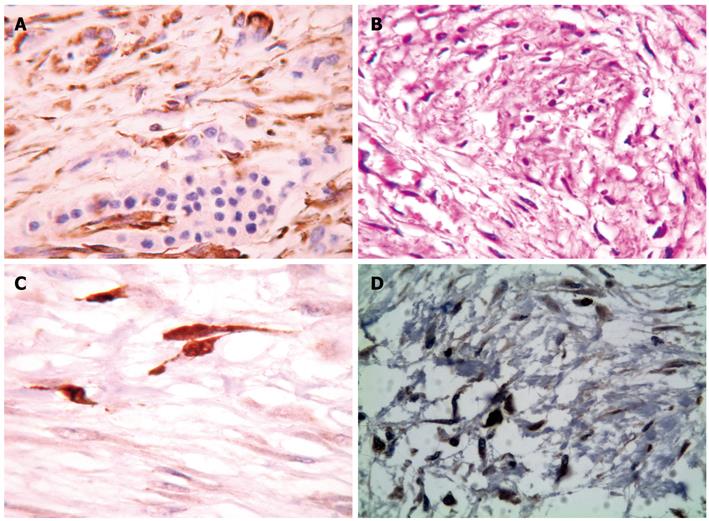
Microscopy showed that tumor cells infiltrated only the serosa of the transverse colon. The nuclei and cytoplasm of the spindle-shaped neoplastic cells were diffuse (Figure 2A) and strongly immunoreactive for S-100 protein (Figure 2B and C). Tumor cells showed increased proliferative activity, with numerous mitotic figures. In the hypercellular areas, we registered > 10 mitotic figures/high-power field. Intense nuclear staining for Ki67 (MIB1) in the neoplastic cells was observed (Figure 2D). There was no cystic component or secondary elements of mature cartilage or bone.
Mesenchymal tumors derived from Schwann cells that envelope peripheral nerves (schwannoma/neurilemmoma) are uncommon. They can be found throughout the body, with the most common localization in the extremities, trunk, head and neck, retroperitoneum, mediastinum and pelvis[1]. Visceral schwannomas, which arise from sympathetic and parasympathetic nerve fibers are very rare[2]. Pancreatic schwannoma is notably uncommon, with only 27 cases reported in the English and Japanese literature to date[3-7]. The patients ranged in age from 41 to 87 years (mean 61 years), with a nearly equal sex distribution. The tumor size ranged from 1.5 to 20 cm (mean: 6.5 cm), with the pancreatic head involved in 44%, and the body and tail in 56% of cases[1]. Schwannomas usually occur as solitary lesions, but are occasionally multiple when associated with von Recklinghausen’s disease[8].
Although three cases of small solid pancreatic schwannomas have been reported[9-11], typical presentation of schwannomas is in the form of cystic, thin-walled, and hemorrhagic masses[3]. In our case, we found a large, solid schwannoma with a well-defined capsule, along with infiltration of the transverse colon.
Typical microscopic features of schwannoma are two microscopic components: a highly ordered cellular component (Antoni A areas), and a loose myxoid component with degenerative changes (Antoni B areas)[12]. Tumor cells are invariably immunoreactive for S100 protein, vimentin and CD56, and negative for cytokeratin AE1/3, CD34, CD117 (c-kit), desmin, and smooth muscle myosin[13].
A review of the literature has revealed only four cases of malignant schwannoma of the pancreas[2,12,14,15]. Malignant transformation of a benign schwannoma is extremely rare[8]. Also, malignant pancreatic schwannomas that were associated with von Recklinghausen’s disease have been reported, but none of the benign pancreatic schwannomas were associated with von Recklinghausen’s disease[2,15].
Clinically, schwannoma is asymptomatic for a long time, or it is accompanied by nonspecific abdominal pain and discomfort. In the late clinical course, compression of the surrounding organs might be noticed.
To establish the diagnosis of pancreatic schwannoma, CT is the initial investigation of choice. CT findings usually show well-defined, round masses with multiple, low-attenuation, cystic necrotic areas. In tumors that are predominantly or exclusively composed of Antoni A areas (cellular component), CT shows inhomogeneous, hypodense, solid masses with contrast enhancement. When the tumor is predominantly composed of Antoni B areas (loose myxoid), CT shows homogeneous cystic masses without significant contrast enhancement[16]. On magnetic resonance imaging, schwannomas are present as masses of low signal intensity on T1-weighted images and of high signal intensity on T2-weighted images[17].
Deep tumors tend to grow larger, therefore, they are more likely to show secondary degenerative changes such as cyst formation, calcification, hemorrhage, and hyalinization, and are known as ancient schwannomas[1]. As a result of these changes and the content of predominantly loose tissue, pancreatic schwannoma is often misdiagnosed as a pseudocyst or other cystic neoplasm of the pancreas.
Definitive preoperative diagnosis is proven using fine-needle aspiration. However, this method correctly diagnoses only one in eight histologically proven schwannomas[18,19]. Definitive diagnosis requires histological examination and complex immunohistochemistry or ultrastructural examination[12]. For the definition of malignancy, which is often difficult in mesenchymal tumors, we use the following criteria: accentuated cell pleomorphism, high mitotic activity, rare stained necrosis, infiltration of the adjacent organs (colon), and locoregional lymph node micrometastases[2,12].
The malignant transformation of pancreatic schwannoma is uncommon, therefore, simple enucleation of benign schwannoma is usually sufficient if the pathology is confirmed before surgery[4].
In the case of the malignant schwannoma, oncological resection is indicated. A review of the literature has revealed one case of unresectable tumor of the pancreatic body, which was resolved by drainage[14]. In the case of pancreatic head localization, simple excision was performed in one case[2], and radical (Whipple) operation was performed in two other cases. However, in the present literature, only short-term follow-up has been reported (maximum, 9 mo), with no data about maximum survival[12,15].
As far as we are aware, this is the first report of radical surgery for malignant schwannoma of the pancreatic body, with infiltration of the transverse colon, with excellent long-term results. Our patient is alive and well 28 mo after the operation.
Radiotherapy has been shown to decrease tumor growth and regression in neurogenic schwannoma, but there have been no previous reports of chemoradiation therapy[20]. However, the role of chemoradiation therapy in the management of pancreatic schwannoma has not been proven. Surgical excision with close follow-up and surveillance remain the mainstay of treatment[3].
In conclusion, pancreatic schwannoma is very rare, but an important pathological condition. It should be considered in the differential diagnosis of cystic neoplasms of the pancreas, although the diagnosis can only be confirmed by microscopic examination. In the case of benign tumors, local excision is adequate, but in the case of malignant schwannoma, oncological standards must be fulfilled.
Peer reviewers: Itaru Endo, MD, PhD, Professor and Chairman, Department of Gastroenterological Surgery, Yokohama City University, Graduate School of Medicine, 3-9 Fukuura, Kanazawa-ku, Yokohama, 2360004, Japan; Dr. Kai Bachmann, Department of Surgery, University Medical Center Hamburg, Martinistrasse 52, Hamburg 22529, Germany
S- Editor Wang JL L- Editor Kerr C E- Editor Ma WH
| 1. | Akiyoshi T, Ueda Y, Yanai K, Yamaguchi H, Kawamoto M, Toyoda K, Hayashi T, Ohuchida J. Melanotic schwannoma of the pancreas: report of a case. Surg Today. 2004;34:550-553. |
| 2. | Coombs RJ. Case of the season. Malignant neurogenic tumor of duodenum and pancreas. Semin Roentgenol. 1990;25:127-129. |
| 3. | Bui TD, Nguyen T, Huerta S, Gu M, Hsiang D. Pancreatic schwannoma. A case report and review of the literature. JOP. 2004;5:520-526. |
| 4. | Tan G, Vitellas K, Morrison C, Frankel WL. Cystic schwannoma of the pancreas. Ann Diagn Pathol. 2003;7:285-291. |
| 5. | Novellas S, Chevallier P, Saint Paul MC, Gugenheim J, Bruneton JN. MRI features of a pancreatic schwannoma. Clin Imaging. 2005;29:434-436. |
| 6. | Morita S, Okuda J, Sumiyoshi K, Taketani M, Moriguchi A, Katsu K, Tanigawa N. Pancreatic Schwannoma: report of a case. Surg Today. 1999;29:1093-1097. |
| 7. | Tofigh AM, Hashemi M, Honar BN, Solhjoo F. Rare presentation of pancreatic schwannoma: a case report. J Med Case Reports. 2008;2:268. |
| 8. | Weiss SW, Goldblum JR. Benign tumors of peripheral nerves. Enzinger and Weiss’s soft tissue tumors. 4th ed. St. Louis: Mosby 2001; 1111-1207. |
| 9. | Burd DA, Tyagi G, Bader DA. Benign schwannoma of the pancreas. AJR Am J Roentgenol. 1992;159:675. |
| 10. | Sugiyama M, Kimura W, Kuroda A, Muto T. Schwannoma arising from peripancreatic nerve plexus. AJR Am J Roentgenol. 1995;165:232. |
| 11. | Feldman L, Philpotts LE, Reinhold C, Duguid WP, Rosenberg L. Pancreatic schwannoma: report of two cases and review of the literature. Pancreas. 1997;15:99-105. |
| 12. | Eggermont A, Vuzevski V, Huisman M, De Jong K, Jeekel J. Solitary malignant schwannoma of the pancreas: report of a case and ultrastructural examination. J Surg Oncol. 1987;36:21-25. |
| 13. | Tan G, Vitellas K, Morrison C, Frankel WL. Cystic schwannoma of the pancreas. Ann Diagn Pathol. 2003;7:285-291. |
| 14. | Móller Pedersen V, Hede A, Graem N. A solitary malignant schwannoma mimicking a pancreatic pseudocyst. A case report. Acta Chir Scand. 1982;148:697-698. |
| 15. | Walsh MM, Brandspigel K. Gastrointestinal bleeding due to pancreatic schwannoma complicating von Recklinghausen’s disease. Gastroenterology. 1989;97:1550-1551. |
| 16. | Ferrozzi F, Zuccoli G, Bova D, Calculli L. Mesenchymal tumors of the pancreas: CT findings. J Comput Assist Tomogr. 2000;24:622-627. |
| 17. | Kim SH, Choi BI, Han MC, Kim YI. Retroperitoneal neurilemoma: CT and MR findings. AJR Am J Roentgenol. 1992;159:1023-1026. |
| 18. | Yu GH, Sack MJ, Baloch Z, Gupta PK. Difficulties in the fine needle aspiration (FNA) diagnosis of schwannoma. Cytopathology. 1999;10:186-194. |
| 19. | Tafe LJ, Suriawinata AA. Cystic pancreatic schwannoma in a 46-year-old man. Ann Diagn Pathol. 2008;12:296-300. |
| 20. | Sawamura Y, Shirato H, Sakamoto T, Aoyama H, Suzuki K, Onimaru R, Isu T, Fukuda S, Miyasaka K. Management of vestibular schwannoma by fractionated stereotactic radiotherapy and associated cerebrospinal fluid malabsorption. J Neurosurg. 2003;99:685-692. |