Published online Jan 7, 2010. doi: 10.3748/wjg.v16.i1.1
Revised: November 18, 2009
Accepted: November 25, 2009
Published online: January 7, 2010
Liver cell transplantation presents clinical benefit in patients with inborn errors of metabolism as an alternative, or at least as a bridge, to orthotopic liver transplantation. The success of such a therapeutic approach remains limited by the quality of the transplanted cells. Cryopreservation remains the best option for long-term storage of hepatocytes, providing a permanent and sufficient cell supply. However, isolated adult hepatocytes are poorly resistant to such a process, with a significant alteration both at the morphological and functional levels. Hence, the aim of the current review is to discuss the state of the art regarding widely-used hepatocyte cryopreservation protocols, as well as the assays performed to analyse the post-thawing cell quality both in vitro and in vivo. The majority of studies agree upon the poor quality and efficiency of cryopreserved/thawed hepatocytes as compared to freshly isolated hepatocytes. Intracellular ice formation or exposure to hyperosmotic solutions remains the main phenomenon of cryopreservation process, and its effects on cell quality and cell death induction will be discussed. The increased knowledge and understanding of the cryopreservation process will lead to research strategies to improve the viability and the quality of the cell suspensions after thawing. Such strategies, such as vitrification, will be discussed with respect to their potential to significantly improve the quality of cell suspensions dedicated to liver cell-based therapies.
- Citation: Stéphenne X, Najimi M, Sokal EM. Hepatocyte cryopreservation: Is it time to change the strategy? World J Gastroenterol 2010; 16(1): 1-14
- URL: https://www.wjgnet.com/1007-9327/full/v16/i1/1.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i1.1
Liver cell transplantation (LCT) is able to correct inborn errors of liver metabolism by supplying viable and functional hepatocytes[1-5]. This innovative therapeutic approach is currently accepted as a bridge to transplantation during the waiting time for a graft. The efficacy of this alternative treatment varies according to the etiology of the disease and remains principally dependent on the quality of the initial liver cell suspension used.
The current great challenge of LCT is the constant availability of the cell suspension. Besides sterility, the cell suspension should present high viability and metabolic activity levels. If freshly isolated cells can be ready to use 5-8 h after the treatment of the organ, data of sterility tests are rarely available before transplantation. Nevertheless, most of the quality control tests performed and documented in the literature reveal a good quality profile of freshly isolated cells, both in vitro and in vivo after transplantation. However, their availability remains significantly limited by ongoing organ shortages. Furthermore, even if freshly isolated cells could be transplanted the same day, we are limited by the quantity of cells that can be infused in one single session. Extensive research based on two different strategies has been developed to efficiently store the isolated liver cells: cold preservation, which could happen during the first 24 h post-isolation and cryostorage. This later strategy remains the sole practical method for the long-term storage of hepatocytes and leads to (1) the development of a readily available cell bank, even in emergency cases, such as metabolic decompensation; (2) the use of fully analysed cell suspensions, including bacterial and viral safety assays; and (3) an efficient planning of future transplantation. In 1999 an “international panel of experts” recognized that ‘‘research should continue to improve the liver cell cryopreservation procedures”[6]. Ten years later, only a few cryopreservation protocol improvements have been documented, and hepatocyte post-thawing quality remains poor.
The aim of this review is to discuss current developments regarding the major cryopreservation/thawing (C/T) protocols used in the field. Pre-C/T management of the cell suspension and post-thawing in vitro and in vivo analyses will be discussed and reviewed. Understanding the biophysical properties of the cryopreservation protocol might supply key and useful information to build efficient strategies. Intracellular ice formation (IIF) or exposure to hyperosmotic solutions, which remain the major C/T damages initiators will be reviewed in detail, regarding their effects on the decrease or loss of cell function, and on cell damages and cell death. Finally, technological developments, such as vitrification, which avoids the crystalline state, or encapsulation, which confers mechanical protection, are currently considered to be exciting new perspectives for the improvement of the cell suspension quality dedicated to clinical LCT.
An initial high quality cell suspension after isolation remains essential prior to cryopreservation. Indeed, key factors that compromise the quality of the isolated hepatocytes include high liver fat content, prolonged warm ischemia and/or storage of the organ[6].
Liver cell isolation is mainly performed using the two-step collagenase perfusion protocol. At 37°C, the first solution, which contains a calcium chelating agent, is perfused to weaken the intercellular junctions of liver cells by removing extracellular calcium ions. The second solution contains collagenase and calcium, essential for the collagenase activity, and disaggregates the extracellular compartment to easily release both non-parenchymal and parenchymal cell fractions. The isolated hepatocyte suspension is obtained after mechanical dissociation, filtration and low speed centrifugation[7].
Isolation is thus the first cause of cell trauma, probably due to oxidative stress, as demonstrated in ischemia/reperfusion of the liver, with impaired mitochondrial functions, consequent intracellular adenosine triphosphate (ATP) depletion (personal unpublished data), and production of reactive oxygen species, leading to hepatocyte death. Addition of anti-oxidant molecules to the isolation medium, such as curcumin, ameliorates the post-isolation quality in terms of metabolic activity and plating. However, such compounds did not show any beneficial effect after cryopreservation/thawing[8].
Detachment from the extracellular matrix has also been shown to promote apoptosis, called anoikis (loss of adhesion molecule). This early cell death could not be totally reversed after in vitro culture of hepatocytes because cells already engaged in this process will die in the hours following the isolation procedure[9,10]. Anoikis is possibly a consequence of the recently described isolation oxidative stress. In conclusion, cell damage, due to the isolation process itself, is already evidenced prior to C/T. However, if plated, the cells have the opportunity in culture to recover and maintain a good metabolic activity.
Suspension pre-culture: To allow the hepatocytes to recover from the isolation stress and improve their quality post-isolation, authors proposed culturing (no attachment culture conditions) freshly isolated hepatocytes prior to C/T. Cold (4°C) or warm (37°C) non-attached, stirred, culture conditions (bio-artificial liver) were developed to avoid later addition deleterious detachment of plated cells. Using pig hepatocytes, Darr et al[11,12] demonstrated that 24 h pre-culture in a spinner bioreactor at 37°C leads to a detectable increase in albumin production after C/T as compared to non pre-cultured hepatocytes. This beneficial effect decreased after 48 h of pre-culture, showing that the recovery of cell quality post-thawing remains difficult. Indeed, non-attached culture conditions might, over time, lead to additional cell damage. Furthermore, albumin production levels remained markedly lower following cryopreservation as compared to freshly isolated cells, even with 24 h pre-incubation. The utility of pre-culture was confirmed by Gómez-Lechón et al[13] who demonstrated that high post-thawing quality hepatocytes of other species (rat, dog and human hepatocytes) were possible using non-attached pre-culture. Criteria such as viability, adaptation of hepatocytes to culture, drug-metabolizing capability and cytochrome P450 (CYP) activity were assessed. The influence of a non-supplemented pre-culture step on hepatocyte quality was not confirmed by data published by Lloyd et al[14] with pig hepatocytes cultured in a bioartificial liver.
Pre-incubation with antioxidants: ATP cellular boosters or antioxidants have been proposed to supplement pre-incubation medium and to potentialize the beneficial effects of pre-culture. Several pre-culture conditions, type of ATP booster and culture at 4°C or 37°C, were evaluated by Terry et al[15,16]. Two hours hyperosmotic glucose 100-300 mmol/L pre-incubation has been shown to improve the viability and attachment efficiency of rat hepatocytes, as well as the viability of human hepatocytes post-thawing. Fructose 100-300 mmol/L pre-incubation also improved the viability and attachment efficiency of rat hepatocytes. On human hepatocytes, fructose improved their attachment efficiency, but not their viability. Pre-incubation with the anti-oxidant alpha-lipoic acid at 0.5-5 mmol/L improved the viability and attachment efficiency of both rat and human hepatocytes. The beneficial effects of this pre-treatment (at lower concentration: 15 mmol/L glucose for 30 min at 37°C) in human hepatocytes were demonstrated by Silva et al[17]. They found that the response of CYP enzymes to typical inducers was significantly improved in the pre-incubated rat and human hepatocytes. The pre-incubated hepatocytes showed a significantly higher plating efficiency compared with hepatocytes cryopreserved without pre-incubation. Finally, Gómez-Lechón et al[18] recently demonstrated that the optimal preservation of isolated cells (cell viability, attaching capacity, and functionality, particularly GSH and glycogen levels, as well as drug-metabolizing cytochrome P450 enzymes) was found in media supplemented with 2 mmol/L N-acetyl-cystein (anti-oxidant molecule) and 15 mmol/L glucose, confirming the importance of anti-oxidant protection after isolation.
In conclusion, based on the literature and on our experience, we believe that liver cell isolation represents an important oxidative stress, potentially controlled by the addition of anti-oxidants to the isolation media and/or by non-attached time-limited culture in an anti-oxidant supplemented medium. Quality of cells prior to C/T is therefore increased. However, all the damage related to C/T is not avoided by these pre-C/T steps. Furthermore, in clinical settings, pre-culture adaptation is difficult.
After isolation and related oxidative stress, the obtained cell population is re-suspended in cryopreservation media and distributed at specific concentrations in special freezing vials. The hepatocytes are then ready for the cooling process and storage in liquid nitrogen. In this chapter, we will review the literature data for liver cell freezing solution as well the documented cooling and thawing process.
In most studies, the hepatocyte concentration varied from 106 to 107 cells/mL[19]. In this range, Lloyd et al[14] did not find any significant superiority of cell concentrations investigated, when evaluating porcine hepatocytes after thawing. This was confirmed by analyzing, hepatocyte attachment, lactate dehydrogenase (LDH) leakage, bilirubin conjugation, and CYP3A4 activity. However, De Loecker et al[20,21] revealed that a decreased cell density of rat hepatocytes correlated with an increased post-thawing viability, as estimated by viability trypan blue exclusion assay. These data suggest that higher cell densities might increase membrane-membrane contacts and subsequent cell damage. Therefore, unless high cell density will save space and is useful for the development of cell banks, cryopreservation at a low cell density (less than 107/cell) is recommended.
Besides cell density, type and volume of vials used for liquid nitrogen storage might also influence post-thawing cell quality; however, few data are available in the literature. Based on the trypan blue exclusion test, cytochrome activity, and tetrazolium inner salt assays, bags of 50 mL seem to give better quality pig hepatocytes, post-thawing, than bags of 100 mL[22].
The lack of recently published data on the concentration of hepatocytes is considered as a minor point for reaching the best post-thawing quality.
We also confirm that, in our hands, in several different volumed vials (from 2 to 100 mL) and varying cell densities, cell density does not influence post-thawing cell quality.
Cryopreservation medium: University of Wisconsin solution (UW) is the gold standard cryopreservation medium for isolated hepatocytes. It was originally developed as a cold storage solution for transplant organs. The principal cryoprotectants of the UW solution are Lactobionate (100 mmol/L), a large molecular weight anion impermeable to most membranes and supposed to suppress hypothermia-induced cell swelling, and Raffinose (30 mmol/L), which allows additional osmotic support[23]. Dexamethasone, another compound in UW solution, is used to stabilise cell membranes[24].
The superior beneficial effect of UW solution was demonstrated by comparing UW to three other freezing media [all were supplemented with 12% dimethylsulfoxide (DMSO)], Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and commercial solutions, Cell Banker 1 and Cell Banker 2. Parameters including viability, plating efficiency, LDH release, ammonia removal test, and lentiviral gene transfer were shown to be highly maintained in hepatocytes cryopreserved with UW solution[25].
The effectiveness of UW solution as a cryoprotectant agent suggests that metabolic, as well as ultrastructural, factors might be important in the effective cryopreservation of isolated hepatocytes[26]. However, UW solution also has limitations, principally its cost and, as demonstrated in the “Parameters for the evaluation of hepatocytes quality after cryopreservation/thawing” paragraph, its incomplete cell protection.
HypoThermosol (HTS), a recently developed freezing solution, is a dextran-based intracellular-type solution. It is used as the carrier solution of the freezing medium. Freshly isolated rat hepatocytes cryopreserved in HTS supplemented with 10% DMSO have been shown to present high viability levels, good long-term maintenance of hepatospecific functions, and good quality response to cytokine challenge at the post-thawing level compared to other supplemented culture media[27]. Further studies confirmed the utility of HTS, allowing a decrease in the DMSO levels within the cryopreservation solution. However, no data comparing UW to HTS are available in the literature[28,29].
Cryoprotectants: Cryoprotectants are essential components of freezing solutions. Two classes of cryoprotectants are described, those that permeate the cell membrane (DMSO, Glycerol) and those that do not such as polymers (Dextran), oligosaccharides (Trehalose), and sugars (Glucose, sucrose or fructose).
DMSO is an important polar permeating aprotic solvent which is less toxic than other members of this class. The use of DMSO in medicine dates from around 1963, when an University of Oregon Medical School team discovered it could penetrate the skin and other membranes without damaging them and could carry other compounds into a biological system. It is able to enter cells and reduce injury by moderating the increase in solute concentration during freezing. In most studies, DMSO is the ideal cryoprotectant, notably giving the best plating efficiency[27,30-37]. Classically, a final concentration of 10% DMSO is described in many protocols, with some exceptions, although higher levels are potentially toxic due to high osmolarity[38]. The rate of addition of the cryoprotectant also appears important to the outcome of cryopreservation. Freezing must be commenced as soon as possible after addition of the cryoprotectant to reduce the possibility of toxicity at ambient temperatures. Hence, DMSO should be added at 4°C, as toxicity of DMSO was demonstrated at 25°C or 37°C. Some authors proposed adding permeating cryoprotectants slowly to the cell suspension to avoid damages related to osmotic shock and cellular dehydration[33,39]. However, this seems to be a minor point[38].
The use of oligosaccharides with higher molecular weights resulted in greatest improvement in viability. Their combination with DMSO has been shown to allow efficient hepatocyte cryopreservation. Both rat and human hepatocytes exhibit significantly higher viability (as estimated by trypan blue exclusion assay) than hepatocytes previously cryopreserved without oligosaccharides. Moreover, attachment and survival rates in plastic dishes of rat hepatocytes were greater after freezing in the presence of di-, tri-, and tetrasaccharides. Such plating amelioration was not confirmed with human hepatocytes[40]. Metabolic activity was also evaluated after cryopreservation with oligosaccharides. When trehalose was combined with DMSO for the cryopreservation of human hepatocytes, a significant increase in total protein level and secretion of albumin was observed after thawing, as well as decreased levels of aspartate aminotransferase[41].
Those works were inspired from data demonstrating the influence of trehalose on cell quality on bull sperm[42,43]. Similarly, several authors recommended adding sucrose to the cryopreservation medium, with or without trehalose, to ameliorate the quality of the cells after thawing. This allows the concentration of DMSO to be decreased while ameliorating the quality of cells. However, it was evaluated on hematopoietic stem cells and fetal liver hematopoietic stem/progenitor cells[44,45], not on hepatocytes.
The beneficial role of a non-metabolizable glucose derivative as a cryoprotectant that mimicked the natural cryoprotective adaptations observed in freeze-tolerant frogs was also investigated. Primary rat hepatocytes were loaded with 3-O-methyl glucose (3OMG) through endogenous glucose transporters without evident toxicity and cryopreserved according to a controlled rate freezer program down to -80°C before storage in liquid nitrogen. In this study, hepatocytes cryopreserved with a relatively small amount of intracellular 3OMG (< 0.2 mol/L) showed high post-thaw viability and maintained long-term hepatospecific functions, including synthesis, metabolism, and detoxification. Metabolite uptake and secretion rates were also largely preserved in the cryopreserved hepatocytes, showing that 3OMG must be considered as an interesting cryoprotectant[46].
An interesting report proposed that wheat protein extracts permitted long-term storage and recovery of large quantities of healthy cells that maintain high hepatospecific functions, via an osmotic modulation effect[47]. In post-thawing culture, the morphology of hepatocytes cryopreserved with wheat protein extracts was similar to that of fresh cells. Furthermore, hepatospecific functions, such as albumin secretion and biotransformation of ammonium to urea, were well maintained during four-days post-plating. Inductions of CYP1A1 and CYP2B in hepatocytes cryopreserved with wheat extracts were similar to those in fresh hepatocytes. Additional data confirmed the utility of wheat extracts as efficient, non-toxic, economic natural cryoprotectants, superior to DMSO, which has limitations due to potential cellular toxicity[47-49].
Finally, human application does not tolerate the use of animal origin products because of possible zoonosis contamination and/or immune response to animal proteins[50]. Fetal calf serum (FCS) or human albumin, are classical ingredients of the cryopreservation solution, in a proportion of 10% to 90 %. No significant differences in classical viability or drug metabolizing enzyme activities were noted while varying the percentage of serum for (human, pig, and rat) hepatocyte cryopreservation in most of the published studies[26,30,33,36,51-53]. We think that a minimal concentration of serum is required for optimal cryopreservation, even if some authors have also successfully cryopreserved porcine hepatocytes without serum. They showed that, after thawing, in appropriate conditions and without serum, the addition of conditioned medium derived from hepatic non-parenchymal cells improved attachment and function of hepatocytes (urea production and CYP activity)[54].
In conclusion, UW solution remains the best and most studied freezing medium and must be supplemented with a permeating cryoprotectant; DMSO remains the gold standard. The addition of a non-permeating cryoprotectant to this solution must also be considered.
Slow freezing protocols are considered to be the best strategy for cryopreserving mature isolated hepatocytes. All the protocols described in this paragraph were developed using DMSO as the cryoprotectant. First protocols included the use of an isopropranolol cooler device, placed in a -80°C freezer, giving a constant temperature decrease of -1°C/min down to -70°C or -80°C, before storing in liquid nitrogen[55]. Other slow freezing protocols are described in the literature, varying from -1°C/min to -5°C/min up to -40°C or -80°C, before storing at -196°C[56]. A decrease in temperature at -1.9°C/min from 4 to -30°C and then -30°C/min from -30°C to -150°C was also adopted by many authors[51,57,58]. More specific protocols were developed by Diener et al and by Hengstler et al[39,59,60]. Several cooling process protocols, where the temperatures of the vial and of the cryopreserving solution were controlled, were tested on rat hepatocytes. Firstly, shock freezing in liquid nitrogen dramatically decreased cell viability, despite the presence of 10% DMSO. Secondly, a slow freezing protocol with -2°C/min led to much better recovered viability than a cooling rate of -38°C/min. While using the slow freezing protocol, the authors determined that the cell suspension becomes supercooled around -20°C. Indeed, when crystallisation starts, the latent heat of fusion is released and the cell sample is warmed. This heat release may be deleterious; therefore, a freezing program with shock cooling was developed. Analysis of post-thawing viability did not show significant differences of hepatocyte viability (86% viability vs 79% according to the slow linear protocol). The same cooling shock can be obtained by clamping the vials, with forceps cooled in liquid nitrogen[61]. However, studies from Lloyd et al[14] (measuring LDH release, cell return, attachment, and biochemical assays) and from our team[62] did not show any difference between computer-controlled freeze rate (without frozen shock), the Nalgene propan-2-ol device or simply using -20°C and -80°C freezers.
Storage of hepatocytes at -20°C or -80°C remains deleterious for cells functions as several proteases might be active at those temperatures. At -130°C, no chemical reaction can occur as there is no more thermal energy. Furthermore, at this temperature, no water, which is at the vitreous or crystalline state, is present at the liquid state. Therefore, -140/-150°C is the minimum acceptable temperature for long-term storage of cryopreserved hepatocytes[6,34,57,63]. At -140°C (the vapour phase of liquid nitrogen) or -196°C (the liquid phase of liquid nitrogen), cells can be stored for long periods[6,34,51,57]. A summary of freeze rate comparison studies is presented in Table 1. The passage of water from one state to another, IIF, is the critical point that might modulate the cell quality. The limitations of these cooling processes will be discussed later in the IIF paragraph.
| Species | Cryoprotectant | Freeze rate | Storage temperature | Ref. |
| Human | DMSO | -1°C/min | -80°C | [55] |
| Rat | DMSO | -1°C/min to -38°C (with cooling shock) then liquid nitrogen | Liquid nitrogen | [61] |
| Dog, monkey, human | DMSO | -1.9°C/min from 4°C to -30°C, then -30°C from -30°C to -150°C | Liquid nitrogen | [57] |
| Rat | DMSO | -38°C/min | Liquid nitrogen | [59] |
| -2°C/min | ||||
| Slow variable | ||||
| Optimized variable rate | ||||
| Human | DMSO | -1.9°C/min from 4°C to -30°C, then -30°C from -30°C to -150°C | Liquid nitrogen | [51] |
| Rat | DMSO | Cooling in 10 min down to 0°C, 8 min at 0°C, in 4 min down to -8°C, in 0.1 min down to -28°C, in 2 min down to -33°C, in 2 min up to -28°C, in 16 min down to -60°C, in 4 min down to -100°C (variable rate) | Liquid nitrogen | [60] |
| Human | DMSO | Variable rate | Liquid nitrogen | [6] |
| Dog, monkey, human | DMSO | -1.9°C/min from 4°C to -30°C, then -30°C from -30°C to -150°C | Liquid nitrogen | [58] |
| Rat | DMSO | Variable rate | Liquid nitrogen | [39] |
| Pig | DMSO | Optimized[59] | Liquid nitrogen | [22] |
| Modified variable |
The critical point of this procedure is to avoid the deleterious phenomenon, IIF. Rapid thawing at 37°C to minimize cellular damage due to reformation of intracellular ice will significantly enhance cell viability. As for cooling, a slow dilution of the cryoprotectant at 4°C is recommended, to avoid osmotic shock and the toxicity of the cryoprotectant[38].
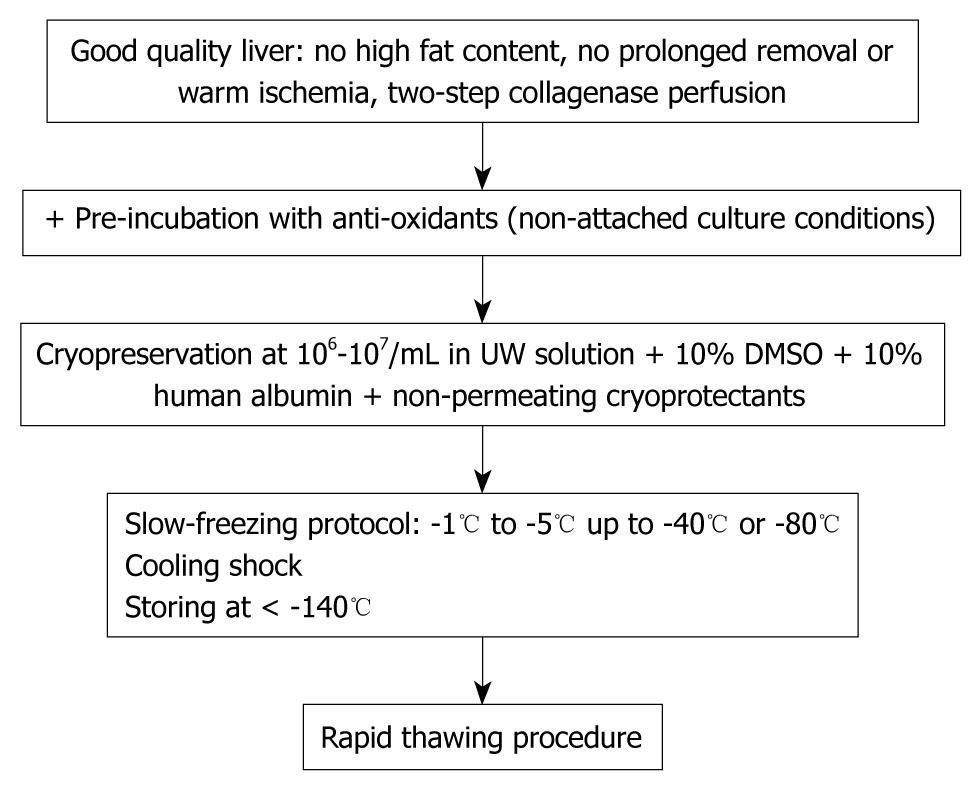
The standardized cryopreservation/thawing protocol is summarized in Figure 1.
The aim of this paragraph is to review the principal assays that may help to standardize the post-thawing evaluation step process.
The viability assays (Trypan blue exclusion test, LDH release, mitochondrial functions and necrotic/apoptotic markers) are important quality markers. Most of the “metabolic” assays investigate only some specific hepatocyte functions, notably drug metabolizing enzymes activities, essential for drug industry application; but also the hepatocytes’ capacity to plate to collagen coated dishes. Other assays should be developed to allow a rapid evaluation of LCT-related critical parameters.
Necrosis was first described to occur following C/T[31], whereby intracellular organelles, most notably the mitochondria, and the entire cell swell and rupture (cell lysis). This phenomenon begins with an impairment of the cell’s ability to maintain homeostasis, leading to an influx of water and extra-cellular ions. Due to the breakdown of the plasma membrane, the cytoplasmic contents, including lysosomal enzymes, are released into the extracellular fluid. This can be tested by LDH release from the cytoplasm to the extracellular medium and reflects cell membrane integrity. However, we found that LDH release was unaffected after C/T of both human and mice hepatocytes and does not adequately assess viability after C/T[62].
Apoptosis, a programmed cell death which has been well characterized, both at the morphological and biochemical levels, was also described following C/T. Annexin V staining, in situ TUNEL assay combined with confocal laser scanning microscopy, or deoxyribonucleic acid (DNA) fragmentation are the most popular apoptosis assays[28,31,64]. These assays should be carefully interpreted if performed in vitro or in vivo.
The mitochondrion is a key player in the initiation of apoptosis and recent studies highlighted its role in C/T-induced cellular damage[65]. Disruption of mitochondrial membrane potential (ΔΨ) was reported following C/T, which is followed, within hours after thawing, by cytochrome c extra-mitochondrial release, caspase-3 activation, and DNA fragmentation. Addition of caspase inhibitors (IDN-1965 or ZVAD-fmk) to the medium during cryopreservation and static culture rescued cells from apoptosis and was associated with increased phase 1 and phase 2 metabolism[64,66].
As mitochondria are the major source of reactive oxygen species (ROS), induction of apoptosis by oxidative stress was also proposed to be involved in the impairment of hepatocytes after C/T[67,68]. In fact, the combination of antioxidant medium containing a caspase inhibitor allowed significant improvements in viability and function in treated rat hepatocytes[68]. Similarly, other authors proposed the addition of S-adenosylmethionine to the cryopreservation medium, to avoid glutathione and viability decrease during cold preservation or cryopreservation in liquid nitrogen[69]. Finally, C/T decreased mitochondria-related cellular respiration and oxygen consumption rate. This effect was evidenced on oligomycin (ATP synthase inhibitor)-sensitive respiration, suggesting that it could result from alteration of a mitochondrial process linked to ATP synthesis, rather than an intrinsic modification of mitochondrial membrane proton permeability (leak). In permeabilized hepatocytes, a marked impairment of mitochondrial oxidative phosphorylation following C/T was observed in in situ mitochondria with substrates for complex 1, under basal mitochondrial respiratory rate. Interestingly, the inhibition of basal mitochondrial respiration was not revealed with complex 2 substrates[62]. The respiratory-chain complex 1 is one of the largest known membrane protein complexes, and is also the major source of mitochondrial ROS[70,71]. Thus, specific alterations of complex 1 subunit(s), which comprise the hydrophilic domain containing the redox centres of the enzyme, and/or deregulation of ROS production leading to oxidative stress, could constitute one of the start-points of the C/T-induced damage. This could explain the oxygen consumption and ∆Ψ decrease, leading to ATP depletion and later cytochrome c release. The intracellular ATP concentration, which is an indirect mitochondrial marker, is probably the easiest and most rapidly measurable parameter for detecting early cellular damages related to cryo-storage.
Detailed information regarding cryopreserved hepatocyte death in situ after transplantation remains poorly investigated in LCT in vivo models.
Attachment of isolated hepatocytes in vitro (collagen coated dishes) is widely used for the evaluation of their quality. The low plating efficiency is often documented in cryopreserved cells. This remains a major problem because engraftment of the transplanted hepatocytes in the recipient liver parenchyma is also dependent on the proteins involved in extracellular matrix adhesion mechanisms[17,23,25,63,72]. Structural membrane damage observed after cryopreservation might contribute to such alterations. Recently, it was demonstrated that the process of cryopreservation leads to down-regulation of cell adhesion at the gene and the protein level (β1-integrin and E-cadherin, amongst others)[73]. This is relevant and probably begins to explain the observed low plating efficiency. Another team was able to demonstrate that when hepatocytes are cryopreserved with wheat extracts instead of DMSO, there was a clear protective effect against loss of β1-integrin, E-cadherin, and β-catenin[74]. We must also recognize the high plating variability from one liver to another.
Conjugation and secretion of biliary acids seems to be maintained following C/T of human hepatocytes. The uptake of taurocholate in cryopreserved hepatocytes of was found to range from 10% to 200% of that observed in freshly isolated cells immediately after thawing at 37°C[75].
The characterization of freshly isolated and C/T monkey hepatocytes demonstrated the maintenance of various hepato-specific functions, but at a low level. The ability to synthesize proteins, glucose, and glucose-6 phosphatase activity was decreased after deep-freeze storage[30]. Concerning protein synthesis, data from the literature show that this important hepatic function is often impaired in hepatocytes after C/T. De Loecker et al[61] demonstrated that cryopreserved human hepatocytes albumin production was reduced to half that of freshly isolated hepatocytes. Glycogen synthesis in cryopreserved porcine hepatocytes was reduced by about 30% after 24 h of culture and about 47% after 48 h of culture compared to freshly isolated hepatocytes. Reduced basal levels of glycogen and of glycogen synthesis could be explained by an increased energy demand in cryopreserved hepatocytes to repair damage caused by cryopreservation. Glycogenolysis was reduced to about 50% in cryopreserved hepatocytes and gluconeogenesis to about 40% of the glucose production in freshly isolated hepatocytes at day 1 and 2 post-thawing. Incubation with glucagon (90 min) increased the glucose production from glycogenolysis and gluconeogenesis in both freshly isolated and cryopreserved hepatocytes[76].
Urea production also seems to be reduced following C/T, according the majority of the papers[30].
There is no apparent significant change in drug metabolizing enzyme activities between freshly isolated and cryopreserved hepatocytes for the major drug-metabolizing pathways. The cryopreservation of human hepatocytes isolated from 17 donors was shown not to alter their capability to metabolize substrates for the major CYP isoforms (CYP1A2, CYP2A6, CYP2C9, CYP2Cl9, CYP2D6, and CYP3A4), as well as the phase II enzymes UDP glucuronyltransferase, and 7-HC sulfation for sulfotransferase[77]. Steinberg et al[78] showed that phase I drug-metabolizing enzyme activities analyzed in cryopreserved human, rat, and mouse hepatocytes were very similar to those of freshly isolated hepatocytes; while phase II enzyme activities were affected by cryopreservation.
Other studies show better stability of drug metabolizing activities in monkey than in rodent hepatocytes. After thawing, Phase I and Phase II activities (CYP, ethoxycoumarin-O-deethylase, aldrin epoxidase, epoxide hydrolase, glutathione transferase, glutathione reductase, and glutathione peroxidase) were well preserved[79]. The decrease in the activity of phase II enzymes, documented in several studies, might be related to the loss of the corresponding cofactors; however, the hypothesis of physical cell alteration is not excluded[78]. The cytosolic enzymes, notably glutathione S-transferase, are more exposed to intracellular ice formation and related C/T damages, even if some mechanical protection can be given by microsomal membranes[55].
Finally, if thawed hepatocytes cultures are sensitive to CYP inducers (rifampicin, rifabutin, phenobarbital, omeprazole, and β-naphthoflavone) the induced activity remains lower as compared to freshly isolated cells, with an increased delay induction time[6,17,60,80-83]. This is summarized in Table 2.
| Hepatocyte in vitro model | Cryopreservation protocol | Parameters evaluated: impairment following C/T | Parameters evaluated: no impairment following C/T | Ref. |
| Rat and human | Pre-incubation | Plating | CYP induction | [17] |
| -20°C, -70°C, liquid nitrogen | ||||
| Porcine | Slow freezing protocol up to -80°C | Trypan blue exclusion test | [23] | |
| Plating | ||||
| Ammonia clearance | ||||
| Rat | Slow freezing protocol up to -80°C | Trypan blue exclusion test | [25] | |
| Plating | ||||
| Ammonia clearance | ||||
| Human | 20% DMSO, 40% FCS | Trypan blue exclusion test | ATP | [63] |
| Slow freezing protocol | Plating | Urea synthesis | ||
| LDH release | ||||
| MTT | ||||
| Porcine | Freezing boxes or slow freezing protocol | CYP | Plating | [72] |
| Glycogen synthesis | ||||
| Glycogenolysis | ||||
| Gluconeogenesis | ||||
| Rat and mouse | Slow freezing protocol | Plating | [41] | |
| Uptake of neutral red | ||||
| Protein synthesis | ||||
| Porcine | Immediate cryopreservation | Protein synthesis | Trypan blue exclusion test | [30] |
| Serum free | Gluconeogenesis | |||
| CYP activity | ||||
| Urea synthesis | ||||
| Rat (monolayer culture post-thawing) | Not available | Protein synthesis | [61] | |
| Human | Storage in liquid nitrogen | Conjugation and secretion of biliary acids | [75] | |
| Human, rat, rabbit, dog and monkey | Slow freezing protocol | CYP activity | [77] | |
| Phase 2 enzymes | ||||
| Human, rat and mouse | Slow freezing protocol | Phase 2 enzymes | Phase 1 enzymes | [78] |
| Monkey | Slow freezing protocol | LDH release | Phase 1 enzymes | [79] |
| Plating | Phase 2 enzymes | |||
| Human | Storing at -80°C | Cytosolic enzymes: glutathione S-transferase | CYP activity | [55] |
| Rat | Slow freezing protocol | CYP induction | [60] | |
| Human | Slow freezing protocol | CYP induction | [80] | |
| Rat | Slow freezing protocol | CYP induction | [81] | |
| Human | Not available | CYP induction | [82] | |
| Human | Not available | CYP induction | [83] |
In conclusion, the standardized “literature based” C/T protocols have limitations, as demonstrated by the collected data in the last four paragraphs. If some progress were made in the assessment of specific hepatic functions, then the true effects of pre-C/T incubation with anti-oxidants or the addition of non-permeating cryoprotectants to the freezing solution on the poor quality of hepatocyte post-C/T could be properly tested. Finally, cell death, probably not a reversible mechanism following C/T, is initiated due to mitochondrial impairment. ATP concentration evaluation, as a mitochondrial operation marker, is a crucial test to evaluate the quality of C/T cells.
Post-thawing cell quality remains poor. How can we explain the observed damages? The passage from a liquid stage of the intracellular and extracellular water, to a crystalline state probably holds the key to understanding C/T damages. In the cryopreservation of cells or tissues, each system has its specific optimal cooling rate, showing a decreased survival at both too low (slow cooling damage) and too high cooling rates (fast cooling damage). During freezing, the transition phase of water leads to a decrease of the extracellular water content. Water can pass through the plasma membrane, which will in turn lead to water efflux and cell dehydration. Slow cooling damage has been attributed to such phenomena as the increase in the external and internal solute (salt) concentration, the small size of the channels of unfrozen solution, or the mechanical stress of cell shrinkage and destabilisation of membranes and proteins at low water potential. At high cooling rates, the intracellular dehydration (by water efflux) cannot keep pace with the extracellular dehydration (by phase transition of water). As a consequence, higher cooling rates result in higher levels of intra-cellular supercooling, higher trans-membrane differences in osmotic pressure and solute concentrations, and higher rates of water efflux through the membrane. This fast cooling damage seems to be due particularly to IIF[84-89].
We recently demonstrated that cryopreservation at -20°C for 20 min followed by rapid thawing induced a dramatic drop of ATP levels in cryopreserved/thawed hepatocytes, correlated with a decreased oxygen consumption rate and altered mitochondrial complex 1 activity (personal unpublished data).
These results suggest that during the cryopreservation damage, complex 1 impairment occurred early in the cryopreservation process by mechanical alteration of mitochondria due to IIF or exposure to hyperosmotic solutions. However, IIF might also occur during the thawing process.
According to the data available in the literature, the ability of cryopreserved/thawed hepatocytes to engraft and to repopulate the recipient liver is not definitively demonstrated. In the eighties, Fuller et al[90] described that fewer cryopreserved (slow freezing protocol in DMSO) autologous hepatocytes cells were detected one month post-transplantation in the recipient liver as compared to freshly isolated cells. David et al[91] in Nagase analbuminemic transplanted rats, found few clusters of C/T cells three months post-transplantation as compared to freshly isolated hepatocytes, with no significant production of albumin. Dunn et al[92] showed in a Dalmatian dog model that sequential intrasplenic LCT can provided a significant but transient, 22 d, correction of urinary uric acid excretion that was similar with either freshly isolated or C/T (slow freezing protocol in DMSO, post-thawing viability around 60%) hepatocytes. The protocol of Papalois et al[93] has demonstrated that cryopreserved pig hepatocytes, at -20°C without cryoprotective medium in Hank’s solution, have adequate viability (around 60%) after one month of storage to support hepatic function in animals with severe acute liver failure by hepatoproliferative factors produced by the hepatocytes engrafted in the spleen.
Besides metabolic supply, intrasplenic transplantation of C/T hepatocytes (at -80°C in UW and DMSO) in rats pre-treated with D-galactosamine improved survival to 60% after seven days (as compared to 100% obtained using freshly isolated hepatocytes)[23].
However, in a transgene-induced liver disease model, an environment that is permissive for clonal expansion of donor cell populations, C/T hepatocytes (stored up to 32 mo in liquid nitrogen) have been shown to possess clonal replicative potential identical to that of freshly isolated hepatocytes. C/T hepatocytes constituting 0.1% of the total adult hepatocyte number in the recipient could repopulate a mean of 32% of recipient liver parenchyma[94]. Furthermore, transplantation of woodchuck hepatocytes into the liver of urokinase-type plasminogen activator/recombination activation gene-2 mice demonstrated that cryopreserved (slow freezing protocol in DMSO, viability up to 70%-80%) cells, retained the ability to divide and to repopulate a xenogenic liver three months post-transplantation. Notably, in vivo susceptibility to infection with woodchuck hepatitis B virus and the proliferative capacity of frozen/thawed woodchuck hepatocytes in recipient mice were identical to those observed by transplanting freshly isolated hepatocytes[95].
The efficiency of C/T (slow freezing protocol in DMSO and HTS, post-thawing viability around 60%) for human hepatocytes was also evaluated in an animal NOD/SCID mice model. Cho et al[96] demonstrated that transplanted cryopreserved human liver cells engrafted in the peritoneal cavity as well as the liver, retained hepatic function (glycogen storage and Glucose-6 phosphatase activity) and proliferated in response to liver injury by carbon tetrachloride. This effect was greater two hours and three days post-transplantation as compared to 7, 14 and 40 d post-transplantation, suggesting some loss of transplanted cells at later times.
Based on the above data, we may conclude that C/T hepatocytes, in a favourable environment, are transiently able to maintain hepatocyte function in vivo, engraft the liver and proliferate at low levels, as compared to freshly isolated cells.
Metabolic diseases: Immediate and medium term metabolic efficacies, decrease of the ammonia levels and urea synthesis were observed in our hands in two urea cycle disorder patients using C/T hepatocytes[97,98]. This was correlated, in one case, with effective demonstration of engraftment up to one year after cell infusion, using Fluorescence In Situ Hybridization (FISH) for the Y chromosome. This four year-old arginosuccinate-lyase deficiency girl was transplanted with C/T cells and underwent a first liver biopsy after infusion of C/T male hepatocytes, which showed a XX/XXYY chimerism, with 4.7% Y-positive cells. This cell lineage was further described on several post-transplant biopsies, reaching more than 10% of the recipient cells, while she received additional fresh and C/T hepatocyte infusions. At King’s College Hospital, one patient with an inherited factor VII deficiency was entirely transplanted with C/T hepatocytes, which led to a transient reduction to 20 percent of the requirements for factor VII therapy[99]. To our knowledge, all other published case reports used an infusion protocol at least partially comprising freshly isolated hepatocytes, preventing any conclusion regarding the respective efficiency of C/T vs freshly isolated cells.
In conclusion, as in animal models and based on few reported data, C/T hepatocytes seem able to transiently support deficient hepatocyte function, justifying their use to stabilise metabolically unstable patients while waiting for a liver graft.
In this final section, we will analyze and discuss several ways to ameliorate the C/T protocols. We think that these new techniques applicable to C/T protocols are the best hopes for changing the future of cryopreservation/thawing.
Encapsulation-in vitro, in vivo: Encapsulation, by conferring a mechanical protection, was investigated with success for hepatocyte cryopreservation protocols.
Firstly, and before considering LCT, several in vitro studies showed that encapsulation of freshly isolated hepatocytes in specially designed multi-component capsules (alginate, cellulose sulphate, and poly (methylene-co-guanidine) hydrochloride) retained their specific functions (transaminase activity, urea synthesis and protein secretion) over the first days of culture. Furthermore, most detoxifying enzymes were also expressed (in cryopreserved alginate-entrapped hepatocytes) at levels close to those in unfrozen encapsulated hepatocytes[100]. Long-term, up to 120 d of cryopreservation, preservation of drug metabolism and transport activities was demonstrated using microencapsulated rat hepatocytes[101]. Moreover, cold-induced apoptosis in hepatocytes can be significantly reduced following their entrapment within alginate gel beads, as demonstrated by measurement of caspase-3-like activity[102]. Finally, cryomicroscopy studies showed that the alginate microencapsulation technique protected the hepatocytes from physical damage caused by the growth of extracellular ice crystals[103].
How can we adapt the encapsulation of cells to the LCT protocol? In 1993, in the Gunn rat model, the authors proposed intraperitoneal transplantation of cryopreserved alginate-encapsulated hepatocytes, allowing significantly reduced hyperbilirubinemia, as well as freshly isolated encapsulated hepatocytes, up to 28 d following transplantation[104]. In a severe liver failure model, two-stage 95% hepatectomy, with xenogenic hepatocytes and without immunosuppression, the authors demonstrated the utility of intrasplenic encapsulated hepatocytes[105].
Intraperitoneal transplantation of cryopreserved or fresh encapsulated rat hepatocytes significantly increased the survival rate to 66% and 80% in the ALF model (acetaminophen administration and 30% hepatectomy). Intraperitoneal transplantation of cryopreserved or fresh encapsulated immortalized hepatocytes improved survival, in this model, to 50% and 55%, respectively. Histopathology revealed that encapsulated hepatocytes were viable, but for a limited period (up to two weeks post-transplantation)[106]. Recently, Baldini et al[107] showed the retention of biological activity and significant viability of porcine encapsulated hepatocytes transplanted intraperitoneally in rats without immunosuppression, confirming the utility of encapsulation to avoid rejection. Moreover, Aoki et al[108] demonstrated that poly-L-lysine entrapped cryopreserved human hepatocytes survived and expressed albumin in rat spleen after transplantation. Finally, Mei et al[109] confirmed these data by showing an increased rate of survival in a mouse model of fulminant hepatic failure after xenogenic transplantation of pig hepatocytes.
In conclusion, cryopreservation of encapsulated hepatocytes is a promising tool for the establishment of banks for the supply and storage of hepatocytes, by mechanically conferring protection. However, the main problem of this technique remains the adaptation to LCT, the problem of injection site and adaptation to the treatment indication (size of capsule pore). Furthermore, this can be only be proposed for ALF or metabolic unstable patients, as the efficacy of the transplantation remains time-limited. Repeated injections must therefore be considered. The time-limited effect is notably due to the hepatocyte de-differentiation, observed with freshly isolated or C/T cells, in this kind of configuration. New projects must evaluate the utility of co-encapsulation of hepatocytes with mesenchymal bone marrow cells or pancreatic islets, as a new type of feeder cells to avoid de-differentiation.
Vitrification (from the Latin, vitreus, glassy) is essentially the solidification of a supercooled liquid by adjusting the composition (high concentration of cryoprotectant) and cooling rate (fast freezing protocol) such that the crystal phase is avoided. The process involves a progressive and marked increase in viscosity during cooling and prevention of ice nucleation and growth. The system is stabilized in the glassy state as translational molecular motion is essentially halted. Vitrification eliminates the biologically damaging effects associated with freezing. No appreciable degradation occurs over time in living matter trapped within a vitreous matrix. Vitrification is potentially applicable to all biologic systems. As the major problem with the current protocols remains IIF, alternatives such as vitrifying hepatocytes are an interesting strategy for attaining the best post-thawing cell quality. Vitrification of precision cut-slices, tissue engineered pancreatic substitute, jugular veins/vessels constructs, and embryonic kidneys has already been performed, allowing the absence of ice into the vitrified samples and an excellent post-thawing quality and/or viability[110-115].
Classically, tissues (vessels constructs and embryonic kidneys) are vitrified at cooling rates of > 40°C/min in a specific solution, comprising DMSO, formamide and 1,2-propanediol in EuroCollins solution (VS55) or a polyethylene formulation consisting of propanediol, DMSO and polyethyleneglycol 400[110,113]. Best viability results were obtained with the VS55 solution. To obtain cooling rates of > 40°C, tissues contained in vials, are cooled to -100°C in an isopentane bath (conductive cooling, freezing point -160°C) placed in a -135°C freezer, removed from the 2-methylbutane bath and vitrified to -120°C in the -135°C freezer (convective cooling).
Re-warming is performed under controlled conditions, and the chemicals removed in a stepwise manner. However de-vitrification might occur during warming from the vitrified state. To prevent de-vitrification, the vitrified material must be warmed uniformly as fast as possible [slowly re-warmed to -100°C using convection followed by rapid re-warming achieved by placing the vial in a DMSO/H2O mixture at room temperature (225°C/min)] so that ice does not have the opportunity to form in significant quantities.
Vitrification of encapsulated hepatocytes in M or G-collagen was recently proposed as an alternative freezing protocol[116]. Wu et al[117] proposed a rapid stepwise introduction of microencapsulated hepatocytes to vitrification solution (40 % v/v ethylene glycol, 0.6 mol/L sucrose in the medium) and their direct immersion in liquid nitrogen. Using this technique, they obtained 100% retention of hepatocyte functions, correlated with excellent viability, and no detectable damage to the microcapsules. If vitrification was also proposed as successful cryopreservation protocol for isolated cells, as has been done for human amnion derived mesenchymal stem cells[118]; however, vitrification of non-encapsulated hepatocytes has not yet been studied. Therefore, further investigations are needed to confirm the potential of vitrification for LCT protocols.
Using current protocols, C/T of hepatocytes induces cell alteration. In vitro functions of C/T hepatocytes remain poorer than those of freshly isolated hepatocytes, while the efficacy in vivo seems to be time-limited, both in animal models and in humans. Hepatocyte mitochondria are very sensitive to C/T, with marked complex 1 activity impairment following thawing. This leads to low intracellular ATP concentration, an excellent and easily obtaining post C/T viability marker. Related cytochrome c release induces cell death within hours by apoptosis.
The IIF or exposure to hyperosmotic solutions are probably the start point of the observed damage. New adapted cryopreservation protocols have therefore to be urgently developed. Interesting perspectives such as vitrification, to avoid the crystalline state, with or without encapsulation, conferring a mechanical protection, must be validated in the future, while considering the problem of their clinical translation.
Peer reviewers: Seong Gyu Hwang, Professor, MD, Department of Internal Medicine, CHA Bundang Medical Center, CHA university, #351, Yatap-Dong, Bundang-Gu, Seongnam, Gyeonggi-Do 463-712, South Korea; Michael S Lan, PhD, Professor of Pediatrics, LSUHSC, The Research Institute for Children, Children’s Hospital, 200 Henry Clay Avenue, Research Building, Rm. 2211, New Orleans, LA 70118, United States; Dr. Matilde Bustos, Department of Hepatology and Gene Therapy, CIMA (Center for Applied Medical Research), University of Navarra, Avda Pio XII, 55, Pamplona 31008, Spain
S- Editor Wang YR L- Editor Stewart GJ E- Editor Lin YP
| 1. | Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317-318. |
| 2. | Najimi M, Sokal E. Update on liver cell transplantation. J Pediatr Gastroenterol Nutr. 2004;39:311-319. |
| 3. | Najimi M, Sokal E. Liver cell transplantation. Minerva Pediatr. 2005;57:243-257. |
| 4. | Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, Bernard Otte J, Evrard V, Latinne D, Vincent MF. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735-738. |
| 5. | Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, Posner MP. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559-569. |
| 6. | Li AP, Gorycki PD, Hengstler JG, Kedderis GL, Koebe HG, Rahmani R, de Sousas G, Silva JM, Skett P. Present status of the application of cryopreserved hepatocytes in the evaluation of xenobiotics: consensus of an international expert panel. Chem Biol Interact. 1999;121:117-123. |
| 7. | Mitry RR, Hughes RD, Dhawan A. Progress in human hepatocytes: isolation, culture & cryopreservation. Semin Cell Dev Biol. 2002;13:463-467. |
| 8. | Illouz S, Alexandre E, Pattenden C, Mark L, Bachellier P, Webb M, Berry D, Dennison A, Richert L. Differential effects of curcumin on cryopreserved versus fresh primary human hepatocytes. Phytother Res. 2008;22:1688-1691. |
| 9. | Smets FN, Chen Y, Wang LJ, Soriano HE. Loss of cell anchorage triggers apoptosis (anoikis) in primary mouse hepatocytes. Mol Genet Metab. 2002;75:344-352. |
| 10. | Zvibel I, Smets F, Soriano H. Anoikis: roadblock to cell transplantation? Cell Transplant. 2002;11:621-630. |
| 11. | Darr TB, Hubel A. Freezing characteristics of isolated pig and human hepatocytes. Cell Transplant. 1997;6:173-183. |
| 12. | Hubel A, Conroy M, Darr TB. Influence of preculture on the prefreeze and postthaw characteristics of hepatocytes. Biotechnol Bioeng. 2000;71:173-183. |
| 13. | Gómez-Lechón MJ, Lahoz A, Jiménez N, Vicente Castell J, Donato MT. Cryopreservation of rat, dog and human hepatocytes: influence of preculture and cryoprotectants on recovery, cytochrome P450 activities and induction upon thawing. Xenobiotica. 2006;36:457-472. |
| 14. | Lloyd TD, Orr S, Berry DP, Dennison AR. Development of a protocol for cryopreservation of hepatocytes for use in bioartificial liver systems. Ann Clin Lab Sci. 2004;34:165-174. |
| 15. | Terry C, Dhawan A, Mitry RR, Lehec SC, Hughes RD. Preincubation of rat and human hepatocytes with cytoprotectants prior to cryopreservation can improve viability and function upon thawing. Liver Transpl. 2005;11:1533-1540. |
| 16. | Terry C, Dhawan A, Mitry RR, Lehec SC, Hughes RD. Preincubation of rat and human hepatocytes with cytoprotectants prior to cryopreservation can improve viability and function upon thawing. Liver Transpl. 2006;12:165-177. |
| 17. | Silva JM, Day SH, Nicoll-Griffith DA. Induction of cytochrome-P450 in cryopreserved rat and human hepatocytes. Chem Biol Interact. 1999;121:49-63. |
| 18. | Gómez-Lechón MJ, Lahoz A, Jiménez N, Bonora A, Castell JV, Donato MT. Evaluation of drug-metabolizing and functional competence of human hepatocytes incubated under hypothermia in different media for clinical infusion. Cell Transplant. 2008;17:887-897. |
| 19. | Terry C, Dhawan A, Mitry RR, Hughes RD. Cryopreservation of isolated human hepatocytes for transplantation: State of the art. Cryobiology. 2006;53:149-159. |
| 20. | De Loecker P, Fuller BJ, Koptelov VA, Grischenko VI, De Loecker W. Cryopreservation of isolated rat hepatocytes: effects of iron-mediated oxidative stress of metabolic activity. Cryobiology. 1997;34:150-156. |
| 21. | De Loecker W, Koptelov VA, Grischenko VI, De Loecker P. Effects of cell concentration on viability and metabolic activity during cryopreservation. Cryobiology. 1998;37:103-109. |
| 22. | Wu L, Sun J, Wang L, Wang C, Woodman K, Koutalistras N, Horvat M, Sheil AG. Cryopreservation of primary porcine hepatocytes for use in bioartificial liver support systems. Transplant Proc. 2000;32:2271-2272. |
| 23. | Kunieda T, Maruyama M, Okitsu T, Shibata N, Takesue M, Totsugawa T, Kosaka Y, Arata T, Kobayashi K, Ikeda H. Cryopreservation of primarily isolated porcine hepatocytes with UW solution. Cell Transplant. 2003;12:607-616. |
| 24. | Southard JH, van Gulik TM, Ametani MS, Vreugdenhil PK, Lindell SL, Pienaar BL, Belzer FO. Important components of the UW solution. Transplantation. 1990;49:251-257. |
| 25. | Arikura J, Kobayashi N, Okitsu T, Noguchi H, Totsugawa T, Watanabe T, Matsumura T, Maruyama M, Kosaka Y, Tanaka N. UW solution: a promising tool for cryopreservation of primarily isolated rat hepatocytes. J Hepatobiliary Pancreat Surg. 2002;9:742-749. |
| 26. | Adams RM, Wang M, Crane AM, Brown B, Darlington GJ, Ledley FD. Effective cryopreservation and long-term storage of primary human hepatocytes with recovery of viability, differentiation, and replicative potential. Cell Transplant. 1995;4:579-586. |
| 27. | Sosef MN, Baust JM, Sugimachi K, Fowler A, Tompkins RG, Toner M. Cryopreservation of isolated primary rat hepatocytes: enhanced survival and long-term hepatospecific function. Ann Surg. 2005;241:125-133. |
| 28. | Baust JM, Van Buskirk, Baust JG. Cell viability improves following inhibition of cryopreservation-induced apoptosis. In Vitro Cell Dev Biol Anim. 2000;36:262-270. |
| 29. | Baust JM, Van Buskirk R, Baust JG. Modulation of the cryopreservation cap: elevated survival with reduced dimethyl sulfoxide concentration. Cryobiology. 2002;45:97-108. |
| 30. | Chen Z, Ding Y, Zhang H. Cryopreservation of suckling pig hepatocytes. Ann Clin Lab Sci. 2001;31:391-398. |
| 31. | Fu T, Guo D, Huang X, O'Gorman MR, Huang L, Crawford SE, Soriano HE. Apoptosis occurs in isolated and banked primary mouse hepatocytes. Cell Transplant. 2001;10:59-66. |
| 32. | Hewitt NJ, Fischer T, Zuehlke U, Oesch F, Utesch D. Metabolic activity of fresh and cryopreserved cynomolgus monkey (Macaca fascicularis) hepatocytes. Xenobiotica. 2000;30:665-681. |
| 33. | Loretz LJ, Li AP, Flye MW, Wilson AG. Optimization of cryopreservation procedures for rat and human hepatocytes. Xenobiotica. 1989;19:489-498. |
| 34. | Rijntjes PJ, Moshage HJ, Van Gemert PJ, De Waal R, Yap SH. Cryopreservation of adult human hepatocytes. The influence of deep freezing storage on the viability, cell seeding, survival, fine structures and albumin synthesis in primary cultures. J Hepatol. 1986;3:7-18. |
| 35. | Smith DJ, Schulte M, Bischof JC. The effect of dimethylsulfoxide on the water transport response of rat hepatocytes during freezing. J Biomech Eng. 1998;120:549-558. |
| 36. | Son JH, Kim KH, Nam YK, Park JK, Kim SK. Optimization of cryoprotectants for cryopreservation of rat hepatocyte. Biotechnol Lett. 2004;26:829-833. |
| 37. | Swales NJ, Utesch D. Metabolic activity of fresh and cryopreserved dog hepatocyte suspensions. Xenobiotica. 1998;28:937-948. |
| 38. | Hengstler JG, Utesch D, Steinberg P, Platt KL, Diener B, Ringel M, Swales N, Fischer T, Biefang K, Gerl M. Cryopreserved primary hepatocytes as a constantly available in vitro model for the evaluation of human and animal drug metabolism and enzyme induction. Drug Metab Rev. 2000;32:81-118. |
| 39. | Utesch D, Diener B, Molitor E, Oesch F, Platt KL. Characterization of cryopreserved rat liver parenchymal cells by metabolism of diagnostic substrates and activities of related enzymes. Biochem Pharmacol. 1992;44:309-315. |
| 40. | Miyamoto Y, Suzuki S, Nomura K, Enosawa S. Improvement of hepatocyte viability after cryopreservation by supplementation of long-chain oligosaccharide in the freezing medium in rats and humans. Cell Transplant. 2006;15:911-919. |
| 41. | Katenz E, Vondran FW, Schwartlander R, Pless G, Gong X, Cheng X, Neuhaus P, Sauer IM. Cryopreservation of primary human hepatocytes: the benefit of trehalose as an additional cryoprotective agent. Liver Transpl. 2007;13:38-45. |
| 42. | Bucak MN, Ateşşahin A, Varişli O, Yüce A, Tekin N, Akçay A. The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen Microscopic and oxidative stress parameters after freeze-thawing process. Theriogenology. 2007;67:1060-1067. |
| 43. | Woelders H, Matthijs A, Engel B. Effects of trehalose and sucrose, osmolality of the freezing medium, and cooling rate on viability and intactness of bull sperm after freezing and thawing. Cryobiology. 1997;35:93-105. |
| 44. | Petrenko YA, Jones DR, Petrenko AY. Cryopreservation of human fetal liver hematopoietic stem/progenitor cells using sucrose as an additive to the cryoprotective medium. Cryobiology. 2008;57:195-200. |
| 45. | Rodrigues JP, Paraguassú-Braga FH, Carvalho L, Abdelhay E, Bouzas LF, Porto LC. Evaluation of trehalose and sucrose as cryoprotectants for hematopoietic stem cells of umbilical cord blood. Cryobiology. 2008;56:144-151. |
| 46. | Sugimachi K, Roach KL, Rhoads DB, Tompkins RG, Toner M. Nonmetabolizable glucose compounds impart cryotolerance to primary rat hepatocytes. Tissue Eng. 2006;12:579-588. |
| 47. | Hamel F, Grondin M, Denizeau F, Averill-Bates DA, Sarhan F. Wheat extracts as an efficient cryoprotective agent for primary cultures of rat hepatocytes. Biotechnol Bioeng. 2006;95:661-670. |
| 48. | Grondin M, Hamel F, Sarhan F, Averill-Bates DA. Metabolic activity of cytochrome p450 isoforms in hepatocytes cryopreserved with wheat protein extract. Drug Metab Dispos. 2008;36:2121-2129. |
| 49. | Grondin M, Hamel F, Averill-Bates DA, Sarhan F. Wheat proteins improve cryopreservation of rat hepatocytes. Biotechnol Bioeng. 2009;103:582-591. |
| 50. | Selvaggi TA, Walker RE, Fleisher TA. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood. 1997;89:776-779. |
| 51. | Dou M, de Sousa G, Lacarelle B, Placidi M, Lechene de la Porte P, Domingo M, Lafont H, Rahmani R. Thawed human hepatocytes in primary culture. Cryobiology. 1992;29:454-469. |
| 52. | Fautrel A, Joly B, Guyomard C, Guillouzo A. Long-term maintenance of drug-metabolizing enzyme activities in rat hepatocytes after cryopreservation. Toxicol Appl Pharmacol. 1997;147:110-114. |
| 53. | Stevenson DJ, Morgan C, Goldie E, Connel G, Grant MH. Cryopreservation of viable hepatocyte monolayers in cryoprotectant media with high serum content: metabolism of testosterone and kaempherol post-cryopreservation. Cryobiology. 2004;49:97-113. |
| 54. | Müller P, Aurich H, Wenkel R, Schäffner I, Wolff I, Walldorf J, Fleig WE, Christ B. Serum-free cryopreservation of porcine hepatocytes. Cell Tissue Res. 2004;317:45-56. |
| 55. | Coundouris JA, Grant MH, Engeset J, Petrie JC, Hawksworth GM. Cryopreservation of human adult hepatocytes for use in drug metabolism and toxicity studies. Xenobiotica. 1993;23:1399-1409. |
| 56. | Lloyd TD, Orr S, Skett P, Berry DP, Dennison AR. Cryopreservation of hepatocytes: a review of current methods for banking. Cell Tissue Bank. 2003;4:3-15. |
| 57. | de Sousa G, Langouët S, Nicolas F, Lorenzon G, Placidi M, Rahmani R, Guillouzo A. Increase of cytochrome P-450 1A and glutathione transferase transcripts in cultured hepatocytes from dogs, monkeys, and humans after cryopreservation. Cell Biol Toxicol. 1996;12:351-358. |
| 58. | Skett P, Roberts P, Khan S. Maintenance of steroid metabolism and hormone responsiveness in cryopreserved dog, monkey and human hepatocytes. Chem Biol Interact. 1999;121:65-76. |
| 59. | Diener B, Utesch D, Beer N, Dürk H, Oesch F. A method for the cryopreservation of liver parenchymal cells for studies of xenobiotics. Cryobiology. 1993;30:116-127. |
| 60. | Hengstler JG, Ringel M, Biefang K, Hammel S, Milbert U, Gerl M, Klebach M, Diener B, Platt KL, Böttger T. Cultures with cryopreserved hepatocytes: applicability for studies of enzyme induction. Chem Biol Interact. 2000;125:51-73. |
| 61. | De Loecker P, Fuller BJ, Koptelov VA, De Loecker W. Metabolic activity of freshly prepared and cryopreserved hepatocytes in monolayer culture. Cryobiology. 1993;30:12-18. |
| 62. | Stéphenne X, Najimi M, Ngoc DK, Smets F, Hue L, Guigas B, Sokal EM. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex 1. Cell Transplant. 2007;16:409-419. |
| 63. | Ostrowska A, Bode DC, Pruss J, Bilir B, Smith GD, Zeisloft S. Investigation of functional and morphological integrity of freshly isolated and cryopreserved human hepatocytes. Cell Tissue Bank. 2000;1:55-68. |
| 64. | Yagi T, Hardin JA, Valenzuela YM, Miyoshi H, Gores GJ, Nyberg SL. Caspase inhibition reduces apoptotic death of cryopreserved porcine hepatocytes. Hepatology. 2001;33:1432-1440. |
| 66. | Matsushita T, Yagi T, Hardin JA, Cragun JD, Crow FW, Bergen HR 3rd, Gores GJ, Nyberg SL. Apoptotic cell death and function of cryopreserved porcine hepatocytes in a bioartificial liver. Cell Transplant. 2003;12:109-121. |
| 67. | Duval M, Plin C, Elimadi A, Vallerand D, Tillement JP, Morin D, Haddad PS. Implication of mitochondrial dysfunction and cell death in cold preservation--warm reperfusion-induced hepatocyte injury. Can J Physiol Pharmacol. 2006;84:547-554. |
| 68. | Fujita R, Hui T, Chelly M, Demetriou AA. The effect of antioxidants and a caspase inhibitor on cryopreserved rat hepatocytes. Cell Transplant. 2005;14:391-396. |
| 69. | Vara E, Arias-Díaz J, Villa N, Hernández J, García C, Ortiz P, Balibrea JL. Beneficial effect of S-adenosylmethionine during both cold storage and cryopreservation of isolated hepatocytes. Cryobiology. 1995;32:422-427. |
| 70. | Pearce LL, Epperly MW, Greenberger JS, Pitt BR, Peterson J. Identification of respiratory complexes I and III as mitochondrial sites of damage following exposure to ionizing radiation and nitric oxide. Nitric Oxide. 2001;5:128-136. |
| 71. | Sammut IA, Thorniley MS, Simpkin S, Fuller BJ, Bates TE, Green CJ. Impairment of hepatic mitochondrial respiratory function following storage and orthotopic transplantation of rat livers. Cryobiology. 1998;36:49-60. |
| 72. | Swales NJ, Luong C, Caldwell J. Cryopreservation of rat and mouse hepatocytes. I. Comparative viability studies. Drug Metab Dispos. 1996;24:1218-1223. |
| 73. | Terry C, Hughes RD, Mitry RR, Lehec SC, Dhawan A. Cryopreservation-induced nonattachment of human hepatocytes: role of adhesion molecules. Cell Transplant. 2007;16:639-647. |
| 74. | Grondin M, Hamel F, Averill-Bates DA, Sarhan F. Wheat proteins enhance stability and function of adhesion molecules in cryopreserved hepatocytes. Cell Transplant. 2009;18:79-88. |
| 75. | Shitara Y, Li AP, Kato Y, Lu C, Ito K, Itoh T, Sugiyama Y. Function of uptake transporters for taurocholate and estradiol 17beta-D-glucuronide in cryopreserved human hepatocytes. Drug Metab Pharmacokinet. 2003;18:33-41. |
| 76. | Loven AD, Olsen AK, Friis C, Andersen B. Phase I and II metabolism and carbohydrate metabolism in cultured cryopreserved porcine hepatocytes. Chem Biol Interact. 2005;155:21-30. |
| 77. | Li AP, Lu C, Brent JA, Pham C, Fackett A, Ruegg CE, Silber PM. Cryopreserved human hepatocytes: characterization of drug-metabolizing enzyme activities and applications in higher throughput screening assays for hepatotoxicity, metabolic stability, and drug-drug interaction potential. Chem Biol Interact. 1999;121:17-35. |
| 78. | Steinberg P, Fischer T, Kiulies S, Biefang K, Platt KL, Oesch F, Böttger T, Bulitta C, Kempf P, Hengstler J. Drug metabolizing capacity of cryopreserved human, rat, and mouse liver parenchymal cells in suspension. Drug Metab Dispos. 1999;27:1415-1422. |
| 79. | de Sousa G, Nicolas F, Placidi M, Rahmani R, Benicourt M, Vannier B, Lorenzon G, Mertens K, Coecke S, Callaerts A. A multi-laboratory evaluation of cryopreserved monkey hepatocyte functions for use in pharmaco-toxicology. Chem Biol Interact. 1999;121:77-97. |
| 80. | Kafert-Kasting S, Alexandrova K, Barthold M, Laube B, Friedrich G, Arseniev L, Hengstler JG. Enzyme induction in cryopreserved human hepatocyte cultures. Toxicology. 2006;220:117-125. |
| 81. | Madan A, DeHaan R, Mudra D, Carroll K, LeCluyse E, Parkinson A. Effect of cryopreservation on cytochrome P-450 enzyme induction in cultured rat hepatocytes. Drug Metab Dispos. 1999;27:327-335. |
| 82. | Reinach B, de Sousa G, Dostert P, Ings R, Gugenheim J, Rahmani R. Comparative effects of rifabutin and rifampicin on cytochromes P450 and UDP-glucuronosyl-transferases expression in fresh and cryopreserved human hepatocytes. Chem Biol Interact. 1999;121:37-48. |
| 83. | Roymans D, Annaert P, Van Houdt J, Weygers A, Noukens J, Sensenhauser C, Silva J, Van Looveren C, Hendrickx J, Mannens G. Expression and induction potential of cytochromes P450 in human cryopreserved hepatocytes. Drug Metab Dispos. 2005;33:1004-1016. |
| 84. | Harris CL, Toner M, Hubel A, Cravalho EG, Yarmush ML, Tompkins RG. Cryopreservation of isolated hepatocytes: intracellular ice formation under various chemical and physical conditions. Cryobiology. 1991;28:436-444. |
| 85. | Hubel A, Toner M, Cravalho EG, Yarmush ML, Tompkins RG. Intracellular ice formation during the freezing of hepatocytes cultured in a double collagen gel. Biotechnol Prog. 1991;7:554-559. |
| 86. | Karlsson JO, Cravalho EG, Borel Rinkes IH, Tompkins RG, Yarmush ML, Toner M. Nucleation and growth of ice crystals inside cultured hepatocytes during freezing in the presence of dimethyl sulfoxide. Biophys J. 1993;65:2524-2536. |
| 87. | Toner M, Cravalho EG, Karel M, Armant DR. Cryomicroscopic analysis of intracellular ice formation during freezing of mouse oocytes without cryoadditives. Cryobiology. 1991;28:55-71. |
| 88. | Toner M, Cravalho EG, Stachecki J, Fitzgerald T, Tompkins RG, Yarmush ML, Armant DR. Nonequilibrium freezing of one-cell mouse embryos. Membrane integrity and developmental potential. Biophys J. 1993;64:1908-1921. |
| 89. | Yuan S, Diller KR. An optical differential scanning calorimeter cryomicroscope. J Microsc. 2005;218:85-93. |
| 90. | Fuller BJ, Lewin J, Sage L. Ultrastructural assessment of cryopreserved hepatocytes after prolonged ectopic transplantation. Transplantation. 1983;35:15-18. |
| 91. | David P, Alexandre E, Chenard-Neu MP, Audet M, Wolf P, Jaeck D, Azimzadeh A, Richert L. Engraftment and function of freshly isolated and cryopreserved Sprague Dawley rat hepatocytes after intrasplenic transplantation in analbuminemic rats. Transplant Proc. 2000;32:2796-2797. |
| 92. | Dunn TB, Kumins NH, Holman DM, Raofi V, Blanchard J, Glimer T, Pollak R, Benedetti E. Multiple sequential transplantation of hepatocytes in the Dalmatian dog model. Transplant Proc. 1999;31:543-544. |
| 93. | Papalois A, Arkadopoulos N, Kostopanagiotou G, Theodorakis K, Peveretos P, Golematis B, Papadimitriou J. Experimental xenotransplantation of fresh isolated and cryopreserved pig hepatocytes: a biochemical and morphological study. Transplant Proc. 1997;29:2096-2098. |
| 94. | Jamal HZ, Weglarz TC, Sandgren EP. Cryopreserved mouse hepatocytes retain regenerative capacity in vivo. Gastroenterology. 2000;118:390-394. |
| 95. | Dandri M, Burda MR, Gocht A, Török E, Pollok JM, Rogler CE, Will H, Petersen J. Woodchuck hepatocytes remain permissive for hepadnavirus infection and mouse liver repopulation after cryopreservation. Hepatology. 2001;34:824-833. |
| 96. | Cho JJ, Joseph B, Sappal BS, Giri RK, Wang R, Ludlow JW, Furth ME, Susick R, Gupta S. Analysis of the functional integrity of cryopreserved human liver cells including xenografting in immunodeficient mice to address suitability for clinical applications. Liver Int. 2004;24:361-370. |
| 97. | Stéphenne X, Najimi M, Smets F, Reding R, de Ville de Goyet J, Sokal EM. Cryopreserved liver cell transplantation controls ornithine transcarbamylase deficient patient while awaiting liver transplantation. Am J Transplant. 2005;5:2058-2061. |
| 98. | Stéphenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317-1323. |
| 99. | Dhawan A, Mitry RR, Hughes RD, Lehec S, Terry C, Bansal S, Arya R, Wade JJ, Verma A, Heaton ND. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation. 2004;78:1812-1814. |
| 100. | Canaple L, Nurdin N, Angelova N, Saugy D, Hunkeler D, Desvergne B. Maintenance of primary murine hepatocyte functions in multicomponent polymer capsules--in vitro cryopreservation studies. J Hepatol. 2001;34:11-18. |
| 101. | Koizumi T, Aoki T, Kobayashi Y, Yasuda D, Izumida Y, Jin Z, Nishino N, Shimizu Y, Kato H, Murai N. Long-term maintenance of the drug transport activity in cryopreservation of microencapsulated rat hepatocytes. Cell Transplant. 2007;16:67-73. |
| 102. | Mahler S, Desille M, Frémond B, Chesné C, Guillouzo A, Campion JP, Clément B. Hypothermic storage and cryopreservation of hepatocytes: the protective effect of alginate gel against cell damages. Cell Transplant. 2003;12:579-592. |
| 103. | Kusano T, Aoki T, Yasuda D, Matsumoto S, Jin Z, Nishino N, Hayashi K, Odaira M, Yamada K, Koizumi T. Microencapsule technique protects hepatocytes from cryoinjury. Hepatol Res. 2008;38:593-600. |
| 104. | Dixit V, Darvasi R, Arthur M, Lewin K, Gitnick G. Cryopreserved microencapsulated hepatocytes--transplantation studies in Gunn rats. Transplantation. 1993;55:616-622. |
| 105. | Aoki T, Jin Z, Nishino N, Kato H, Shimizu Y, Niiya T, Murai N, Enami Y, Mitamura K, Koizumi T. Intrasplenic transplantation of encapsulated hepatocytes decreases mortality and improves liver functions in fulminant hepatic failure from 90% partial hepatectomy in rats. Transplantation. 2005;79:783-790. |
| 106. | Mai G, Huy NT, Morel P, Mei J, Bosco D, Berney T, Majno P, Mentha G, Trono D, Buhler LH. Treatment of fulminant liver failure by transplantation of microencapsulated primary or immortalized xenogeneic hepatocytes. Transplant Proc. 2005;37:527-529. |
| 107. | Baldini E, Cursio R, De Sousa G, Margara A, Honiger J, Saint-Paul MC, Bayer P, Raimondi V, Rahmani R, Mouiel J. Peritoneal implantation of cryopreserved encapsulated porcine hepatocytes in rats without immunosuppression: viability and function. Transplant Proc. 2008;40:2049-2052. |
| 108. | Aoki T, Koizumi T, Kobayashi Y, Yasuda D, Izumida Y, Jin Z, Nishino N, Shimizu Y, Kato H, Murai N. A novel method of cryopreservation of rat and human hepatocytes by using encapsulation technique and possible use for cell transplantation. Cell Transplant. 2005;14:609-620. |
| 109. | Mei J, Sgroi A, Mai G, Baertschiger R, Gonelle-Gispert C, Serre-Beinier V, Morel P, Bühler LH. Improved survival of fulminant liver failure by transplantation of microencapsulated cryopreserved porcine hepatocytes in mice. Cell Transplant. 2009;18:101-110. |
| 110. | Bottomley MJ, Baicu S, Boggs JM, Marshall DP, Clancy M, Brockbank KG, Bravery CA. Preservation of embryonic kidneys for transplantation. Transplant Proc. 2005;37:280-284. |
| 111. | Brockbank KG, Song YC, Khirabadi BS, Lightfoot FG, Boggs JM, Taylor MJ. Storage of tissues by vitrification. Transplant Proc. 2000;32:3-4. |
| 112. | de Graaf IA, Draaisma AL, Schoeman O, Fahy GM, Groothuis GM, Koster HJ. Cryopreservation of rat precision-cut liver and kidney slices by rapid freezing and vitrification. Cryobiology. 2007;54:1-12. |
| 113. | Elder E, Chen Z, Ensley A, Nerem R, Brockbank K, Song Y. Enhanced tissue strength in cryopreserved, collagen-based blood vessel constructs. Transplant Proc. 2005;37:4625-4629. |
| 114. | Song YC, Khirabadi BS, Lightfoot F, Brockbank KG, Taylor MJ. Vitreous cryopreservation maintains the function of vascular grafts. Nat Biotechnol. 2000;18:296-299. |
| 115. | Song YC, Chen ZZ, Mukherjee N, Lightfoot FG, Taylor MJ, Brockbank KG, Sambanis A. Vitrification of tissue engineered pancreatic substitute. Transplant Proc. 2005;37:253-255. |
| 116. | Kuleshova LL, Wang XW, Wu YN, Zhou Y, Yu H. Vitrification of encapsulated hepatocytes with reduced cooling and warming rates. Cryo Letters. 2004;25:241-254. |
| 117. | Wu Y, Yu H, Chang S, Magalhães R, Kuleshova LL. Vitreous cryopreservation of cell-biomaterial constructs involving encapsulated hepatocytes. Tissue Eng. 2007;13:649-658. |
| 118. | Moon JH, Lee JR, Jee BC, Suh CS, Kim SH, Lim HJ, Kim HK. Successful vitrification of human amnion-derived mesenchymal stem cells. Hum Reprod. 2008;23:1760-1770. |