Published online Mar 7, 2009. doi: 10.3748/wjg.15.1105
Revised: January 16, 2009
Accepted: January 23, 2009
Published online: March 7, 2009
AIM: To investigate the changing pattern of different histological parameters occurring in the stomach tissue of Helicobacter pylori (H pylori) infected tribal populations and duodenal ulcer patients among ethnic Bengalis and correlation of the genotypes of H pylori with different histological parameters.
METHODS: One hundred and twelve adult individuals were enrolled into this study between 2002 and 2004. Among them, 72 had clinical features of duodenal ulcer (DU) from ethnic Bengali population and 40 were asymptomatic ethnic tribals. Endoscopic gastric biopsy samples were processed for histology, genotyping and rapid urease test. Histologically, haematoxylin and eosin staining was applied to assess the pathomorphological changes and a modified Giemsa staining was used for better detection of H pylori. For intestinal metaplasia, special stainings, i.e. Alcian blue periodic acid-Schiff and high iron diamine-Alcian blue staining, were performed. PCR was performed on bacterial DNA to characterize the presence or absence of virulence-associated genes, like cagA, and distribution of different alleles of vacA and iceA.
RESULTS: Intraglandular neutrophil infiltration, a hallmark of activity of gastritis, was present in 34 (94%) of tribals (TRs) and 42 (84%) of DU individuals infected with H pylori. Lymphoid follicles and aggregates, which are important landmarks in H pylori infection, were positive amongst 15 (41%) of TRs and 20 (40%) of DU subjects. Atrophic changes were observed in 60% and 27.7%, respectively, among DU cases and tribals (P > 0.003). Metaplastic changes were detected in low numbers in both groups. Moderate to severe density distribution of H pylori in the gastric mucosa was 63% among TRs, whereas it was 62% in DU subjects. There were no significant differences in the distribution of virulence-associated genes like cagA, vacA and iceA of H pylori strains carried by these two populations.
CONCLUSION: Our study showed almost similar distribution of inflammatory cells among asymptomatic tribals and DU Bengali patients. Interestingly, the tribal population are free from any clinical symptoms despite evidence of active histologic gastritis and infection with H pylori strains carrying similar virulence markers as of strains isolated from patients with DU. There was an increased cellular response, especially in terms of neutrophil infiltration, but much lower risk of developing atrophy and metaplastic changes among the tribal population.
-
Citation: Saha DR, Datta S, Chattopadhyay S, Patra R, De R, Rajendran K, Chowdhury A, Ramamurthy T, Mukhopadhyay AK. Indistinguishable cellular changes in gastric mucosa between
Helicobacter pylori infected asymptomatic tribal and duodenal ulcer patients. World J Gastroenterol 2009; 15(9): 1105-1112 - URL: https://www.wjgnet.com/1007-9327/full/v15/i9/1105.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1105
Helicobacter pylori (H pylori) is of growing concern today because of its crucial role in the pathogenesis of chronic gastritis, peptic ulcer diseases and in the multi-step carcinogenic process of gastric cancer[1]. In developing countries, 70%-90% of the population carries H pylori and develop persistent inflammation in their stomachs, which lasts for decades unless treated with antibiotics[2]. About 60%-95% of peptic ulcer diseases are thought to be idiopathic and it is now well established that H pylori is the causative agent of nearly all of these cases in adults. In addition, almost all H pylori infected individuals develop gastritis[3]. Severe gastritis is believed to be the denominator of peptic ulcer diseases and atrophic gastritis, which may lead to gastric cancer[45]. However, it is not clear why a few strains are associated with ulcer formation with relevant clinical symptoms, while others are not associated with any disease manifestation.
H pylori is one of the most genetically diverse of bacterial species, with any given isolate easily distinguished from most others by DNA fingerprinting. Only one-half to two-thirds of US and European strains carry the cag pathogenicity island (cag PAI) and such strains are recovered preferentially from persons with overt disease. In contrast, nearly all-Asian strains carry the cag PAI, independent of disease status. Potentially more significant in terms of host interaction and evolution were the findings that East Asian and Western strains differ markedly in DNA sequence motifs in the vacA and cagA genes., We have previously reported that sequences of the cagA gene in Indian strains show a close match with ethnic European strains, but are distinct from East Asian strains. On the other hand, DNA sequence motifs of an informative middle region of vacA gene from Indian H pylori strains are distinct both from European and East Asian strains. So, there are strong indications of significant geographic differences among strains[67].
Moreover, gastric cancer is more prevalent in Japan and China than any other parts of the world and duodenal ulcer is more common in India as compared to gastric ulcer. The distribution and the nature of gastritis are, thus, major determinants of clinical outcome of H pylori infection. It is, therefore, important to understand the dynamics of gastritis associated with this infection in developing countries like India, where H pylori infection is highly prevalent and H pylori is acquired early in life. Surprisingly, even the healthy individuals in India carry the toxigenic vacA s1, vacA m1 alleles and cag-PAI[8]. However, previous reports do not indicate whether this lack of disease association with putative virulent H pylori strains is due to a lesser bacterial load in gastric mucosa leading to insignificant level of epithelial injury.
Santhals and Oroans are two distinct ethnic tribal groups that had settled in the Birbhum district of West Bengal centuries ago[7]. They constitute less than 5% of the overall population of West Bengal (Census of India, 2001). Ethnically, Santhals are proto-Australoids and speak the Santhali dialect of the Austro-Asiatic language family, while the Oroans are ethnically Dravidian and speak the Khurukh dialect of the Dravidian linguistic family. That is, the two lineages to which they belong have been distinct for millennia. In contrast, the ethnic Bengalis have an Indo-European ancestry, and their Bengali language is derived from Sanskrit. Traditionally, both Santhals and Oroans have been hunters-gatherers, but most have now become settled as agriculturists. Nevertheless, they remain culturally and linguistically distinct from most of Indian society and rarely intermarry with people of other ethnicities. Their separation from mainstream Bengalis and other Indians during much of human history is reflected in genetic differences in autosomal and mitochondrial DNA markers. Our previous study[7] showed that the majority of these tribal communities are infected with H pylori; but, interestingly, none of them shows any symptoms. On the other hand, duodenal ulcer, which is H pylori-associated, is of particular importance in ethnic Bengali populations and is far more common than in most other geographic regions[9].
These considerations and our interest in the dynamics of gastritis associated with this infection motivated the present study. We wanted to investigate the changing pattern of different histological parameters occurring in the stomach tissue of H pylori-infected tribal populations and duodenal ulcer patients among ethnic Bengalis. Our aim was to get insights of the cause for the near absence of H pylori-associated overt disease in these tribal populations and to correlate the H pylori genotypes with the different histological findings.
A total of 112 adult mainstream Bengali and ethnic tribal individuals (72 Bengalis and 40 tribals) of both sexes (aged between 20-65 years) underwent a non-sedated upper gastrointestinal endoscopy (GIF XQ 30, Olympus optical company, Japan) under topical lignocaine anesthesia at the hospital of the Institute of Post Graduate Medical Education and Research, Kolkata, India, throughout the years 2002-2004. Out of 72 suspected duodenal ulcer (DU) cases, the mean age of 43 males and 29 females was 45 ± 11.72 and 42.7 ± 9.16, respectively. Among 40 tribals, the mean age of 24 males and 16 females was 31.4 ± 6.22 and 32.13 ± 6.44, respectively. Seventy-two Bengali patients were chosen for endoscopy from individuals with abdominal pain seeking care at outpatient department as possible DU patients and for comparative analysis, 40 asymptomatic individuals from tribal (TR) population (Santhals and Orans) were recruited. A detailed history was taken, and a physical examination of each subject was carried out prior to endoscopy. The objectives of the study were explained to all. Informed consents were obtained from each individual under protocols approved by the institutional ethical committees of the Post-Graduate Medical Education and Research and National Institute of Cholera and Enteric Diseases, Kolkata, West Bengal, India. None of these asymptomatic individuals reported to have any gastro-duodenal discomfort. Exclusion criteria were: use of antibiotics, antihistamines and proton pump inhibitors during the three months prior to this study. From each participant, four biopsies were obtained, three from the antrum and one from the fundus. Of the three antral biopsy specimens, one was used for an in-house rapid urease test (RUT), one for culture and the third one, along with one biopsy from the fundus, was processed for histologic examination.
Biopsies for culture were taken in 1 mL of brucella broth (Difco) containing 15% glycerol and transported to the National Institute of Cholera and enteric Diseases in ice-cold condition. Biopsy samples in transport medium were vortexed vigorously for 2 min and 200 &mgr;L of the broth were streaked on brain heart infusion (BHI) agar (Difco) enriched with 7% sheep blood, 0.4% IsovitaleX and H pylori selective supplement-Dent (Oxoid, Basingstoke, Hampshire, England). Plates were incubated at 37°C in double-gassed incubator, which maintains 10% CO2, 5% O2 and 85% N2 for 3-6 d. The organisms were identified by their typical colony morphology, appearance on Gram staining and positive reactions in urease, catalase and oxidase tests.
A modification of the method of Murray and Thompson[10] was used for H pylori genomic DNA extraction. In brief, cells from a confluent lawn of bacterial culture on BHI agar plate were collected and resuspended in TE buffer (10 mmol/L Tris-HCl, 1 mmol/L EDTA, pH 8.0), treated with 10% SDS and freshly prepared proteinase K and incubated at 37°C for 1 h. After incubation, CTAB/NaCl (10% cetyl trimethyl ammonium bromide in 0.7 mol/L NaCl) was added and incubated at 65°C for 10 min. The aqueous phase was then treated with phenol-chloroform and DNA pellet was washed with 70% ethanol. The nucleic acid was suspended in TE and treated with RNAse at 37°C for 30 min. Specific PCR was carried out in 20 mL volumes using 10 ng of DNA, 1 U of Taq polymerase (Promega, Madison, Wis.), 10 pmol of each primer per reaction, 0.25 mmol/L (each) deoxynucleoside triphosphate, and 2 to 3 mmol/L MgCl2 in standard PCR buffer for 30 cycles generally under the following conditions: 94°C for 40 s, 55°C for 40 s, and 72°C for a time chosen based on the size of the expected fragment (1 min/kb). The primers are listed in Table 1.
| Region(s) amplified | Primer | Nucleotide sequence | References |
| vacA s1 or vacA s2 | VA1-F | 5'-ATGGAAATACAACAAACACAC | [7] |
| VA1-R | 5'-CTGCTTGAATGCGCCAAAC | ||
| vacA m1 or vacA m2 | VAG-F | 5'-CAATCTGTCCAATCAAGCGAG | [28] |
| VAG-R | 5'-GCGTCAAAATAATTCCAAGG | ||
| cagA (5' end) | cag5c-F | 5'-GTTGATAACGCTGTCGCTTC | [28] |
| cag3c-R | 5'-GGGTTGTATGATATTTTCCATAA | ||
| cag-PAI empty site | Luni 1 | 5'-ACATTTTGGCTAAATAAACGCTG | [7] |
| R5280 | 5'-GGTTGCACGCATTTTCCCTTAATC | ||
| iceA1 | IceA1F | 5'-TATTTCTGGAACTTGCGCAACCTGAT | [7] |
| M.Hpy1R | 5'-GGCCTACAACCGCATGGATAT | ||
| IceA2 | cycSF | 5'-CGGCTGTAGGCACTAAAGCTA | [7] |
| IceA2R | 5'-TCAATCCTATGTGAAACAATGATCGTT |
One biopsy from antrum and one from fundus of the stomach were fixed in 10% buffered formalin overnight, dehydrated in graded series of alcohol and xylene and were processed for paraffin embedding. Serial thin (3-4 &mgr;m) sections were cut by Rotary microtome (Leica 2145, Germany) and stained with haematoxylin and eosin (H&E) stain to see the morphological changes. For better visualization of H pylori, modified Giemsa stain was done in all the cases along with H&E stain, as the sensitivity and specificity of this added stain exceeds 90%[11].
The histologic changes and grading were done according to updated Sydney system[12]. All the biopsy specimens were number-coded and examined by a single pathologist who was unaware of the result of the other tests while examining the slides. H pylori in the biopsy specimens was looked for carefully and then the bacterial density was measured as it may have an impact on disease association and epidemiologic importance. H pylori was measured in the modified Giemsa stained sections by counting the H pylori like organisms on the mucosal surface and in the foveolae[13]. In brief, bacterial density was measured by comparing the histologic presence of bacteria on the gastric surface epithelium using the visual analogue scale. Severe colonization was defined as the presence of large groups of organisms on the surface and upper pits of more than 2/3rd of the mucosal surface examined. Mild colonization was defined as individual organisms or small groups covering les than 1/3rd of the mucosal surface. Moderate colonization was between these two.
Chronic gastritis, activity, atrophy and H pylori density were scored as 0 (absent), 1 (mild), 2 (moderate) and 3 (severe). Intestinal metaplasia and lymphoid follicles/aggregates were graded as 0 (absent) or 1 (present). If antral and fundal biopsy sites showed different grades for any variable, the higher score was used. Intestinal metaplasia was classified as type 1, 11 or 111 by Alcian blue periodic acid-Schiff (AB-PAS) and high iron diamine-Alcian blue (HID-AB) staining.
Among 112 individuals included in the study, 72 had clinical features of DU from ethnic Bengali population and 40 were asymptomatic ethnic tribals. In 50 out of 72 (69%) DU and 36 out of 40 (90%) TR subjects, evidence of H pylori infection was evaluated by three methods (RUT, histology and culture) and was included in further study. All of these subjects had evidence of chronic gastritis. A grade from 0 (absent) to 3 (severe) was assigned for five histological parameters, i.e. inflammation (chronic inflammatory cells), activity (neutrophils), glandular atrophy, intestinal metaplasia and H pylori density, and the grading was done according to the Sydney system[12].
Histologically active chronic gastritis was detected in 34 (94%) TRs and 42 (84%) DU patients (all from antral sites), whereas 30 TRs and 38 DU subjects showed evidence of chronic active gastritis from fundic sites. Intestinal metaplasia was detected in 3 (8.3%) TRs and 7 (14%) DU cases-all from antral sites of biopsy specimens (Table 2). No metaplastic changes were detected at fundic sites. For ease of the correlative analysis of the histological findings with genotypes, only antral biopsy specimens were evaluated further.
| Symptomatic DU (n = 50) | Asymptomatic TR (n = 36) | P-values | OR (95% CI) | |
| Neutrophil infiltration | 42 (84) | 34 (94.4) | 0.124 | 3.24 (0.57-23.76) |
| Lymphoid follicle/aggregates | 20 (40) | 15 (41.7) | 0.876 | 1.07 (0.41-2.80) |
| Atrophy | 30 (60) | 10 (27.7) | 0.0031 | 0.26 (0.10-2.67) |
| Metaplasia | 7 (14) | 3 (8.3) | 0.325 | 0.56 (0.10-2.67) |
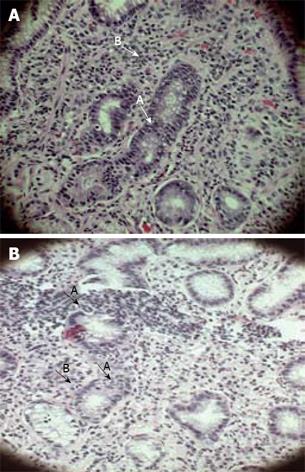
Gastritis is characterized by lymphocytes, plasma cells and scattered polymorphonuclear leucocytes (PMNs) in gastric mucosa. Chronicity or persistent infection was a common feature in all of our study subjects. Chronic gastritis was evident histologically by the presence of inflammatory infiltrates, which was essentially made up of mononuclear cells like macrophages, lymphocytes and plasma cells. Intraglandular neutrophil infiltration, a hallmark of activity of gastritis was present in 34 (94%) of TRs and 42 (84%) of DU individuals infected with single strain of H pylori. Neutrophil infiltration inside glandular epithelium and in the lamina propria of a DU and a TR case are shown in Figure 1A and B, respectively.
Mucus depletion, derangement of normal cellular architecture and foveolar hyperplasia with erosion were noted among TRs; but, frank ulceration in the gastric surface mucosa was not detected. Among most of the DU cases, ulceration, haemorrhage with exudation, loss of surface epithelium at places and atrophic changes were the frequent findings.
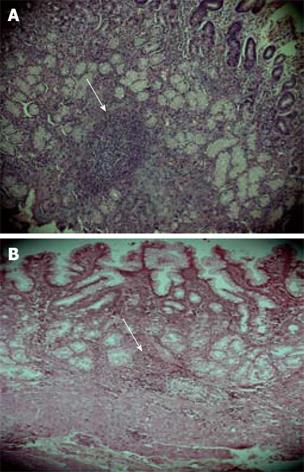
Lymphoid follicles and aggregates were examined carefully as the positivity of this parameter is almost pathognomic of H pylori infection and the follicles/aggregates were positive amongst 15 (41%) of TRs and 20 (40%) of DU subjects. An almost comparable distribution pattern of lymphoid follicles/aggregates was present among DU patients and the tribal population. Mononuclear cells infiltration in the lamina propria with lymphoid follicle in a DU and lymphoid aggregate in a TR case has been represented in Figure 2A-B.
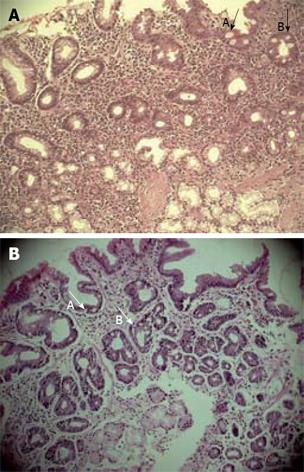
Atrophic changes among 50 DU patients were detected in 30 (60%) cases, where mild form of atrophy was present in 18 (36%) and a moderate form of atrophy was found in 12 (24%). Among 36 asymptomatic TRs, atrophic changes were identified in 10 (27.7%), where 7 (19.4%) showed mild form of atrophy and 3 (8.3%) showed moderate form of atrophy. Although the incidence of active gastritis was quite high, especially among tribals, atrophy and metaplastic changes in the gastric mucosa were much rarer than in the urban DU patients. Metaplasia was recognized morphologically by the presence of goblet cells, absorptive cells and cells resembling colonocytes in the surface epithelium and glands of the gastric mucosa and (Type 1) or complete type of intestinal metaplasia (Figure 3A-B) were detected in 3 TRs (8.3%) and 7 DU (14%) subjects (Table 2).
Moderate to severe density distribution of H pylori at the surface and in the pits of the gastric mucosa was found in 63% of TRs and in 62% of DU subjects.
The presence or absence of the cag PAI was scored by PCR with specific primers using DNA extracted from cultured strains. As shown in Table 3, a 350-bp product indicative of the cag PAI was obtained with primers specific for the cagA gene from each of the 36 strains in tribal population. None yielded a 550-bp product expected of a cag empty site, which would indicate complete absence of the cag PAI. On the other hand, in the symptomatic Bengali population, all yielded band specific for cagA gene and three cases also produced a 550-bp product for cag PAI empty site indicating that these patients had mixed infections with both cagA positive and negative strains. The presence of potentially toxigenic vacAs1 versus nontoxigenic vacAs2 alleles at the 5' end of vacA was determined based on sizes of PCR products (259 bp versus 286 bp, respectively) generated with vacAs region-specific primers. All 36 tribal strains yielded a 259-bp fragment, indicating that they carried s1 alleles; no s2 alleles were found; but, in the Bengali population, three strains produced both s1 and s2 fragment whereas the rest produced a s1 fragment. The alleles of the vacA middle(m) region, which determines the cell type specificity of the vacuolating cytotoxin action, were also studied by PCR. Products were obtained only with vacA m1 primers in 26 of 36 tribal strains, only with vacA m2 primers in seven strains and with both m1 and m2 primers in three strains, indicating a mixed infection. Among the 50 Bengali strains, 31 were positive for m1 while 17 strains had the m2 allele alone and the remaining two had both alleles. PCR was used to test for iceA1, which is virulence-associated in some populations, and the completely unrelated iceA2 gene, which occupies the same chromosomal locus in strains lacking iceA1. The iceA1 gene was found alone in 15 of 36 tribal cultures, iceA2 was found alone in 14 strains, and a mixture of iceA1 and iceA2 alleles (again, indicating mixed infection) was found in seven tribal cultures (Table 3). Among strains isolated from Bengali DU patients, iceA1 and iceA2 were found in 29 and 18 cases, respectively whereas iceA1-iceA2 mixed infections were found in 3 cases. Genotyping results from this study and sequence-based analysis from a previous study[7] clearly indicated that tribal strains are closely matched to those of mainstream Bengalis.
| No. (%) of strain from | ||
| Genotype | DU patients (n = 50) | TRs (n = 36) |
| cagA positive only | 47 (94) | 36 (100) |
| cagA negative only | 1 (2) | 0 (0) |
| Both cagA+ and cagA- | 2 (4) | 0 (0) |
| vacA s1 only | 47 (94) | 36 (100) |
| vacA s2 only | 1 (2) | 0 (0) |
| vacA s1 and s2 mixed | 2 (4) | 0 (0) |
| vacA m1 only | 31 (62) | 26 (72.2) |
| vacA m2 only | 17 (34) | 7 (19.4) |
| vacA m1 and m2 mixed | 2 (4) | 3 (8.3) |
| ice A1 only | 29 (58) | 15 (41.7) |
| ice A2 only | 18 (36) | 14 (38.9) |
| ice A1 and ice A2 mixed | 3 (6) | 7 (19.4) |
χ2 test was employed to compare the histological parameters between symptomatic and asymptomatic subjects to know the status of few important histological parameters like neutrophil infiltration, lymphoid follicle/aggregates formation and atrophy and metaplastic changes. Neutrophil infiltration was almost three times higher in the asymptomatic tribal population compared to urban DU cases, although the difference was not statistically significant. Regarding the atrophic and metaplastic changes, DU subjects were 4 and 2 times at higher risk of developing further disease process than TRs. Atrophic changes among DU cases were statistically significant (Table 2). Genotyping of H pylori strains between DU and TRs were not found statistically significant (Table 3).
H pylori infection is common in the Santhal and Oroan ethnic minorities of West Bengal, whereas symptomatic individuals with H pylori-associated disease are rare in these populations, even though the genotypes of the strains they carry are similar to those for mainstream Bengalis. The near-universality of H pylori infection can be ascribed to relatively low levels of sanitation, hygiene and education, conditions that contribute to a high risk of infection and superinfection, even in adulthood. Hence, although both the tribal communities and Bengali urban population are infected with H pylori with similar genetic make-up, there is a distinct difference regarding the manifestation of the disease. This enigma was previously explained, mostly in Western countries, by reporting the association of certain virulence alleles (vacAs1, cagA and iceA1) with the H pylori-related disease where around 50% of the H pylori strains lack the cagPAI. However, this view needs to be reexamined since in the Asian context an overt disease association with these alleles does not exist[6–8]. The present study investigated the histologic findings observed in gastric mucosa of H pylori-infected asymptomatic TRs and urban DU subjects (which prevail only in Bengali population, but not in the tribal population) to understand the cause for near absence of H pylori associated overt disease in these tribal populations and correlation of the genotypes of H pylori with different histological parameters.
Bacterial density is directly related to inflammation in terms of neutrophil, lymphocytes and plasma cell infiltration of the gastric mucosa and we also noticed moderate to severe degree infiltration of the bacteria in the gastric mucosa[14–16], where inflammatory cells were a marked feature. H pylori-positive biopsy samples were mostly inflamed with chronic superficial gastritis and the inflammatory cells were mononuclear cells with neutrophil infiltration in the epithelium. The amount of inflammation was highly variable, ranging from minimal infiltration in the lamina propria with intact glandular architecture to severe dense inflammation. Mononuclear cells, consisting mainly of macrophages, lymphocytes and plasma cells, were present to a variable degree in all the study cases, indicating a chronic infection among asymptomatic TRs as well as in DU patients. The presence of PMNs signifying ‘a sign of activity’ was more pronounced among the tribal population (94%) than DU patients (84%). It may be due to the host immune response against bacteria among tribals, which are stronger. Tribals are less exposed to environmental pollution and hazardous agents and as PMNs are the first line of defense against bacteria, increased cellularity and more active gastritis is well reflected in our study in H pylori infection.
A H pylori infection with a longer duration leads to loss of gastric glands and development of multifocal atrophic gastritis, which is often accompanied by intestinal metaplasia. In our study population, although the H pylori-associated gastritis as well as its activity is quite high among TRs, atrophic changes and the incidence of metaplasia were lower than in DU patients. One of the characteristics of H pylori infection is the growth of lymphoid follicles/aggregates. Here, 41% of the biopsy specimens were positive for lymphoid follicles and aggregates in TRs and a comparable proportion (40%) was found among DU subjects, although we did not encounter any case associated with lymphoma. In a study conducted by Eidt and Stolte, lymphoid follicles or aggregates were detected in 54% of H pylori infected cases[17], which is higher compared to our data.
It is now well accepted that peptic ulcer diseases have an etiologic link with H pylori; but, not every individual infected by this micro-organism develops the disease clinically[1819]. The actual element responsible for the pathogenesis of H pylori is yet to be determined. The cytotoxin associated gene cagA has been related to ulcerogenicity[20]. The genes in the cag pathogenicity island are supposed to induce epithelial cells to release interleukin-8 production. This, together with other interleukins, attracts neutrophils, which migrate from capillaries through the lamina propria, and emerge between the epithelial cells. However, in our study, cagA was present in TRs as frequently as in DU. Consistent to this finding, polymorphonuclear activity was detected in 97% cases cagA-positive TRs, and 95% cagA-positive DU subjects, which was quite a high proportion. Correlating the histologic findings of gastritis with the genotypes, like iceA, or combination of iceA, vacA and cagA, no particular allelic mosaicism could be identified as responsible for neutrophil and mononuclear cell infiltration, formation of lymphoid follicles and aggregates, atrophic and metaplastic changes and the successive disease outcome. This is in consistent with the findings of few earlier reports[2122].
An apparent correlation has still to be detected between the different genetic features of H pylori strains and the histologic findings of the disease outcome. When the histological changes, such as the presence of neutrophil infiltration, the activity of gastritis, the mononuclear and lymphoid follicle/aggregate formation, the atrophy and the metaplastic changes, were evaluated with respect to the genotypes of the strains of H pylori, the differences between the Bengali ethnic duodenal ulcer patients and the tribal population were not not statistically significant, except for the atrophy. However, a few observations in our study are interesting: (a) the almost similar distribution of inflammatory cells among asymptomatic TRs and DU cases; (b) the fact that, in spite of the evidence of active histologic gastritis, tribal groups were free from any clinical symptoms; (c) the increased cellular response, especially in terms of neutrophil infiltration, but much lower risk of developing atrophy and metaplastic changes among the tribal population.
Finally, the present study suggests that, on average, H pylori infections are less virulent in these ethnic minorities than in mainstream Indians. Such lack of virulence might be due to subtle features of bacterial strains or aspects of the human host environment. Thus, the bacterial genotype, host genetic factors and environmental factors, all may have important influence in the disease outcome of H pylori infected people. At least two reports of decreased virulence apparently being selected in vivo have appeared: one during human infection[23] and another during adaptation of human strains to mice[24]. Lack of virulence might also reflect features of the host. One possibility entails concurrent infection with particular parasites that may down-regulate inflammatory responses to infection, as it has been documented in a mouse infection model[25]. Resistance to pathogenic effects of putatively virulent H pylori strains might also be determined by features of human host genotype[2627]. It will be interesting to study why tribal groups are free from any clinical symptoms in spite of evidence of active histologic gastritis and also to identify the host factors that may provide immunity against the pathogenic effects of putatively virulent H pylori strains. Such kind of studies may uncover new genetic factor/factors that affect human infection, increase our understanding of bacterium-host interactions in colonization and disease, and provide new insights into the evolution of this diverse and globally distributed human pathogen.
Helicobacter pylori (H pylori) infection and duodenal ulcer disease is common among ethnic Bengali population in West Bengal, India. In contrast, although H pylori infection is equally or more common in the ethnic tribal minorities (Santhals and Orans) of West Bengal, symptomatic disease is extremely rare. This study addresses different histological parameters occurring in the stomach tissue of H pylori-infected tribal populations and duodenal ulcer patients among ethnic Bengalis for getting insights of the cause for the near-absence of H pylori-associated overt disease in these tribal populations and correlate the H pylori genotypes with different histological parameters.
Although both the tribal communities and Bengali urban population are infected with H pylori with similar genetic make-up, there is a distinct difference regarding the manifestation of the disease. When the histological changes, such as the presence of neutrophil infiltration, the activity of gastritis, the mononuclear and lymphoid follicle/aggregate formation, the atrophy and the metaplastic changes, were evaluated with respect to the genotypes of the strains of H pylori, the differences between the Bengali ethnic duodenal ulcer patients and the tribal population were not statistically significant, except for the atrophy.
The most interesting observations in the study are: (1) the almost similar distribution of inflammatory cells among asymptomatic tribals and duodenal ulcer patients from Bengali population in Kolkata, (2) the fact that, in spite of evidence of active histologic gastritis, tribal groups were free from any clinical symptoms; and (3) the increased cellular response, especially in terms of neutrophil infiltration, but much lower risk of developing atrophy and metaplastic changes among the tribal population.
The apparent lack of virulence in the tribal group might reflect features of the host. The study raised two important issues: (1) why are tribal groups free from any clinical symptoms, in spite of evidence of active histologic gastritis, and (2) the need to identify the host factors (in tribal patients) that may provide immunity against the pathogenic effects of putatively virulent H pylori strains. Such kind of studies may uncover new genetic factor/factors that affect human infection, increase our understanding of bacterium-host interactions in colonization and disease.
This article investigated the changing pattern of different histological parameters in the stomach tissue of H pylori infected populations. The paper is well written and their results are reliable.
| 1. | Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328-1333. |
| 2. | Taylor DN, Blaser MJ. The epidemiology of Helicobacter pylori infection. Epidemiol Rev. 1991;13:42-59. |
| 3. | NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65-69. |
| 4. | Miehlke S, Bayerdörffer E, Lehn N, Mannes GA, Sommer A, Höchter W, Weingart J, Bästlein E, Hatz R, Stolte M. Risk prediction of duodenal ulcer relapse. Gastroenterology. 1995;108:A167. |
| 5. | Kuipers EJ, Lundell L, Klinkenberg-Knol EC, Havu N, Festen HP, Liedman B, Lamers CB, Jansen JB, Dalenback J, Snel P. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018-1022. |
| 6. | Mukhopadhyay AK, Kersulyte D, Jeong JY, Datta S, Ito Y, Chowdhury A, Chowdhury S, Santra A, Bhattacharya SK, Azuma T. Distinctiveness of genotypes of Helicobacter pylori in Calcutta, India. J Bacteriol. 2000;182:3219-3227. |
| 7. | Datta S, Chattopadhyay S, Balakrish Nair G, Mukhopadhyay AK, Hembram J, Berg DE, Rani Saha D, Khan A, Santra A, Bhattacharya SK. Virulence genes and neutral DNA markers of Helicobacter pylori isolates from different ethnic communities of West Bengal, India. J Clin Microbiol. 2003;41:3737-3743. |
| 8. | Chattopadhyay S, Datta S, Chowdhury A, Chowdhury S, Mukhopadhyay AK, Rajendran K, Bhattacharya SK, Berg DE, Nair GB. Virulence genes in Helicobacter pylori strains from West Bengal residents with overt H. pylori-associated disease and healthy volunteers. J Clin Microbiol. 2002;40:2622-2625. |
| 9. | Lam SK. Differences in peptic ulcer between East and West. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:41-52. |
| 10. | Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321-4325. |
| 11. | Cutler AF, Havstad S, Ma CK, Blaser MJ, Perez-Perez GI, Schubert TT. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology. 1995;109:136-141. |
| 12. | Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6:209-222. |
| 13. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. |
| 14. | Langdale-Brown B, Haqqani MT. Acridine orange fluorescence, Campylobacter pylori, and chronic gastritis. Scand J Gastroenterol. 1990;25:127-133. |
| 15. | Satoh K, Kimura K, Yoshida Y, Kasano T, Kihira K, Taniguchi Y. A topographical relationship between Helicobacter pylori and gastritis: quantitative assessment of Helicobacter pylori in the gastric mucosa. Am J Gastroenterol. 1991;86:285-291. |
| 16. | Chan WY, Hui PK, Leung KM, Thomas TM. Modes of Helicobacter colonization and gastric epithelial damage. Histopathology. 1992;21:521-528. |
| 17. | Eidt S, Stolte M. Prevalence of lymphoid follicles and aggregates in Helicobacter pylori gastritis in antral and body mucosa. J Clin Pathol. 1993;46:832-835. |
| 18. | Isenberg JI, Soll AH. Epidemiology, clinical manifestations, and diagnosis. Cecil textbook of medicine. 20th ed. Saunders: Philadelphia 1996; 664-666. |
| 19. | Peura DA. Helicobacter pylori and ulcerogenesis. Am J Med. 1996;100:19S-25S; discussion 25S-26S. |
| 20. | Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791-5795. |
| 21. | Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K, Graham DY. Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter. 1998;3:241-253. |
| 22. | Wang HJ, Kuo CH, Yeh AA, Chang PC, Wang WC. Vacuolating toxin production in clinical isolates of Helicobacter pylori with different vacA genotypes. J Infect Dis. 1998;178:207-212. |
| 23. | Kersulyte D, Mukhopadhyay AK, Velapatiño B, Su W, Pan Z, Garcia C, Hernandez V, Valdez Y, Mistry RS, Gilman RH. Differences in genotypes of Helicobacter pylori from different human populations. J Bacteriol. 2000;182:3210-3218. |
| 24. | Philpott DJ, Belaid D, Troubadour P, Thiberge JM, Tankovic J, Labigne A, Ferrero RL. Reduced activation of inflammatory responses in host cells by mouse-adapted Helicobacter pylory isolates. Cell Microbiol. 2002;4:285-296. |
| 25. | Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536-542. |
| 26. | Ferrero RL, Fox JG. In vivo modeling of Helicobacter associated gastrointestinal diseases. Helicobacter pylori: physiology and genetics. American Society for Microbiology: Washington 2001; 565-582. |
| 27. | Ferrero RL, Jenks PJ. In vivo adaptation to the host. Helicobacter pylori: physiology and genetics. American Society for Microbiology: Washington 2001; 583-592. |
| 28. | Chattopadhyay S, Patra R, Ramamurthy T, Chowdhury A, Santra A, Dhali GK, Bhattacharya SK, Berg DE, Nair GB, Mukhopadhyay AK. Multiplex PCR assay for rapid detection and genotyping of Helicobacter pylori directly from biopsy specimens. J Clin Microbiol. 2004;42:2821-2824. |