Published online Mar 7, 2009. doi: 10.3748/wjg.15.1057
Revised: January 29, 2009
Accepted: February 4, 2009
Published online: March 7, 2009
AIM: To investigate the mechanism of liver regeneration induced by fusing the omentum to a small traumatic injury created in the liver. We studied three groups of rats. In one group the rats were omentectomized; in another group the omentum was left in situ and was not activated, and in the third group the omentum was activated by polydextran particles.
METHODS: We pre-activated the omentum by injecting polydextran particles and then made a small wedge wound in the rat liver to allow the omentum to fuse to the wound. We monitored the regeneration of the liver by determining the ratio of liver weight/body weight, by histological evaluation (including immune staining for cytokeratin-19, an oval cell marker), and by testing for developmental gene activation using reverse transcription polymerase chain reaction (RT-PCR).
RESULTS: There was no liver regeneration in the omentectomized rats, nor was there significant regeneration when the omentum was not activated, even though in this instance the omentum had fused with the liver. In contrast, the liver in the rats with the activated omentum expanded to a size 50% greater than the original, and there was histologically an interlying tissue between the wounded liver and the activated omentum in which bile ducts, containing cytokeratin-19 positive oval cells, extended from the wound edge. In this interlying tissue, oval cells were abundant and appeared to proliferate to form new liver tissue. In rats pre-treated with drugs that inhibited hepatocyte growth, liver proliferation was ongoing, indicating that regeneration of the liver was the result of oval cell expansion.
CONCLUSION: Activated omentum facilitates liver regeneration following injury by a mechanism that depends largely on oval cell proliferation.
- Citation: Singh AK, Pancholi N, Patel J, Litbarg NO, Gudehithlu KP, Sethupathi P, Kraus M, Dunea G, Arruda JA. Omentum facilitates liver regeneration. World J Gastroenterol 2009; 15(9): 1057-1064
- URL: https://www.wjgnet.com/1007-9327/full/v15/i9/1057.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1057
The omentum has been called the “policeman of the abdomen” because after traumatic injury it migrates to the injured site, adheres to the wound, and promotes healing[12]. These properties have found clinical application where the omentum is surgically brought into contact with injured tissues such as ischemic heart, fractured bones, or injured spinal cord[3–6]. We have recently shown that introducing a foreign body into the peritoneal cavity further enhanced the healing power of the omentum by causing it to expand, surround the foreign body, and transform itself from mostly fatty tissue to tissue abundant in progenitor cells and rich in growth and angiogenic factors (activated omentum)[78].
Because liver regeneration can be brought about by resident stem cells (oval cells) even in the absence of hepatocyte multiplication[910], we attempted to use the activated omentum to facilitate liver regeneration. The procedure involved removing a small wedge of tissue (traumatic injury) in rats and allowing the omentum to adhere to the wound in order to supply the liver with stem cells. We also studied two other groups of rats (controls); one in which the omentum was left in its native state (inactivated omentum), and the other in which the omentum was surgically removed (omentectomized), and focused on the cellular and developmental gene activation at the site of injury and omental adhesion.
Animal experimentation was conducted according to the approval of the Institutional Animal Care and Use Committee (IACUC).
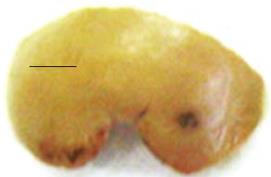
Under general anesthesia, male Sprague-Dawley rats (200-250 g) were laparotomized and the most anterior and prominent of the liver lobes lying in the middle of the abdominal cavity was exposed. Using a pair of fine scissors a small V-shaped cut was made in the lobe (3-4 mm on each side) and the wedge of liver was removed and later used as normal tissue for immunostaining and quantitative reverse transcription polymerase chain reaction (RT-PCR) (Figure 1). The rats were divided into three groups. In the activated omentum group, before the incision was sutured, 5 mL of polydextran particle slurry (Biogel P-60, 120 &mgr;mol/L; Biorad Laboratories, Richmond, CA, USA) (1:1 in normal saline) was introduced into the abdominal cavity to activate the omentum. The inactivated omentum group underwent similar hepatic wedge injuries. However, polydextran slurry was not placed in the abdomen and, thus the omentum was not activated (inactivated omentum). The omentectomized group underwent similar hepatic wedge resections, in addition to an omentectomy. An omentectomy was performed by surgically excising the entire omentum from the lower curvature of the stomach.
The animals were maintained on normal rat chow and water ad libitum from three to twenty eight days. At the time of sacrifice, the livers were examined, wholly removed, and weighed. Liver mass was expressed conventionally as a percent ratio: liver weight/body weight. Pieces of the re-grown liver from the point of omental fusion, and at 0.5 cm and 1.0 cm away from the wound as well as portions from an uninjured lobe were collected for immunostaining and quantitative RT-PCR.
To test whether liver regeneration by omental intervention depended upon hepatocyte proliferation, rats were injected intraperitoneally daily for four days with 2-acetyl-amino-fluorene, which inhibits hepatocyte proliferation (2-AAF; 30 mg/kg dissolved in M400 polyethylene glycol (Avg. MW = 400); both chemicals were obtained from the Sigma Chemical Company, St Louis, MO, USA), followed by liver wounding and omental activation (at day 5 by intraperitoneal injection of polydextran), and further daily injections of 2-AAF for four days to inhibit expansion of hepatocytes. The rats were sacrificed 14 d after liver wounding and the livers were examined, wholly removed, and weighed.
Pieces of normal and regenerated liver (including the omental attachment) were fixed for histology and immunostaining by immersion in Histochoice® (Amersco Inc., Solon, OH, USA). Following dehydration and paraffin embedment, tissues were sectioned (5 &mgr;m thick) and stained with hematoxylin-eosin (HE) or Trichrome stain. Immunostaining was carried out by first pressure-cooking the sections for 10 min in a solution of BorgDecloaker® (Biocare Medical; Walnut Creek, CA, USA) for antigen enhancement. For immunofluorescent staining the sections were incubated with monoclonal (mouse) anti-rat cytokeratin-19 (Sigma Chem. Co, St Louis, MO, USA) followed by washing and re-incubating with fluorescein (FITC) labeled anti-mouse IgG antibody (Sigma Chem. Co., St Louis, MO, USA). The slides were washed and wet-mounted in glycerol-PBS. For immunoperoxidase staining, sections were sequentially incubated with monoclonal (mouse) anti-rat cytokeratin-19, anti-mouse IgG-biotin conjugate, avidin-horse radish peroxidase and finally developed with diaminobenzidine-H2O2 (brown color) (Vector Laboratories, Inc., Burlingame, CA, USA). The slides were examined either by epifluorescent or light microscopy and digitally photographed (Nikon Inc., New York, NY, USA).
Liver tissues at the point of omental attachment or wound edge (in omentectomized rats), at 0.5 and 1.0 cm away from the omental attachment, and from a remote uninjured lobe were tested for expression of developmental genes. The specific genes and their respective forward and reverse primer sequences are listed in Table 1. The liver tissue was cleared of the attached omental tissue and processed for total RNA extraction by Trizol using a RNA purification kit (Invitrogen, CA, USA). The RT-PCR procedure was carried out in one step using 3 &mgr;g of total tissue RNA and primers using the Invitrogen RT-PCR system (Invitrogen, CA, USA). The system uses Superscript II reverse transcriptase for first strand synthesis and Taq DNA polymerase for second strand cDNA synthesis and amplification (30 cycles). β-actin amplification was performed from the total RNA preparations (60 ng) as a control. The RT-PCR products were quantitated as the ratio of gene band density/β-actin band density by image analysis using MIPAV software (JAVA imaging software inspired by the National Institutes of Health).
| Gene1 | Primer | Predicted size (bp) | Accession number2 | Entrez gene ID2 |
| β-actin | F (926) 5'-TCATGAAGTGTGACGTTGACATCCGT-3'3 | |||
| R (1210) 5'-CCTAGAAGCATTTGCGGTGCACGATG-3' | 285 | NM_031144 | 81822 | |
| Wnt-4 | F (127) 5'-GAAACGTGCGAGAAGCTCAAAG-3' | |||
| R (513) 5'-AAAGGACTGTGAGAAGGCTACG-3' | 387 | NM_053402 | 84426 | |
| WT-1 | F (1059) 5'-TGAGAAACCATACCAGTGTGAC-3' | |||
| R (1458) 5'-GTAGGTGAGAGGGAGGAATTTC-3' | 400 | NM_031534 | 24883 | |
| Nanog | F (541) 5'-ATCCATTGCAGCTATTCTCAGG-3' | |||
| R (850) 5'-CTTCCAAATTCGCCTCCAAATC-3' | 310 | XM_575662 | 414065 | |
| AFP | F (1421) 5'-CAGTGAGGAGAAACGGTCCG-3' | |||
| R (1672) 5'-ATGGTCTGTAGGGCTCGGCC-3' | 252 | NM_012493 | 24177 | |
| Oct-4 | F (633) 5'-GGAGATATGCAAATCGGAGACC-3' | |||
| R (984) 5'-CGAGTAGAGTGTGGTGAAATGG-3' | 352 | NM_001009178 | 294562 | |
| HNF-6 | F (1698) 5'-AAGACCAGGACCTCAAGATAGC-3' | |||
| R (2001) 5'-GCAGTGTGGTGGAACAGATAAG-3' | 304 | NM_022671 | 25231 |
Quantitative data presented in Figures 2 to 6, which compare the differences between different groups, were analyzed by student’s t test. The differences were considered significant when P < 0.05.
In all rats in which the liver was traumatically wounded and the omentum was intact, whether activated (n = 24) or inactivated (n = 12), there was fusion between the omentum and the wound edge of the liver, and the omentum remained attached to the injury site for up to 28 d. On gross inspection, by day 14 new tissue filled the resected wedge, and the location of the resection site was only identifiable by the omental attachment. In omentectomized rats (n = 12), there was an absence of omental attachment and of liver growth at the wound, making the wound edges noticeable until at least day 28 (Figure 1).
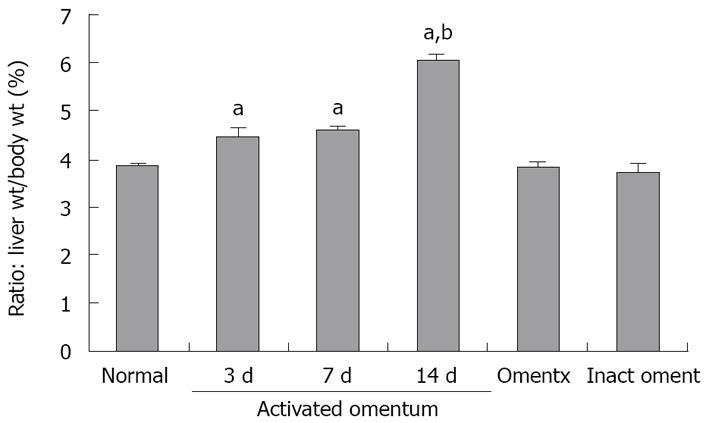
In rats with activated omentum, there was additional liver growth, especially in the wounded lobe at the point of omental attachment. There was also growth in other lobes which was suggested by alterations in the natural contours of the edges of the uninjured liver lobes. Figure 2 shows the liver mass (as a percent ratio to body weight) at different times after wounding and fusing of the omentum to the wound. The percent ratio in normal rats was established to be 3.85 ± 0.07 [Normal (n = 15); Figure 2]. In the activated omentum group, the liver grew to 110% of its original mass by day 3 (percent ratio: 4.4 ± 0.24) and to a maximum size of 150% by day 14 (percent ratio: 6.0 ± 0.16) (n = 6 at days 3, 7, 14 and 28; Figure 2). From day 14 to day 28, the liver did not grow any further, but remained enlarged (data not shown).
In rats with inactivated omentum, growth was observed at the site of omental fusion and filled the resected site with new tissue, however, the overall liver mass did not increase at any of the time points compared with the established normal liver mass [n = 3 at days 3, 7, 14 and 28 (day 14 data shown in Figure 2)].
In omentectomized rats, the liver did not grow and the resection site remained visible up to day 28. The percent ratio of liver wt/body wt in this group was similar to that of normal rats at all time points [n = 3 at days 3, 7, 14 and 28 (day 14 data shown in Figure 2)].
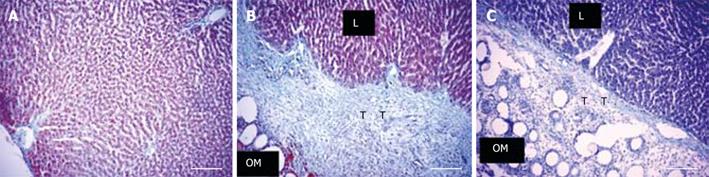
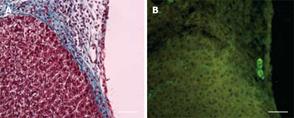
In the activated omentum group, in which liver tissue grew to more than normal size, histological examination at the site of injury revealed normal hepatic architecture up to the point of omental fusion (Figure 3B). At the site of fusion there was a wide and compact band of interlying tissue between the omentum and the growing edge of the liver (Figure 3B). On one side of the interlying tissue lay the liver tissue and on the other side was the omental tissue with the embedded polydextran gel particles. The compactness and the width of the interlying tissue was maximal between 3 and 7 d after liver injury (400-600 &mgr;mol/L) (Figure 3B) and became thinner (100-150 &mgr;mol/L) and less compact by day 14 (Figure 3C). In the interlying tissue, small islands of liver tissue, probably newly formed, were seen (Figure 4G). By day 28 the interlying tissue was barely visible and appeared like a tissue septum (not shown).
In the inactivated omentum group in which liver growth was minimal, although the adherent omental tissue was clearly visible, a thinner interlying tissue than that seen in the activated omentum group was observed (Figure 5A). In the omentectomized group, the void left by the injury was visible at all time points. The injury site lacked omental attachment, but showed a layer of dead tissue (approx. five cells thick) which sloughed off from the wound edge by day 14 (not shown).
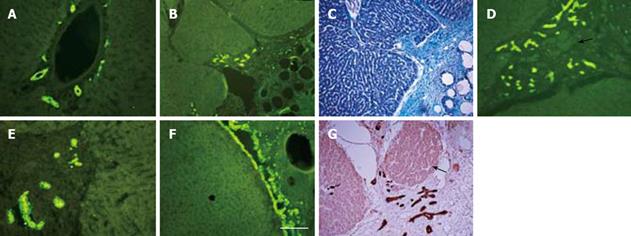
Immunostaining of normal adult rat liver for cytokeratin-19, a well-known marker for bile ducts as well as oval cells (oval cells are presumed to be liver stem cells), showed the expected widespread presence of oval cells in the lining of bile ducts lying in the liver triads (Figure 4A). In the liver with or without omental attachment, cytokeratin-19 positive bile duct staining in the triads of the uninjured lobes was similar in intensity to that seen in the normal liver (not shown). In the liver tissue with activated omentum, remarkably, cytokeratin-19 positive bile ducts in the regenerated liver (day 3 or 7) extended into the interlying area between the liver and the omentum (Figure 4B-E). Occasionally, the growing edge of the liver at the interlying tissue was seen to be entirely covered with cytokeratin-19 positive cells (Figure 4F). Islands of liver tissue, probably newly formed, were present in the interlying tissue (Figure 4G).
In the inactivated omentum group, the interlying tissue attached to the wound edge also showed extensions of the cytokeratin-19 positive bile ducts (at days 3 and 7) (Figure 5B), although these were much less frequent than those seen in the activated omentum group. In omentectomized rats, the wound edge was devoid of omental attachment, and as expected no cytokeratin-19 positive bile ducts were seen outside the liver tissue (not shown).
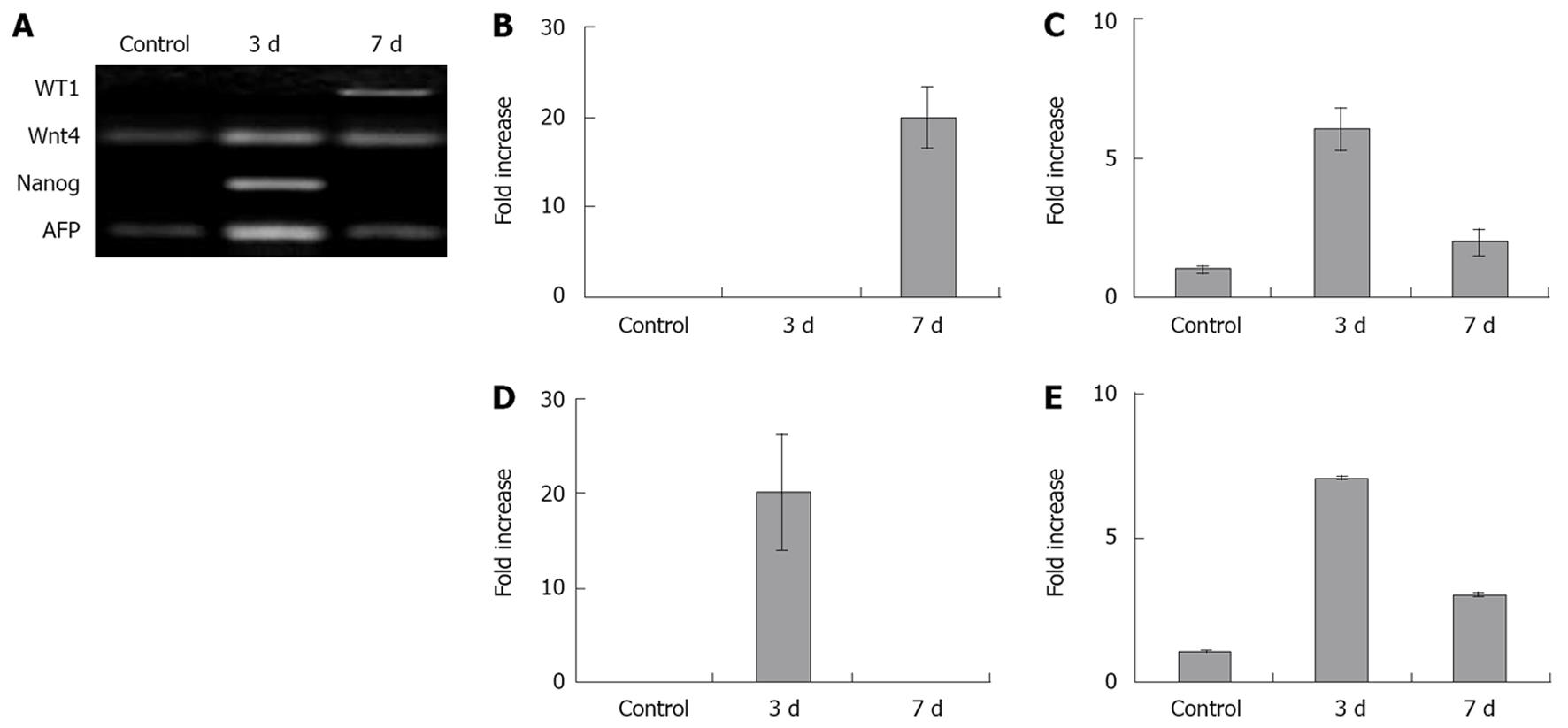
To further investigate if activated omentum fused to the injured liver triggered developmental events in the adult liver, we determined the expression levels of several important genes associated with (1) pluripotent embryonic stem cell activity (Nanog, Oct-4), (2) liver differentiation (WT1, Wnt-4, HNF-6) and (3) fetal liver synthetic activity (α-fetoprotein; AFP) at days 3 and 7 after wounding using RT-PCR. Comparisons between normal rat adult liver and the regenerated liver attached to the activated omentum showed high expression levels (7-20 fold) of four of these genes (WT-1, Wnt-4, Nanog, AFP) in the regenerated liver tissue (Figure 6) (Oct-4 and HNF-6 levels were negative; not shown). Wnt-4, Nanog, and AFP were maximally activated at day 3, while WT-1 showed maximal activation at day 7.
Regenerated liver tissue from sites further away from the injured area (0.5 cm and 1.0 cm away from the activated omentum in the same lobe, and tissue from an uninjured lobe), showed reduced expression of WT-1, Wnt-4, and AFP genes (although higher in all cases compared to normal adult liver (Nanog, Oct-4, AFP did not change), suggesting that the regeneration stimulus ‘rippled’ from the injured area further into the liver tissue (data not shown).
In contrast, in the inactivated omentum group (compared to normal adult liver) the expression level of WT-1, Wnt-4 and Nanog increased to a much smaller degree than that observed in the activated omentum group at days 3 and 7 (WT-1 by 1.5-fold, Wnt-4 by 1.9-fold and Nanog by 1.2-fold), while AFP decreased by 0.8-fold (P < 0.05 in all cases; no detectable changes were seen in Oct-4 and HNF-6).
In the omentectomized group, while the expression levels of WT-1 and Nanog did not change, the levels of Wnt-4, Oct-4, AFP and HNF-6 decreased by 0.78-fold- undetectable levels compared with normal adult liver (P < 0.05 in all cases).
Because liver mass can increase due to either progenitor cell activation or hepatocyte multiplication, we performed the wedge wound experiment in a small group of rats in which hepatocyte multiplication was blocked by treatment with 2-AAF. This was performed to confirm that liver regeneration was via progenitor cells and not by hepatocyte expansion in our model. Fourteen days after wounding, 2-AAF treated rats showed complete healing of the wedge wound and increased the liver mass to 135% of the original mass [liver weight/body weight ratios: 5.1 ± 0.2 in 2-AAF treated (n = 4) versus 3.85 ± 0.07 in normal controls (n = 15); P < 0.05], confirming that omentum-assisted liver regeneration was not mediated by hepatocyte expansion, but by progenitor cell activation.
For many years the omentum was believed to have no specific function. During the course of the 19th century, however, several investigators recognized that it possessed healing properties. These were later exploited in a variety of surgical procedures designed to facilitate the healing of bone fractures, spinal cord injuries, and heart ischemia[1–6]. In previous studies we investigated this process and found that these properties can be enhanced by physically expanding the omentum with foreign particles. Under such conditions, the expanded omentum becomes rich in growth and angiogenic factors, and has abundant progenitor cells[78]. In a separate study we used this approach to generate new β-cells from the diabetic pancreas[11].
Here we applied these findings in an attempt to regenerate liver tissue by creating a surgical wound and allowing the omentum to fuse with the wound. We studied groups of rats with (1) no omentum (omentectomized), (2) inactivated omentum, and (3) omentum pre-activated by foreign polydextran particles. We found no liver growth in omentectomized rats. In rats with inactivated omentum, the omentum fused with the injured site and although new growth was noted, this did not result in a significant increase in liver mass. However, in rats with activated omentum, following omental fusion, the liver grew to fill the wound and continued to grow, both at the wound site and globally, to a level 50% greater than the original mass. These findings suggest that the omentum plays an important role in bringing about growth and regeneration of the injured liver. The amount of liver growth induced by the omentum was proportional to the degree of omental activation, consistent with our previous observations that the concentration of growth factors and the number of progenitor cells in the omentum increase with increased activation[78].
Clinicians have long known that the liver has the ability to regenerate. Experimentally, a 70% hepatectomy (either surgically or chemically) induces a form of liver regeneration in which growth is largely due to hepatocyte proliferation[9–13]. When hepatectomy is carried out following the administration of drugs which inhibit hepatocyte proliferation, the regeneration is mainly due to the expansion of oval cells[14–17]. In these various models, there is a massive loss of functional liver tissue, which then systemically triggers a cascade of cytokines (such as tumor necrosis factors-α, IL-6 and growth factors). In our model, the injury was so slight that regeneration would not occur unless the omentum was activated, as shown in our omentectomized control rats.
In further studies we attempted to understand the mechanism by which activation of the omentum causes liver regeneration. Histologically, at the fusion site between the activated omentum and the liver, we found a wide and compact interlying band of tissue into which tubular structures resembling bile ducts extended and proliferated. On staining, these structures were strongly positive for cytokeratin-19, a known marker for oval cells, believed to be liver progenitor cells[18–20]. At an early stage of regeneration the oval cells in the interlying tissue were seen near small islands of liver tissue; later these islands became integrated into the native liver, so that the border between the native and the new liver could no longer be discerned in stained sections. Because proliferation of the progenitor oval cells took place in the omentum rather than in the liver, they may have had more room to proliferate and expand, accounting for the robust, supra normal liver growth.
Because liver tissue can also regenerate by hepatocyte proliferation we repeated our experiment in rats treated with 2-AAF, a drug commonly used to inhibit hepatocyte multiplication. Our finding that the activated omentum continued to exert its regenerative property in 2-AAF-treated rats by increasing the liver mass to 135% of the native mass, further suggested that the proliferation of progenitor oval cells rather than proliferation of hepatocytes was responsible for liver regeneration in our model.
As genes and proteins involved in liver development (Wnt-4, WT-1, HNF-6, AFP) may become re-activated during liver regeneration[15–1721–23], we tested these developmental genes and also Nanog and Oct-4, markers of early progenitor cells[2425], to see if they were altered in regenerating liver tissue. We found that many of these genes, silent in the adult liver, were highly up-regulated (7 to 20-fold) after fusion of the activated omentum. Nanog was up-regulated by 20-fold three days after omental fusion and returned to undetectable levels by day seven, suggesting the transient presence of early progenitor cells. Gene expression of Wnt-4 and AFP was highest at day 3 and decreased by day 7, in contrast to WT-1 which was unchanged at day 3 and highest at day 7. These findings are consistent with the transient activation of these transcription factors (WT-1 and Wnt-4) known to occur in liver re-modeling and differentiation[162627]. HNF-6, a marker strongly associated with hepatocyte proliferation[28], was unchanged, as also noted in a previous study of non-hepatocyte mediated (but progenitor cell mediated) liver regeneration[16]. This was not surprising because liver growth by omental fusion was via oval cells and not dependent on hepatocyte proliferation. Interestingly, we also found a few selected genes (WT-1, Wnt-4 and AFP) to be activated in regions of the native liver 0.5 cm and 1.0 cm from the wound edge. The level of activation decreased as the distance from the wound edge increased, suggesting that a paracrine effect was exerted by the omentum. Importantly, we found lower activation levels of genes in the inactivated omentum group (1.2-1.9-fold), consistent with reduced liver growth seen in these rats. Furthermore, in omentectomized rats where there was no liver growth, a decrease in gene expression levels was observed compared with normal liver.
The present study is the first to demonstrate the unique role of the omentum in traumatic wound healing of the liver. We have shown previously that omental derived factors stimulate wound healing and can be upregulated by pre-activating the omentum. By bringing the omentum into close contact with injured liver we observed a vigorous regeneration of liver tissue. Although the liver is known to regenerate to the original size following a significant loss of hepatic tissue, there are no reports of liver regeneration up to 150% of the original size as noted in our study. As both cytokeratin-19 positive cells and expression of developmental genes were increased, we postulate that both growth factors and stem cells are conveyed to the site of injury by omental fusion. It may be argued that bringing the omentum into contact with damaged organs after controlled deliberate wounding may have immediate clinical applicability. Also, the use of progenitor cells isolated from the activated omentum or of the growth factors secreted by these cells holds further promise of other exciting therapeutic possibilities.
Although the liver is a unique tissue that can regenerate after an acute injury, it has been a challenge to induce such regeneration after chronic liver disease. It is, therefore, important to study mechanisms of liver regeneration in order to devise new approaches for regeneration following damage by chronic disease. Although embryonic stem cells have the power to regenerate liver tissue, their use is hampered by ethical, political and safety concerns. In that regard, the use of adult stem cells derived from the patient’s own tissue to regenerate the liver is free of such concerns and, therefore an alternative approach.
Stem cells have been derived from several adult organs such as bone marrow, skin, hair, kidney and dental pulp. Although these cells express stem cell markers and differentiate to other cell phenotypes in culture they seem to lack the potency to regenerate an organ in vivo. Identifying a source of adult stem cells that could regenerate liver or other organs would be an immense advantage.
Singh and his colleagues devised a methodology to harness adult stem cells to regenerate the liver by first activating the omentum using a foreign body to increase its content of stem cells and growth factors. They then cut and removed a small piece of the liver tissue and let the activated omentum adhere to the wound in order to supply stem cells to the injured liver. They found that the liver of these rats with an activated omentum expanded to a size 50% greater than the original, an outcome never reported before. This approach represents an application of adult stem cells to regenerate an organ in vivo.
This method of liver regeneration is novel and could be attempted in patients with liver failure in order to regenerate new liver tissue.
Activated omentum, which is central to this methodology of liver regeneration, was created by injecting polydextran particles (foreign body) into the abdominal cavity. As the omentum naturally grows to encapsulate the particles individually it expands 20-30 times its original size and has abundant stem cells and growth factors, which appear to be the basis of the regenerating power of the omentum.
Reviewers considered the use of the omentum to regenerate the liver as meritorious and interesting. Further they thought the paper was well written, results were clear, and the data supported the conclusions reached by the authors.
| 1. | Vernik J, Singh AK. Omentum: power to heal and regenerate. Int J Artif Organs. 2007;30:95-99. |
| 2. | Liebermann-Meffert D. The greater omentum. Anatomy, embryology, and surgical applications. Surg Clin North Am. 2000;80:275-293, xii. |
| 4. | Vineberg AM, Kato Y, Pirozynski WJ. Experimental revascularization of the entire heart. Evaluation of epicardiectomy, omental graft, and/or implantation of the internal mammary artery in preventing myocardial necrosis and death of the animal. Am Heart J. 1966;72:79-93. |
| 5. | Nottebaert M, Lane JM, Juhn A, Burstein A, Schneider R, Klein C, Sinn RS, Dowling C, Cornell C, Catsimpoolas N. Omental angiogenic lipid fraction and bone repair. An experimental study in the rat. J Orthop Res. 1989;7:157-169. |
| 6. | Goldsmith HS. Brain and spinal cord revascularization by omental transposition. Neurol Res. 1994;16:159-162. |
| 7. | Litbarg NO, Gudehithlu KP, Sethupathi P, Arruda JA, Dunea G, Singh AK. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res. 2007;328:487-497. |
| 8. | Singh AK, Patel J, Litbarg NO, Gudehithlu KP, Sethupathi P, Arruda JA, Dunea G. Stromal cells cultured from omentum express pluripotent markers, produce high amounts of VEGF, and engraft to injured sites. Cell Tissue Res. 2008;332:81-88. |
| 11. | Singh AK, Gudehithlu KP, Litbarg NO, Sethupathi P, Arruda JA, Dunea G. Transplanting fragments of diabetic pancreas into activated omentum gives rise to new insulin producing cells. Biochem Biophys Res Commun. 2007;355:258-262. |
| 12. | Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545-1559. |
| 13. | Higgins GM, Anderson RM. Experimental pathology of the liver. 1. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;112:186-202. |
| 14. | Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433-445. |
| 15. | Dabeva MD, Laconi E, Oren R, Petkov PM, Hurston E, Shafritz DA. Liver regeneration and alpha-fetoprotein messenger RNA expression in the retrorsine model for hepatocyte transplantation. Cancer Res. 1998;58:5825-5834. |
| 16. | Gordon GJ, Coleman WB, Grisham JW. Temporal analysis of hepatocyte differentiation by small hepatocyte-like progenitor cells during liver regeneration in retrorsine-exposed rats. Am J Pathol. 2000;157:771-786. |
| 17. | Kuhlmann WD, Peschke P. Hepatic progenitor cells, stem cells, and AFP expression in models of liver injury. Int J Exp Pathol. 2006;87:343-359. |
| 19. | Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477-1487. |
| 20. | Walkup MH, Gerber DA. Hepatic stem cells: in search of. Stem Cells. 2006;24:1833-1840. |
| 21. | Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288-295. |
| 22. | Kanato K, Hosen N, Yanagihara M, Nakagata N, Shirakata T, Nakazawa T, Nishida S, Tsuboi A, Kawakami M, Masuda T. The Wilms' tumor gene WT1 is a common marker of progenitor cells in fetal liver. Biochem Biophys Res Commun. 2005;326:836-843. |
| 23. | Nava S, Westgren M, Jaksch M, Tibell A, Broome U, Ericzon BG, Sumitran-Holgersson S. Characterization of cells in the developing human liver. Differentiation. 2005;73:249-260. |
| 24. | Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643-655. |
| 25. | Gerrard L, Zhao D, Clark AJ, Cui W. Stably transfected human embryonic stem cell clones express OCT4-specific green fluorescent protein and maintain self-renewal and pluripotency. Stem Cells. 2005;23:124-133. |
| 26. | Plescia C, Rogler C, Rogler L. Genomic expression analysis implicates Wnt signaling pathway and extracellular matrix alterations in hepatic specification and differentiation of murine hepatic stem cells. Differentiation. 2001;68:254-269. |
| 27. | Jiang F, Parsons CJ, Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol. 2006;45:401-409. |
| 28. | Tan Y, Yoshida Y, Hughes DE, Costa RH. Increased expression of hepatocyte nuclear factor 6 stimulates hepatocyte proliferation during mouse liver regeneration. Gastroenterology. 2006;130:1283-1300. |