Published online Feb 21, 2009. doi: 10.3748/wjg.15.888
Revised: December 29, 2008
Accepted: January 6, 2009
Published online: February 21, 2009
Signet-ring cell carcinoma (SRCC) of ampulla of Vater is extremely uncommon, and less than 15 cases have been reported so far in literature. It mainly occurs in elderly people (median age 57 years). We report a rare case of SRCC of the ampulla of Vater in a 38-year-old woman who presented with a small tumor at the Vater, discovered by the contrast-enhanced ultrasound (CEUS). Histopathological examination showed prominent signet-ring features. We also describe the imaging features of SRCC of ampulla of Vater in CEUS.
- Citation: Gao JM, Tang SS, Fu W, Fan R. Signet-ring cell carcinoma of ampulla of Vater: Contrast-enhanced ultrasound findings. World J Gastroenterol 2009; 15(7): 888-891
- URL: https://www.wjgnet.com/1007-9327/full/v15/i7/888.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.888
Signet-ring cell carcinoma (SRCC) usually occurs in the gastrointestinal tract. The World Health Organization (WHO) defines it as a special type or a variant of gastrointestinal adenocarcinoma. SRCs may exist alone or coexist with any other types of malignant gastrointestinal tumors. SRCC is very rarely found among carcinomas of the ampulla of Vater. Here, we describe one patient with SRCC in the ampulla of Vater, which was found by contrast-enhanced ultrasound (CEUS). This is the first case reported in literature, which was successfully diagnosed with CEUS.
A 38-year-old woman was hospitalized because of pruritus for 13 d, and dermatic and scleral jaundice with urine the color of bean oil for 5 d. The stool s had a silver color. The patient had nausea but without vomiting, fever and abdominal pain. She lost weight of about 3 kg in 1 mo. She had a history of surgery for left breast adenoma at another institution several years ago.
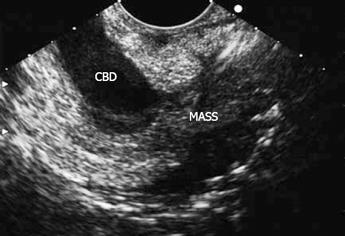
Physical examination revealed mucocutaneous jaundice without tenderness in the epigastrium. The laboratory test results showed that white blood cells and hemoglobin were normal. Biochemical tests demonstrated the presence of glutamate-pyruvate transaminase at 446.5 IU/L (normal range, 0-40), glutamic-oxal (o) acetic transaminase at 277.3 IU/L (normal range, 5-34), alkaline phosphatase at 744.1 IU/L (normal range, 40-150), γ-glutamyltransferase at 1687.2 IU/L (normal range, 9-64), total bilirubin at 186.6 mg/dL (normal range, 3.4-20.5), direct bilirubin at 154.2 mg/dL (normal range, 0-8.6), and indirect bilirubin at 32.4 mg/dL (normal range, 3.4-11.9). The tumor markers of carcinoembryonic antigen were 4.74 ng/mL (normal range, 0-5), alpha fetoprotein 3.87 ng/mL (normal range, 0-9), and carbohydrate antigen 19-9 143.13 ng/mL (normal range, 0-37). Endoscopic ultrasound (Figure 1) showed a heterogenic, hypoechoic mass with ill-defined margins at the junction of the common bile duct (CBD) and the main pancreatic duct (PMD)- ampulla of Vater.
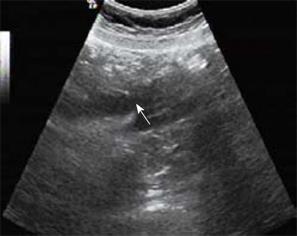
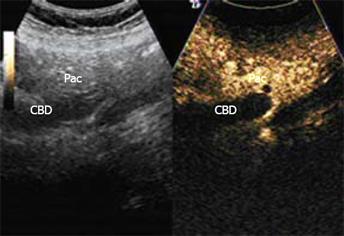
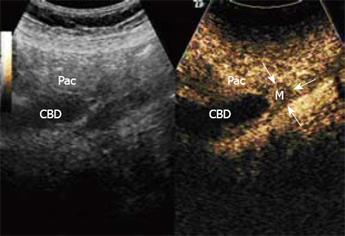
Conventional gray-scale ultrasound using a Logiq 9 scanner (GE, USA) equipped with a C2-4 transducer with a central frequency of 3.5 MHz revealed that the intrahepatic bile duct was dilated with a diameter of 0.8 cm, and the initial and intermediate portion of the CBD were dilated with a maximal diameter of 2.0 cm. The end part of the CBD suddenly became narrow, with a diameter of 0.7 cm, and there was no exact mass at the end of the CBD (Figure 2). CEUS was performed with low acoustic power, providing real-time imaging using low-mechanical index modes. Contrast-specific CEUS mode of contrast pulse sequencing was applied. The contrast agent, SonoVue (Brocca, Milan, Italy) (2.4 mL) was administered. The wall of the CBD began to enhance at 12 s after contrast agent was administered (Figure 3), while there was no obvious hyper-enhanced or hypo-enhanced lesion in the ampulla of Vater. A hypo-enhanced lesion about 1.7 cm × 1.6 cm with blurred borders in the ampulla of Vater was found from 20 s to 180 s, compared with the adjacent pancreas (Figure 4). At delayed phase (120 s after contrast agent administration), we scanned the whole liver and no abnormal enhanced lesions were found, indicating that there was no metastases in the liver.
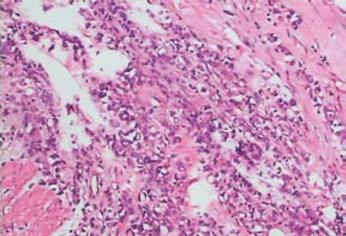
The patient underwent a pancreato-duodenectomy with an extended lymphadenectomy and gastrectomy of 1/4 of the normal stomach. The mass was located in the ampulla of Vater with a size about 2.0 cm × 2.0 cm, brittle and protruding to the cavity of the duodenum. It had infiltrated to the periphery pancreatic tissue and adhered to the inferior vena cava. Lymph nodes of No. 16 were tumescent. The final pathological examination (Figure 5) showed that the cancer cells were widespread and polygonal, and the nuclei of the cells were located on one side, which are prominent signet-ring features. Final pathology confirmed an SRCC of the ampulla of Vater, and the pancreas and the whole wall of the duodenum were infiltrated with carcinoma. No distal or nodal metastases were identified. There was no evidence of lymphatic and vascular invasion. The ampullary cancer was diagnosed as T3N0M0, stage II A according to American Joint Committee on Cancer TNM classification[1] and International Union Against Cancer TNM classification[2].
Our patient did not receive adjuvant therapies such as chemotherapy or radiotherapy after the operation. The patient remained well and had no evidence of recurrence and distant metastases during the 6-mo follow-up.
SRCC can arise in many organs, but it usually occurs in the gastrointestinal tract, especially in the stomach. It has been reported that 90% of SRCC occurs in the stomach, with the rest arising in several other organs, including the breast, gallbladder, pancreas, urinary bladder and colon[3]. It is extremely uncommon in the ampulla of Vater. Less than 15 cases have been described in the literature. Akatsu[4] has summarized the previous 14 cases (eight men and six women) and concluded that the median age at diagnosis was 57 years (range, 32-83 years), approximately 15 years older than SRCC of the stomach, but similar to the median age for SRCC of the large bowel. Our case was a 38-year-old woman. It is very rare[5]. The origin for SRCC of the ampulla of Vater remains controversial; one theory is that these tumors may originate from heterotopic gastric mucosa. Another theory holds that these carcinomas arise from areas of gastric-type metaplastic epithelia, which are considered to be a protective response to elevated acidity and are observable in the duodenal bulb of peptic ulcer patients[6]. However, there was no history of peptic ulcer disease in our patient and no ectopic gastric epithelium was found in the tumor and peritumoral tissues. Surgery is the the first choice for the treatment of such disease. As for the prognosis, poorly differentiated adenocarcinoma is more frequently associated with an advanced tumor stage and poor prognosis in cases of ampullary carcinoma. SRCC elsewhere in the gastrointestinal tract has a poor prognosis. However, it is unclear whether the prognosis of a patient with SRCC is worse than that of patients with ordinary carcinoma occurring in the ampulla of Vater, because of the small number of cases so far reported. A patient has been reported to survive for 7.5 years after radical resection, and the author has suggested that long-term survival is possible in ampullary SRCC without nodal involvement[4]. A 58-year-old patient lived for 134 mo after resection and had no evidence of recurrence[7].
As for diagnosis of SRCC, helical computed tomography (CT) only showed a dilated CBD without a mass lesion in the ampulla of Vater in some cases[57–9]. Upon ultrasound, it may present as an abnormal echoic mass in the ampulla of Vater[6], or obstruction and dilation of the CBD at the level of the pancreas[89]. Our case is unique because we underwent a special examination with CEUS before surgery and found the lesion, which was not discovered by conventional gray-scale ultrasound.
CEUS has gained increasing interest in recent years. The properties of SonoVue and the high sensitivity of recent ultrasound equipment to the presence of microbubbles have shown that CEUS is potentially very useful in revealing many organs and vascular structures. It has been a rapidly evolving technique for clinical application. CEUS allows the assessment of the macrovasculature and microvasculature in different parenchymas, and the identification and characterization of lesions in organs. It has been reportes that CEUS produced results very similar to those obtained with contrast-enhanced CT and magnetic resonance imaging in the characterization of various liver lesions. The causes of obstructive jaundice can be divided into two categories: tumorous and non-malignant stenosis. For non-malignant stenosis, such as acute or chronic inflammation of the papilla, fibroid stenosis at the end of the CBD can be irritated by cholesterol calculi or sludge at the end of the CBD; blood clots at the end of the CBD may cause obstructive jaundice too. However, the main cause of obstructive jaundice is the tumors arising from the ampulla of Vater, and mostly are malignant. In the diagnosis of obstructive jaundice, the emphasis should be laid on excluding the non-malignant reasons: non-shadowing stones, blood clots and sludge. This may influence the selection of therapy. The non-shadowing stones, blood clots and sludge may appear non-enhanced by CEUS because of an absence of blood supply. In the present case, the carcinoma showed iso-enhancement at an early stage after contrast agent administration, and obvious hypo-enhancement at the delayed phase, because it had intravital tissue with blood supply; microbubbles are distributed within the blood and appear wherever there is a blood supply. Our case showed that CEUS may provide an effective means of diagnosis of ampullary carcinomas. CEUS could offer real-time imaging of the microcirculation in the lesions. By CEUS, the lesion may be displayed much clearer than by conventional gray-scale ultrasound. It can also offer a good method in the discrimination of ampullary carcinoma from non-malignant lesions.
| 1. | Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC cancer staging handbook: TNM classification of malignant tumors. 6th ed. Springer-Verlag: New York 2002; 171-177. |
| 2. | Di Giorgio A, Alfieri S, Rotondi F, Prete F, Di Miceli D, Ridolfini MP, Rosa F, Covino M, Doglietto GB. Pancreatoduodenectomy for tumors of Vater's ampulla: report on 94 consecutive patients. World J Surg. 2005;29:513-518. |
| 3. | Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Kikuchi S, Yamauchi H. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998;186:121-130. |
| 4. | Akatsu T, Aiura K, Takahashi S, Kameyama K, Kitajima M, Kitagawa Y. Signet-ring cell carcinoma of the ampulla of Vater: report of a case. Surg Today. 2007;37:1110-1114. |
| 5. | Purohit RC, Kant K, Bhargava N, Kothari N, Purohit V. Signet ring cell carcinoma of ampulla of Vater in a young adult. Indian J Gastroenterol. 2005;24:222-223. |
| 6. | Ramia JM, Mansilla A, Villar J, Muffak K, Garrote D, Ferron JA. Signet-ring-cell carcinoma of the Vater's ampulla. JOP. 2004;5:495-497. |
| 7. | Bloomston M, Walker M, Frankel WL. Radical resection in signet ring carcinoma of the ampulla of Vater: report of an 11-year survivor. Am Surg. 2006;72:193-195. |
| 8. | Li L, Chen QH, Sullivan JD, Breuer FU. Signet-ring cell carcinoma of the ampulla of Vater. Ann Clin Lab Sci. 2004;34:471-475. |
| 9. | Eriguchi N, Aoyagi S, Jimi A. Signet-ring cell carcinoma of the ampulla of Vater: report of a case. Surg Today. 2003;33:467-469. |