Published online Feb 21, 2009. doi: 10.3748/wjg.15.829
Revised: January 4, 2009
Accepted: January 11, 2009
Published online: February 21, 2009
AIM: To retrospectively analyze the imaging features of solid-pseudopapillary tumors (SPTs) of the pancreas on multi-detector row computed tomography (MDCT) and define the imaging findings suggestive of malignant potential.
METHODS: A total of 24 consecutive cases with surgically and pathologically confirmed SPTs of the pancreas underwent preoperative abdominal MDCT studies in our hospital. All axial CT images, CT angiographic images, and coronally and sagittally reformed images were obtained. The images were retrospectively reviewed at interactive picture archiving and communication system workstations.
RESULTS: Of the 24 cases of SPTs, 11 cases (45.8%) occurred in the pancreatic head and seven (29.1%) in the tail. Eighteen were pathologically diagnosed as benign and six as malignant. MDCT diagnosis of SPTs was well correlated with the surgical and pathological results (Kappa = 0.6, P < 0.05). The size of SPTs ranged from 3 to 15 cm (mean, 5.8 cm). When the size of the tumor was greater than 6 cm (including 6 cm), the possibilities of vascular (8 vs 1) and capsular invasion (9 vs 0) increased significantly (P < 0.05). Two pathologically benign cases with vascular invasion and disrupted capsule on MDCT presented with local recurrence and hepatic metastases during follow-up about 1 year after the resection of the primary tumors.
CONCLUSION: Vascular and capsular invasion with superimposed spread into the adjacent pancreatic parenchyma and nearby structures in SPTs of the pancreas can be accurately revealed by MDCT preoperatively. These imaging findings are predictive of the malignant potential associated with the aggressive behavior of the tumor, even in the pathologically benign cases.
- Citation: Wang DB, Wang QB, Chai WM, Chen KM, Deng XX. Imaging features of solid pseudopapillary tumor of the pancreas on multi-detector row computed tomography. World J Gastroenterol 2009; 15(7): 829-835
- URL: https://www.wjgnet.com/1007-9327/full/v15/i7/829.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.829
Solid pseudopapillary tumors (SPTs) of the pancreas are rare and predominantly occur in young women in the second and third decades of life, with only about 8.3% of all cases reported in males[12]. SPT is usually a benign tumor with low-grade malignant potential; however, about 14.7% of cases demonstrate malignant behavior with recurrence and metastasis[3]. In contrast to the ductal adenocarcinoma of the pancreas, complete resection of the tumor could provide a more than 90% cure rate[45].
Currently, the imaging modalities such as computed tomography (CT) including multi-detector row computed tomography (MDCT), magnetic resonance imaging (MRI), and ultrasonography (US) have been used for detection and characterization of pancreatic tumors in clinical practice[67]. It is well known that MDCT can provide more accurate delineation of normal and abnormal pancreatic morphology[6]. Furthermore, MDCT can be performed intrinsically for CT angiography (CTA) scanning, and the CTA images can be conveniently obtained by post-processing techniques at the dedicated workstation. However, to the best of our knowledge, there has been no published literature regarding the imaging features implying the malignant potential of the SPTs of the pancreas on MDCT.
This study aimed to retrospectively analyze the imaging features of SPTs of the pancreas on MDCT, and define the imaging findings suggestive of the malignant potential associated with aggressive behavior.
From June 2001 to June 2008, a total of 24 consecutive patients with surgically and pathologically confirmed SPTs of the pancreas, ranging in age from 11 to 64 years (mean 34.27 years; 21 females, three males), underwent preoperative abdominal MDCT studies in our hospital. Abdominal pain or discomfort was the major indication to undergo the imaging studies (87.5%; 21/24). The youngest female patient (11 years old) and a 21-year-old woman both presented with a big mass in the upper abdomen, while the remaining one demonstrated jaundice and elevation of liver enzymes, suggestive of impairment of liver function. The patients have been followed up for 3 mo to 7 years. Institutional review board approval and waiver of informed consent for this retrospective study were obtained.
All abdominal MDCTs were performed on a 4-slice or 16-slice multi-detector row CT scanner (LightSpeed QX/I or Lightspeed 16; GE Medical Systems, Milwaukee, WI, USA). All axial CT images were obtained during breath-holding before (non-enhanced), 25 s (arterial phase), 60 s (portal phase), and 90 s (hepatic parenchyma phase) after the initial administration of contrast materials. Contrast-enhanced MDCT was performed after the intravenous injection of iohexol (Omnipaque 300; Amersham, Shanghai, China) at a dose of 2 mL/kg body weight through an antecubital vein using a power injector (LF CT 9000; Liebel-Flarsheim, Cincinnati, OH) at a flow rate of 2.5 to 3.0 mL/s. The major scanning parameters were as follows: beam pitch, 1.5; beam collimation, 4-2.5 mm; gantry rotation time, 0.5-0.8 s; section thickness, 3.75 mm; reconstruction interval, 3.75 mm; and table speed, 15.0 mm/rotation. Axial images were reconstructed with a soft tissue algorithm. The CTA and reformed images were obtained using maximum intensity projection (MIP) or multiplanar volume reformation (MPVR) technique on the workstation. Besides MDCT, 15 cases underwent MRI, and 18 cases underwent US.
All images were retrospectively reviewed at interactive picture archiving and communicating system (PACS) workstations by two experienced abdominal radiologists (Wang DB and Chai WM) in consensus. Disputes in readings were resolved through consultation with a third experienced abdominal radiologist (Chen KM). The items included in the imaging analysis were: (1) size, location, and density, as well as the hemodynamics of the tumor; (2) vascular or capsular invasion, invasion of tumor into the adjacent pancreatic parenchyma, and involvement of nearby vessels as well as metastases in lymph nodes and other places in the upper abdomen. All the reviewers were unaware of the pathological nature regarding the benignity and malignancy for each case. Based on the imaging findings of the SPTs of the pancreas on MDCT, each case was given a diagnosis of either benign or malignant SPT.
Histopathological and immunohistochemical studies were performed in all the cases. The diagnosis of SPTs by MDCT was compared with the pathological results.
The Fisher’s exact test and Kappa test were introduced for comparison of imaging diagnosis and pathological results. A P value less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using the SPSS computer software (Version 13.0, Chicago, IL, USA).
Of the 24 cases, 18 were diagnosed as benign SPTs while the other six as malignant on pathological study. The size of SPTs ranged from 3 to 15 cm (mean 5.8 cm). One of the malignant SPTs metastasized to the liver unifocally and the regional lymph nodes. The metastatic lesions were also resected simultaneously during the operation of the primary tumor in the pancreatic head. There were another two cases of malignant SPT with regional lymph node metastasis found in surgery. The involvement of nearby vessels (n = 8, including two benign cases), adjacent organs including spleen (n = 1), duodenum (n = 1), and stomach (n = 1), and abutting pancreatic parenchyma (n = 6), as well as the peripancreatic fat (n = 6), was revealed during the operations (Table 1). One of them had a thrombus in the portal vein. Complete resection was performed for each case in this group.
| Imaging features on MDCT | Surgical and pathological results | |
| Benign (n = 18) | Malignant or potentially malignant (n = 6) | |
| Location | ||
| Head | 8 (33.3) | 3 (12.5) |
| Neck | 1 (4.2) | 0 |
| Body | 2 (8.3) | 1 (4.2) |
| Tail | 5 (20.8) | 2 (8.3) |
| Body-neck | 1 (4.2) | 0 |
| Body-tail | 1 (4.2) | 0 |
| Size | ||
| < 6 cm | 10 (41.7) | 1 (4.2) |
| ≥ 6 cm | 8 (33.3) | 5 (20.8) |
| Density | ||
| Cystic | 5 (20.8) | 0 |
| Solid | 3 (12.5) | 2 (8.3) |
| Solid-cystic | 10 (41.7) | 4 (16.6) |
| With calcifications | 3 (12.5) | 2 (8.3) |
| Capsule | ||
| Intact | 15 (62.5) | 0 |
| Un-intact | 3 (12.5) | 6 (25) |
| Enhancement | ||
| Mild | 6 (25) | 0 |
| Moderate | 9 (37.5) | 3 (12.5) |
| Marked | 3 (12.5) | 3 (12.5) |
| Enhancing pattern and hemodynamics | ||
| Peripheral and persistent enhancement | 17 (70.8) | 6 (25) |
| Central and persistent enhancement | 1 (4.2) | 0 |
| Pancreatic or peripancreatic invasion | ||
| Adjacent pancreatic invasion | 3 (12.5) | 6 (25) |
| Pancreatic duct dilatation | 1 (4.2) | 1 (4.2) |
| Peripancreatic fat invasion | 1 (4.2) | 6 (25) |
| Nearby vessels involvement | 2 (8.4) | 6 (25) |
| Direct invasion into adjacent organs | 0 | 3 (12.5) |
| Regional lymph nodes metastasis | 0 | 3 (12.5) |
| Liver metastasis | 0 | 1 (4.2) |
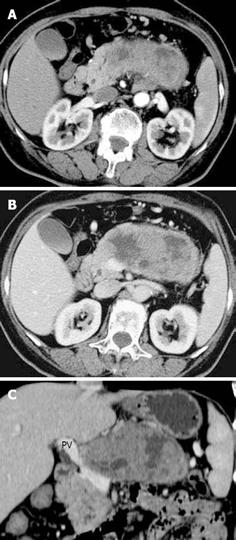
Table 1 summarizes the imaging features of SPTs on MDCT in comparison with pathological outcome. Of the 24 cases of SPTs, 11 (45.8%) occurred in the pancreatic head and seven (29.1%) in the tail (Table 1). The calcifications were identified in five cases (three benign, two malignant). On MDCT, the tumors were divided into three types according to the density: cystic, solid, and solid-cystic (mixed) (Figure 1). The mixed type comprised 58.3% (14/24) of the cases of SPT. Six malignant and three benign cases were identified with a disrupted capsule, whereas the other cases had an intact and smooth capsule. In this group, 50% (12/24) of the SPTs demonstrated moderate enhancement of the tumors after administration of contrast agents. Nine of the 12 cases were benign. However, among the six cases with marked enhancement, three were malignant. Peripheral and persistent enhancement in solid components occurred in 95.8% (23/24) of the cases. The MDCT identified all surgically and pathologically confirmed tumoral invasion into the adjacent structures and organs (spleen, duodenum and stomach) and metastases (lymph nodes, n = 3; liver metastasis, n = 1) in six malignant cases (Table 1). However, the adjacent pancreatic invasion (n = 3), pancreatic duct dilatation (n = 1), peripancreatic fat invasion (n = 1), and nearby vascular involvement (n = 2) in benign cases were also depicted on MDCT. The subsequent pancreaticoduodenectomy operation was performed on four of these cases, and pathological study revealed the benign nature in spite of encasement of the nearby portal vein. Therefore, eight cases were diagnosed as malignant SPTs by MDCT, including six categorized as malignant by pathology (75%; 6/8). Finally, MDCT diagnosis of SPTs was well correlated with the surgical and pathological results (Kappa = 0.6, P < 0.05) (Table 2).
| MDCT | Pathologic results | Total | |
| Malignant or malignant potential | Benign | ||
| Malignant | 5 | 3 | 8 |
| Benign | 1 | 15 | 16 |
| Total | 6 | 18 | 24 |
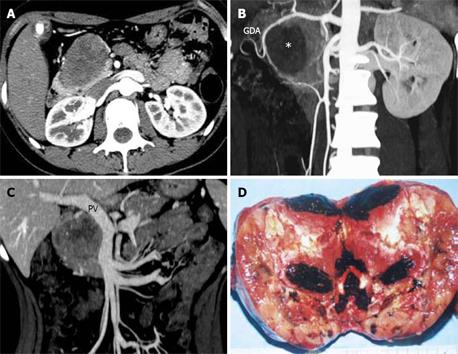
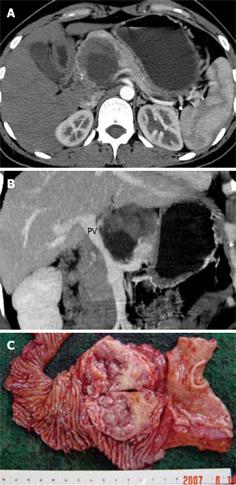
When the size of the tumor was ≥ 6 cm, the possibilities of vascular involvement (8 vs 1) and capsular invasion (9 vs 0) increased significantly (P < 0.05) (Tables 1 and 3). The disrupted capsule detected on MDCT was attributed to the tumoral invasion into adjacent pancreatic parenchyma or peripancreatic structures by pathologically confirmed SPT in this group (Figure 2). Table 4 demonstrates that the portal vein, superior mesenteric vein, and splenic vein were vulnerable to being involved. Two pathologically benign cases with vascular involvement and disrupted capsule on MDCT presented with local recurrence and hepatic metastases during follow-up, about 1 year after resection of the primary tumors (Figure 3).
| Tumor size | Vascular involvement | Metastasis | Capsular invasion | |||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| < 6 cm | 1 | 10 | 0 | 11 | 0 | 6 |
| ≥ 6 cm | 8 | 5 | 13 | 10 | 9 | 4 |
SPT of the pancreas is rare, mainly occurring in young women. It was first described by Frantz in 1959[8] and the tumor was named the Frantz tumor after the author. Over time, it has been designated with various names such as: solid and papillary tumor[9]; papillary cystic tumor[10]; solid-cystic tumor[11]; and solid and papillary epithelial neoplasm[12]. However, these names do not exactly reflect what is present either at the microscopic or macroscopic level. WHO in 1996 proposed the name ‘solid-pseudopapillary tumor’, which can depict two major histological features in the tumor: solid and papillary components[13]. In fact, SPTs have been subdivided by the WHO classification into: solid-papillary neoplasm with borderline malignant potential, and solid-papillary carcinoma[14]. To date, more than 700 cases have been reported in the English literature[15]. About 15% are known to present with metastasis or recurrence[3]. However, based on the conventional histopathology, it has been difficult to establish the criteria that are suggestive of aggressive behavior including recurrence and metastasis[141617].
CT is the imaging modality of choice for detection and characterization of SPTs of the pancreas, while the MRI can be more accurate in differentiating the cystic or solid component inside the tumor[7]. If MRI reveals an encapsulated mass lesion with solid and cystic component as well as hemorrhage without obvious internal septations, SPT of the pancreas should be suspected[7]. According to Yu[7], the MRI findings of SPTs were well correlated with the pathological patterns. Fine needle aspiration biopsy guided by endoscopic ultrasound can help distinguish SPTs from other pancreatic lesions, since the masses are commonly localized in the pancreatic head, thus playing an important role in preoperative planning[18]. Therefore, the imaging modalities are very useful in preoperative assessment of this disease and could provide strong evidence for treatment protocol planning. It is well known that MDCT can provide more accurate delineation of normal and abnormal pancreatic morphology[6]. Furthermore, MDCT can be performed intrinsically for CTA scanning, and the CTA images can be conveniently obtained by post-processing techniques at the dedicated workstation, meanwhile, the coronal and sagittal images can be reformed using axial source images. Thus, axial CT and CTA images can be simultaneously obtained during a single scanning.
The SPT is characterized with imaging features of a well-circumscribed mass, which is surrounded by a clear-depicted peritumoral capsule, and vulnerable to hemorrhage and cystic changes, resulting in the heterogeneously cystic central component and solid periphery. The overview of the clinicopathological characteristics of the SPTs in our series (Table 1) is almost identical to the data reported in the literature[16]. Our MDCT images demonstrated that peripheral and persistent enhancement in solid components occurred in 95.8% (23/24) of all cases. As is well documented in the literature[19], the enhancement of SPTs was not significant in the adjacent pancreatic parenchyma. However, 50% (3/6) of cases in this group with marked enhancement turned out to be malignant. It seems that the hypervascular feature on MDCT could imply an aggressive nature, since half of the malignant cases were categorized as having marked enhancement. Further study is needed to confirm this.
The accuracy of MDCT diagnosis of SPTs was well correlated with the surgical and pathological results (Kappa = 0.6, P < 0.05) (Table 2). As a matter of fact, some of the pathologically benign cases with local invasion detected by MDCT in this group might turn out to be malignant during follow-up, as was reported in two cases herein. Although the axial and coronal reformed images, as well as CTA images, could reveal the aggressive behavior of SPTs, the combination of all these images seems to add value to the assessment of the status of tumoral invasion into the adjacent major vasculature (Table 4). However, statistical analysis was not carried out for the capability of identification of vascular invasion by different images, since the limited number of cases included would bias the results.
In this group, when the mass was more than 6 cm (including 6 cm) in diameter, the tumor was more vulnerable to having aggressive behavior such as metastasis and recurrence. The mean mass size in our series was 5.8 cm. When we set up the threshold of tumor size at 6 cm, the nearby vascular involvement (8 vs 1) and peritumoral capsular invasion (9 vs 0) were more common in SPTs bigger than 6 cm (including 6 cm), which indicated the biologically aggressive nature revealed by MDCT (P < 0.05, Table 3). Interestingly, if the mass was smaller than 6 cm, there was no case categorized as having capsular invasion in this group, which may be wrong if the same size threshold were applied to other groups, since different imaging modalities were employed. As a result of invasion beyond the capsule, invasion into the adjacent pancreatic parenchyma was identified in nine cases, including six malignant cases. Two of the three cases in this settings regarded as benign pathologically had aggressive behavior of vascular involvement and capsular invasion on MDCT, and subsequently presented with local recurrence and hepatic metastasis during follow-up after surgery of the primary tumor. In this context, the findings on MDCT could predict the possible aggressive nature of SPTs of the pancreas, based on our data. Complete surgical resection can be proposed for SPTs and the prognosis is supposed to be excellent[15]. Up to the time of writing, no patient died during the follow-up in our group.
In conclusion, based on our SPT series, local invasion, including vascular and capsular invasion with superimposed spread into the adjacent pancreatic parenchyma and peripancreatic structures and organs, can be accurately depicted by MDCT preoperatively. The findings suggestive of malignancy on MDCT were well correlated with aggressive behavior of the tumor, even in the pathologically benign cases. Therefore, the value of imaging features implying aggressive behavior of SPTs needs to be emphasized; it seems that the imaging findings are predictive of the malignant potential associated with aggressive nature, and are probably beneficial to the patient’s surgical protocol planning.
The solid-pseudopapillary tumor (SPT) of the pancreas is rare, mainly occurring in young women. To date, more than 700 cases have been reported in the English literature. About 15% are known to present with metastasis or recurrence. However, based on conventional histopathology, it has been difficult to establish the criteria that are suggestive of the aggressive behavior including recurrence and metastasis.
No pathological factors including mitotic rate, nuclear pleomorphism and vascular invasion were found to correlate with the prognosis of SPT. Also, the histopathological features suggestive of malignant potential were non-specific. Aggressive behavior may not be completely excluded, even in the absence of pathological features suggesting malignant potential. CT is the imaging modality of choice for detection and characterization of SPTs of the pancreas while MRI can be more accurate in differentiating the cystic from solid component inside the tumor.
To the best of our knowledge, there is no published literature regarding the imaging features implying the malignant potential of SPTs of the pancreas on multi-detector row computed tomography (MDCT). In our series, when the size of the tumor was greater than 6 cm (including 6 cm), the possibilities of vascular (8 vs 1) and capsular invasion (9 vs 0) increased significantly (P < 0.05). Two pathologically benign cases with vascular invasion and disrupted capsule on MDCT presented with local recurrence and hepatic metastases during follow-up, about 1 year after resection of the primary tumors. Vascular and capsular invasion with superimposed spread into the adjacent pancreatic parenchyma and nearby structures in SPTs of the pancreas could be accurately revealed by MDCT preoperatively. These imaging findings could be predictive of the malignant potential associated with aggressive behavior of the tumor, even in the pathologically benign cases.
The findings suggestive of the malignancy on MDCT are well correlated with aggressive behavior of the tumor, even in the pathologically benign cases. Therefore, the value of imaging features implying aggressive behavior of SPTs needs to be emphasized. It seems that the imaging findings are predictive of the malignant potential associated with aggressive nature and are beneficial to the patient’s surgical protocol planning.
“Solid pseudopapillary”: Encompasses the two most conspicuous histological features, which are also depicted macroscopically, the cystic center and the solid periphery of the mass. “MDCT”: Multiple detectors applied to CT. This modality can improve the scanning speed and spatial resolution dramatically. Furthermore, MDCT can be performed intrinsically for CT angiography scanning. “Aggressive behavior”: The tumor with aggressive behavior means that it has local invasion, local recurrence, or metastasis.
This is a retrospective study of the radiological features of SPT upon MDCT in 24 consecutive cases. Radiological changes have been correlated with operative and pathological findings. These imaging findings could be predictive of the malignant potential associated with aggressive behavior of the tumor, even in pathologically benign cases.
| 1. | Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002;9:35-40. |
| 2. | Tien YW, Ser KH, Hu RH, Lee CY, Jeng YM, Lee PH. Solid pseudopapillary neoplasms of the pancreas: is there a pathologic basis for the observed gender differences in incidence? Surgery. 2005;137:591-596. |
| 3. | Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005;29:512-519. |
| 4. | Moholkar S, Sebire NJ, Roebuck DJ. Solid-pseudopapillary neoplasm of the pancreas: radiological-pathological correlation. Pediatr Radiol. 2005;35:819-822. |
| 5. | Klimstra DS, Wenig BM, Heffess CS. Solid-pseudopapillary tumor of the pancreas: a typically cystic carcinoma of low malignant potential. Semin Diagn Pathol. 2000;17:66-80. |
| 6. | Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB Jr. MDCT in Pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol. 2004;182:419-425. |
| 7. | Yu CC, Tseng JH, Yeh CN, Hwang TL, Jan YY. Clinicopathological study of solid and pseudopapillary tumor of pancreas: emphasis on magnetic resonance imaging findings. World J Gastroenterol. 2007;13:1811-1815. |
| 8. | Frantz VK. Tumors of the pancreas. Atlas of Tumor Pathology, 1st series. Washington DC: US Armed Forces Institute of Pathology 1959; 32-33. |
| 9. | Kuo TT, Su IJ, Chien CH. Solid and papillary neoplasm of the pancreas. Report of three cases from Taiwan. Cancer. 1984;54:1469-1474. |
| 10. | Boor PJ, Swanson MR. Papillary-cystic neoplasm of the pancreas. Am J Surg Pathol. 1979;3:69-75. |
| 11. | von Herbay A, Sieg B, Otto HF. Solid-cystic tumour of the pancreas. An endocrine neoplasm? Virchows Arch A Pathol Anat Histopathol. 1990;416:535-538. |
| 12. | Balthazar EJ, Subramanyam BR, Lefleur RS, Barone CM. Solid and papillary epithelial neoplasm of the pancreas. Radiographic, CT, sonographic, and angiographic features. Radiology. 1984;150:39-40. |
| 13. | Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. Histological typing of tumors of the exocrine pancreas. International Histological Classification of Tumours, 2nd ed. Springer: New York 1996; 120-128. |
| 14. | Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP. 2006;7:131-136. |
| 15. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. |
| 16. | Kang CM, Kim KS, Choi JS, Kim H, Lee WJ, Kim BR. Solid pseudopapillary tumor of the pancreas suggesting malignant potential. Pancreas. 2006;32:276-280. |
| 17. | Shaikh S, Arya S, Ramadwar M, Barreto SG, Shukla PJ, Shrikhande SV. Three cases of unusual solid pseudopapillary tumors. Can radiology and histology aid decision-making? JOP. 2008;9:150-159. |
| 18. | Nadler EP, Novikov A, Landzberg BR, Pochapin MB, Centeno B, Fahey TJ, Spigland N. The use of endoscopic ultrasound in the diagnosis of solid pseudopapillary tumors of the pancreas in children. J Pediatr Surg. 2002;37:1370-1373. |