CASE REPORT
A 59-year-old Chinese woman who was previously healthy presented to our clinic with a 2-mo history of intermittent abdominal pain accompanying general fatigue, subjective low-grade fever, weight loss and night sweats, which were not improved after treatment with over-the-counter antibiotics and traditional Chinese herbal drugs. Ten days before she visited our clinic, she felt her symptoms getting worse with nausea and vomiting, and weight loss of 10 kg within 2 mo. Thereafter, she failed to pass gas or stools which prompted her to seek medical care. She was admitted to 263 Hospital of PLA. A biochemical test showed 6500/mL white blood cells (WBCs), 78% N, 90 g/L hemoglobin, normal tumor markers (including carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, CA125, alpha fetoprotein (AFP), erythrocyte sedimentation rate (ESR) 148 mm/h), and 135.8 mg/L CRP. Abdominal plain film displayed ascites and incomplete intestinal obstruction. Chest X-ray showed a high density of WBCs in the right middle outer lobe and lymph node calcification in the right trachea bifurcation. Abdominal paracentesis was arranged for further evaluation. Routine test of ascites showed that yellow fluid was translucent, 95% mononuclear cells, 5% polynuclear cells, 6400/&mgr;L erythrocytes and 210/&mgr;L WBCs, and 1.010 gravity. Protein concentration was 44.7 g/L, glucose was 1.82 mmoL/L, lactate dehydrogenase was 334 U/L, and adenosine deaminase was 15 U/L. Acid-fast bacillus could not be found in the fluid. Culture of bacteria was negative. Cytology of the fluid was also negative. CEA and AFP of ascites were normal. Ten days after treatment with antibiotics, her abdominal pain was aggravated, and she was transferred to our hospital.
The patient was a cotton spinner prior to retirement and underwent appendectomy 30 years ago. She denied any history of hepatitis or alcohol, tobacco or recreational drug use and had no knowledge of sick contacts. Physical examination showed symptoms of tenacious abdominal wall, deep and rebound tenderness, decreased intestinal sound, but no actual palpable abdominal mass. Liver and spleen were not palpable, shifting dullness was negative.
After admission, tuberculin was positive. Ultrasonography and pelvic cavity computed tomography (CT) showed ascites and mesentery thickening and peritonitis could not be excluded. Blood test results were 100/mL WBCs, 83.8% N, 85 g/L hemoglobin, and 545000/mL platelets. Routine urine and feces tests were normal. Feces occult blood tests was negative (140 mm/h ESR, 218 mg/L CRP, and 29.6 g/L albumin). Two-thirds of anti-tuberculosis test parameters were positive. Tumor markers (CEA, CA199, CA125, and AFP) were normal. CA, CYFRA, FRER, and TSGF were 153100 U/mL, 21-1 16.44 ng/mL, > 2000 ng/mL, and 121.45 U/mL, respectively. LTA was positive. Autoimmune antibodies were negative. Abdominal ultrasound showed small encapsulated effusion in abdominal and pelvic cavity. Parietal peritoneum was thick. Ultrasound of the vagina showed metratrophia with no occupying lesions in the pelvic cavity. Chest X-ray showed old tuberculous foci on both lungs and mediastinum. Due to the suspicious appearance of the lesion, a positron emission tomography (PET) scan was performed along with induced peritoneal cavity CT. Diagnostic percutaneous needle biopsy was discussed with the patient and her family, but she denied any invasive tests. PET scan showed an increased metabolic activity in peritoneum, mesentery, and areas of omentum with thick and diffusive lesser tubercles. Local intestinal adhesions or distensions showed incomplete intestinal obstruction.
She began to cough and had a low fever, but biochemical tests could not identify any infectious bacteria in her sputum and feces. Since the patient remained symptomatic, we decided to treat her disease with oral isoniazid, rifabutin, ethambutol, and pyrazinamide. During the first 6 wk, she noticed significant clinical improvements, such as reduction in abdominal pain and ascites, and resolution of tenacious abdominal wall. Body temperature became normal. However, 8 wk after antibiotic therapy, as well as enteral and parenteral nutrition, her condition began to deteriorate. In addition to CT and ultrasonography, the patient underwent percutaneous CT-guided biopsy of the thick peritoneum, which revealed primary papillary serous carcinoma of the peritoneum (PSCP). Although subsequent staging suggested that it was amenable to chemotherapy, the patient was too weak to tolerate the treatment. Finally, therapy failed to improve her condition and she developed liver and kidney failure.
DISCUSSION
The main differential diagnostic considerations of diffuse peritoneal involvement associated with peritonitis include infectious processes (mainly tuberculosis) and malignant neoplastic conditions. Peritoneal involvement is a rare form of abdominal tuberculosis. Peritoneal tuberculosis occurs predominantly in patients aged 20-40 years and is always secondary to other tuberculous lesions and appears to be more common in females than in males. Tuberculosis in females commonly reaches the peritoneum through tubal infection and attacks the tubes during the sexually active period of life[1]. Diagnosis of any extrapulmonary forms of tuberculosis is quite difficult. For example, difficult diagnosis of peritoneal tuberculosis is due to its non-specific clinical manifestations, such as weight loss, abdominal pain, fever, ascites, and vomiting[23]. Positive culture of 7.8% Mycobacterium. tuberculosis has been reported[4]. Although our patient denied previous medical history of pulmonary or extra-pulmonary tuberculosis, tuberculous infection was diagnosed based on her positive tuberculin test and chest X-ray scan.
PSCP is a rare malignancy that predominantly affects postmenopausal women[5]. Reports suggest that approximately 10% of women diagnosed with ovarian, endometrial or sigmoid carcinoma actually have PSCP[6–8]. Multicentric peritoneal involvement is typical, and omental involvement is particularly common. Extensive calcification of omental caking present in many cases is a useful CT finding for excluding mesothelioma. The absence of ovarian mass is critical for excluding metastatic papillary serous ovarian carcinoma, which otherwise has a similar appearance at CT and is histologically identical to its primary peritoneal counterpart[9]. Some reports indicate a poor prognosis for women with peritoneal carcinomatosis[10–13]. Patients suffering from PSCP usually complain of abdominal distention, pain or pressure, associated with ascites and gastrointestinal symptoms (loss of appetite, nausea, vomiting, and change in bowel habits)[14]. On physical examination, the most common finding is ascites. The clinical presentation is usually indistinguishable from advanced ovarian cancer. Reports suggest that approximately 10% of women diagnosed with ovarian carcinoma actually have PSCP[15].
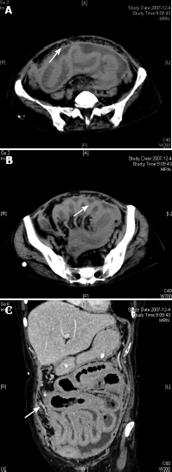
In this case, a moderate amount of ascites located between intestinal canals was observed by ultrasonography, and a thickened intestinal wall and pronounced enhancement of peritoneum were seen at CT scanning. Most nodules coalesced to form large omental plaques (omental cakes) (Figure 1). The largest plaque was located in the left lower quadrant of the abdomen, and extended to the pelvis, but did not involve the ovary. There was no calcification within the masses. The size of ovaries was normal. After treatment with anti-tuberculous drugs, the ascites decreased. Two months later, ascites stopped decreasing, suggesting that tissue biopsy is necessary to help its diagnosis. A delayed diagnosis or inadequate treatment, may promote progression to the malignant disease and the risk of life-threatening complications. To the best of our knowledge, this is the first report on the coexistence of PSCP and tuberculous peritonitis in humans.
Figure 1 CT showing pronounced contrast enhancement of peritoneum and thickened intestinal wall.
A: Most nodules coalesced to form large omental plaques (omental cakes); B: A moderate amount of ascites located between intestinal canals; C: No actual large abdominal masses.