INTRODUCTION
Pneumatosis intestinalis (PI) is a rare disorder characterized by multilocular gas-filled cysts localized in the submucosa and subserosa of the alimentary tract. It can occur in any part of the gastrointestinal tract (GI), from the esophagus to the rectum, but the small intestine is the most common localization[1–5]. PI may be primarily and secondarily associated with a coexisting disease. Eighty-five percent of patients have secondary PI[167]. Gastric PI, defined as air within the wall of the stomach, is an uncommon localization[8–12]. On the other hand, gastric PI, secondary to malignancy, has been very rarely reported in the literature[13]. We described here a case of gastric PI associated with adenocarcinoma localized in duodenum.
CASE REPORT
A 94-year-old man was admitted to our hospital with anorexia, nausea, vomiting, fatigue, constipation, weight loss, abdominal bloating and discomfort which started 1 mo previously, in May 2003. He had a prior history of peptic ulcer 15 years ago. He reported a history of upper GI bleeding in 1990. He had no the other systemic diseases, such as chronic obstructive lung diseases, connective tissue diseases, and no history of abdominal surgery. His family history was not contributory. On physical examination, he was dehydrated and afebrile. His blood pressure was 100/80 mm/Hg, pulse was 84/min, and heart sounds and jugular venous pressure were normal. No murmur was detected. Breath sounds were normal and there were no organomegaly or lympadenomegaly. Epigastric and periumblical sensitivity were noted. There was an abdominal distension and hypertympanism found on epigastric sites.
Initial laboratory results were as follows: blood urea nitrogen 23 mg/dL, creatinine 1.1 mg/dL, sodium 140 mEq/L, potassium 3.8 mEq/L, calcium 9.3 mg/dL, alanine aminotransferase 33 U/L, aspartate aminotransferase 27 U/L, γ- glutamyltransferase 428 U/L (N:7-49) alkaline phosphatase 317 U/L (N:38-155), lactate dehydrogenase 412 IU/L, glucose 105 mg/dL, erythrocyte sedimentation rate 40 mm/h, C-reactive protein 136.4 mg/L, total bilirubin 0.78 mg/dL, direct bilirubin 0.13 mg/dL, white blood cells (WBCs) 12 400/mm3, platelets 217 000/mm3, hematocrit 36.3%, mean corpuscular volume 84.9 fL, Fe 47 &mgr;/dL, total iron binding capacity 214 &mgr;/dL, and ferritin 55.4 ng/mL. Serum protein electrophoresis was normal. The patient’s tumor marker profile was as follows: carbohydrate antigen 19-9 903.1 U/mL (0-37), carcinoembryonic antigen 12.3 ng/mL (0-3.4). Urine analysis revealed a urine specific gravity of 1010, protein negative, pH 5, and two WBCs per high-power field on urine sediment. Hepatitis B and C serology, and anti-HIV assays were negative. Fecal occult blood test was positive for the one stool sample. Other laboratory values were within normal limits.
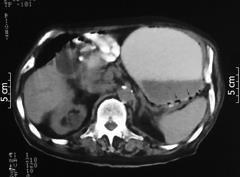
The patient was treated with nasogastric intubation, parenteral fluids, ranitidine i.v. and broad-spectrum antibiotics. Because of elevated tumor markers, a diagnostic work-up for possible malignancy was initiated. The plain abdominal radiography (PAR) was normal. Abdominal ultrasonography (US) revealed sludge within the lumen of the gall bladder with findings of hydrops. The intrahepatic bile ducts were dilated (right, 5.3 mm; left, 4.5 mm). Abdomen and pelvic CT scans showed an enlarged stomach and multilocular gas-filled cysts localized in the wall of the stomach (Figure 1). The diagnosis of PI was made. Subsequent magnetic resonance cholangiopancreatography imaging demonstrated sludge with findings of hydrops and dilatation of intrahepatic bile ducts and choledochus. There was a calculius of 2 cm diameter at the neck of gall bladder.
Figure 1 Abdomino-pelvic CT scan showing big stomach and multilocular gas within the wall of the stomach (arrows).
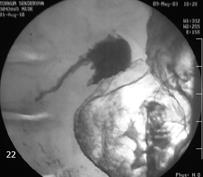
A barium-enema study revealed luminal obstruction of 80% (Figure 2). As a result of these findings, gastroduodenoscopy and biopsy were performed. In the antrum, the ulcer had a 4-5 cm diameter, likely a malignant ulcer, and it was established that the lesion obstructed the lumen in the postbulbar duodenum. Malignancy was not detected in biopsy specimens. After the patient’s general condition markedly improved, exploratory laporatomy was performed in the department of general surgery. An inoperable tumor originating from the duodenum was detected. In addition, the tumor was infiltrated to extra-hepatic bile ducts and vascular structures. Gastrojejunostomy and choledochojejunostomy were carried out. The histopathological examination of biopsy specimens revealed adenocarcinoma infiltration originating from the GI tract. As a result of his age and performance status, we decided to treat with supportive management. Following the surgical procedure, the gas-filled cysts disappeared and his symptoms improved. After 6 mo of follow-up, he died from the the influence of malignancy and poor general condition.
Figure 2 Barium-enema study showing luminal obstruction of 80% in the bulber apex.
DISCUSSION
PI is an uncommon entity in which gas-filled lesions are accumulated within the GI wall. In 42% of the cases with PI, the small intestine is the most common and the large intestine (36%) the second most common localization; in 22% of patients both small and large intestine are affected[126]. On the other hand, gastric PI has been rarely documented[8–12]. It can be classified as either idiopathic or of primary unknown etiology (15%) or the secondary type (85%)[167]. Fifty-five percent of secondary causes are peptic ulcer related to pyloric obstruction, and 3.3% of cases are GI malignancy[2]. In our patient, the gastric secondary type of PI associated with GI malignancy was detected. Gastric PI related to malignancy has been documented extremely rarely[13].
Although the pathogenesis of PI is not well understood, many theories have been proposed. First, the mechanical theory suggests that gas under pressure diffuses into the bowel wall from the intestinal lumen or the pulmonary airway. This theory is probably related to PI caused by trauma, surgery, endoscopy and bowel obstruction. The bacterial theory proposes that bacteria enter the bowel wall and produce gas within the intestinal wall[314]. The pulmonary theory proposes that alveolar rupture results in the diffusion of air through the mediastinum into the retroperitoneal space, through the diaphragm, along major vessels in the mesentery, and into the bowel wall. It may explain the occurrence of PI in patients with chronic obstructive pulmonary disease. Finally, there is the mucosal damage theory. It suggests that gas under pressure is forced into the bowel wall by mucosal disruption. PI associated with peptic ulcer disease, pyloric stenosis and malignancy may probably be explained by this theory[23].
PI is usually asymptomatic, but patients can have gastrointestinal symptoms variying in severity, such as abdominal discomfort, distension and pain, rectal bleeding, constipation, meteorism and weight loss. The symptoms are dependent on the localization of PI and on the presence or non-presence of underlying disease[236]. Our patient also presented with gastrointestinal symptoms and elevated tumor markers. After PI was detected, we initialy started a diagnostic work-up to exclude malignancy as a cause of PI. The work-up revealed an inoperable adenocarcinoma originating from the duodenum. After gastrojejunostomy and choledochojejunostomy, the gas-filled cysts disappeared and the patient improved.
In two-thirds of patients with PI, PAR shows characteristic changes in the bowel. The PAR with our patient was normal, but an abdomino-pelvic CT scan revealed PI. In the literature, additional diagnostic procedures, such as US, CT, MRI, endoscopy and barium contrast studies, have been documented[125]. There is no special treatment for PI. If underlying diseases are present, it is necessary to treat them[6]. Conservative therapy can be causal or symptomatic. Causal treatments consist of normobaric or hyperbaric oxygen therapy, antibiotic treatment, especially metronidazole, endoscopic puncture and cysts sclerotherapy[615–17]. The aim of these therapies is to suppress the etiological mechanisms. Surgical treatments should be avoided unless there are serious diseases, such as malignancy, metabolic acidosis, portal venous gas and severe inflammation[18].
This report constitutes a rare case of gastric PI caused by duodenal adenocarcinoma. In patients with PI, especially localized in the stomach, who have serious GI symptoms and elevated tumor markers, malignancy-induced PI should be considered in the differential diagnosis.