INTRODUCTION
Liver transplantation (LT) in patients with end-stage liver diseases has become an accepted treatment. Advances in the field of percutaneous, radiological, minimally invasive techniques have increased the importance of interventional radiology in the management of patients after LT[1]. In this article, we discuss the possible applications of interventional radiology in the management of adult recipients after deceased donor LT or living related LT (LRLT), including diagnosis of graft disease, treatment of vascular complications and treatment of biliary complications. Techniques used, results and possible complications of interventional radiology procedures are described by reviewing our experience and other protocols present in literature.
DIAGNOSIS OF GRAFT DISEASE
Random liver biopsy is frequently requested after LT. Any alteration of liver function tests, not explained by diagnostic imaging, requires a liver biopsy to exclude rejection and/or other pathologies. Liver biopsy can be performed by a percutaneous approach (blind or ultrasound guided) or, in selected patients, with a transjugular approach. In our practice, in patients without ascites, percutaneous core liver biopsies are performed with an 18-Ga needle under ultrasound guide to avoid entering the bowel or other adjacent organs, and to avoid the perforation of main intra-hepatic vascular structures. If coagulation defects are present (platelets < 50 000 mm3 and/or prothrombin activity < 50%) patients receive infusion of platelets and/or fresh frozen plasma. No antibiotic prophylaxis is performed before the procedure. Complications are infrequent, most of them being minor (pain, decrease in hematocrit value not necessitating treatment). Possible major complications of percutaneous liver biopsies are bleeding, emobilia, arterio-portal fistula and infections, and are reported in up to 3% of cases[2]. Controlled studies have shown that blind percutaneous biopsy carries a higher risk for major complications compared to ultrasound guided liver biopsy[3]. Although it has been reported by Little et al[2] that the presence of perihepatic ascites does not statistically affect the major or minor complications rate of image-guided percutaneous hepatic biopsy, in our center, the transjugular approach is preferred if perihepatic ascites are present. The transjugular approach is considered mandatory if a severe coagulopathy and/or massive amounts of perihepatic ascites are present. This technique reduces the risk of hemorrhage, because a biopsy specimen is acquired through the hepatic vein and any bleeding from the puncture site remains in the vascular space. In addition, if a clinical suspicion of portal hypertension is present, hepatic vein pressure gradient (HVPG) can be measured during the same procedure. The right internal jugular vein approach is usually preferred, but in selected cases, if the right jugular vein is not usable (thrombosis or difficult catheterization), the left internal jugular vein can be used[4]. In our experience, there are no major complications related to transjugular liver biopsies performed in adult liver transplant recipients. Despite transjugular liver biopsy being effective and safe for patients with contraindications to percutaneous liver biopsy, in patients with small liver or in patient with partial LT (from a living donor or deceased donor), subcapsular or intraperitoneal bleeding due to accidental perforation of the capsule is possible. Hemobilia, accidental puncture of kidney, transient dysrhythmias, and hematoma at the puncture site are the most common complications reported in other studies[5–7].
TREATMENT OF VASCULAR COMPLICATIONS
Vascular complications following LT are associated with high morbidity, graft lost and mortality rate. The majority of vascular complications develop within 3 mo of the transplant and possible complications should be considered in any LT patient with alteration of liver function tests. The clinical presentation of vascular complications is often indistinguishable from other post-transplantation complications (biliary complications, rejection, graft dysfunction, infections). Color Doppler ultrasonography (US), multidetector-row computed tomography (MDCT), and magnetic resonance are useful for diagnosis. Vascular complications can affect the hepatic artery, hepatic vein, portal vein and inferior vena cava (IVC).
Hepatic artery thrombosis (HAT)
HAT is a dramatic, potentially life-threatening, complication of LT occurring in 3%-9% of adult liver-transplanted patients[89]. Risk factors for HAT are considered to be surgical technique, small donor vessels, slow flow secondary to hepatic artery stenosis (HAS), ischemia-reperfusion injury, coagulation abnormalities, ABO blood group incompatible transplantation, use of aortic jumping graft and multiple rejection episodes. Ischemia caused by HAT results in severe biliary and parenchyma damage and is associated with high rates of graft loss and mortality. Urgent thrombectomy and revascularization or re-transplantation is currently considered the treatment of choice in case of early diagnosis of HAT[10]. If Doppler US and/or computed tomograghy (CT) suspicious of HAT are present, an arteriogram is usually performed to confirm the imaging finding and, in very early diagnosis of HAT, to try to restore the hepatic flow with selective thrombolytic therapy and eventual treatment of concomitant HAS with balloon angioplasty and/or stent placement. In our practice, selective catheterization of the hepatic artery stump is performed with a microcatheter and an infusion of thrombolytic drugs. In selected patients with concomitant steal syndrome from splenic and/or gastroduodenal arteries, percutaneous splenic and/or gastroduodenal artery embolization can be performed in the same session in order to increase the flow in the hepatic artery (Figure 1). Surgical thrombectomy and/or re-transplantation should be reserved for cases in which percutaneous techniques fail. Saad et al[11] reported successful in re-establishment of arterial flow and uncovered underlying arterial anatomical defects in four out of five patients treated, but none were treated definitively by endoluminal procedures, due to the inability to resolve the underlying arterial stenosis, showing that the treatment of the underlying arterial defect is mandatory. Several cases of successful thrombolytic treatment of HAT in adult transplanted recipients are reported, usually if the diagnosis of HAT is performed a few hours after LT[12–15], but further analysis is needed to understand the correct timing of a possible thrombolysis and if endovascular procedures can be safely considered, a viable option to prevent re-transplantation after HAT. Possible complications of endovascular therapies are dissection and/or rupture of the hepatic artery during the arterial manipulation (requiring the placement of a covered stent) and bleeding from the arterial anastomosis[16].
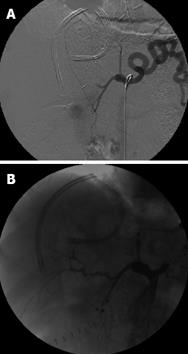
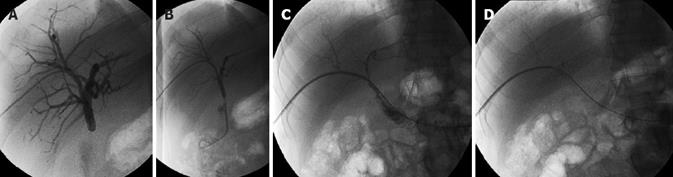
Figure 1 Status post right lobe LRLT in 18-year-old male.
A: Celiac arteriogram showed acute HAT. Local thrombolysis with TPA was performed by placing a microcatheter in the hepatic artery stump; B: Celiac arteriogram after TPA infusion showed patent hepatic artery. In this patient, splenic artery embolization was performed by coils and PVA in order to increase the flow in hepatic artery. Although a good and early flow restoration was achieved, the patient underwent re-transplantation 2 d later for graft failure.
Hepatic artery stenosis (HAS)
HAS is an insidious complication of LT occurring in approximately 5% of patients[17] leading to graft ischemia and possible hepatic artery occlusion as a result of slow flow with high incidence of morbidity and mortality due to hepatic insufficiency, biliary damage and possible sepsis. The majority of HAS arises at the anastomosis site and usually occurs within 3 mo after transplantation. The etiology of HAS can be due to small caliber of arteries or vascular clamp injury. Non-anastomotic stenosis can be present in cases of rejection or hepatic necrosis. The most common complication seen on cholangiography of recipients with HAS is non-anastomotic biliary strictures (BSs) seen in up to 49% of patients[18]. Early detection of HAS is fundamental because many of the stenoses are suitable for percutaneous treatment with angioplasty and/or stent placement or surgical revision, allowing good long-term graft function. In adult recipients with HAS that underwent percutaneous transluminal angioplasty, a 60%-80% patency rate at 1 year is reported[19]. Percutaneous transluminal angioplasty has also been reported to be an effective treatment of HAS after living donor LT, with a success rate of 94% and a complication rate of 6%, with possible HAS recurrence in 33% of patients[20]. In our practice, in a case of suspicious Doppler US or MDCT scan of HAS, a hepatic arteriogram is performed, from a transfemoral approach, with a 5F Cobra 2 or SOS catheter. A coaxial microcatheter is then advanced through the stenosis and the trans-stenotic pressure gradient measured. If a significant pressure gradient is present (> 10 mmHg) then an angioplasty is performed. Before angioplasty, 0.2 mg of nitroglycerine and 2000 UI of heparin are infused into the hepatic artery to reduce the risk of spasm or thrombosis. A 6F guiding catheter is advanced and a balloon catheter advanced over a 0.018 inch or 0.014 inch stiff wire. The diameter of the balloon used varies according to the diameter of the hepatic artery, ranging from 3 to 6 mm. Procedural success is determined by reduction or absence of the stenosis in a final arteriogram with significant reduction of the trans-stenotic pressure gradient (Figure 2). If a good patency is not restored, a metallic stent is deployed. The use of low-profile coronary stents in the treatment of HAS, as a first therapeutic approach, also showed good results with a 1-year patency rate of 45%-53%[2122].
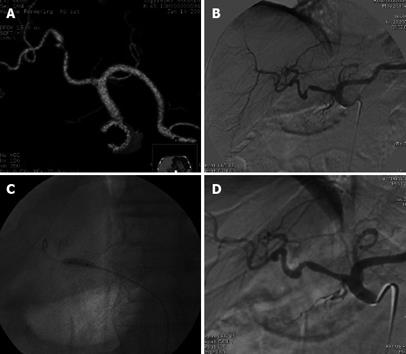
Figure 2 Status post deceased donor LT.
Doppler US performed 5 mo after the transplant showed low intrahepatic resistive index 0.50 and prolonged systolic acceleration time 0.113 s, suspected for HAS. A: MDCT volume rendering 3D reconstruction showed a severe stenosis in the hepatic artery anastomosis; B: Digital subtraction angiography (DSA) celiac arteriogram confirmed the stenosis, trans-stenotic pressure gradient measured by a microcatheter was 50 mmHg; C: DSA percutaneous transluminal angioplasty performed by 4 mm diameter balloon catheter; D: DSA final arteriogram showed good patency of the arterial anastomosis with trans-stenotic pressure gradient reduced to 4 mmHg. Doppler US performed 4 mo later showed regular resistive index 0.70 and systolic acceleration time 0.100 s. Patient currently in good general condition and without biliary tree impairment after 6 mo of follow-up.
Portal vein stenosis (PVS)
PVS is a postoperative complication reported in 3% of patients after LT[23]. Clinical symptoms of hemodynamically significant PVS are related to portal hypertension and are bleeding from varices, splenomegaly and ascites. The portal vein is usually accessed by a transhepatic approach or by a transjugular approach. Percutaneous transhepatic angioplasty is considered an effective treatment and is usually considered as a first, non-surgical, therapeutic approach. Shibata et al[24] in a large series of patients, reported a success rate of 74% with a single session of balloon dilatation and a mean follow up of 24 mo. Recurrent stenoses were detected in 28% of patients and a maximum of three sessions of dilatation were necessary to resolve the stenosis. Funaki et al[25] reported metallic stent placement to treat recurrent and/or non-responsive, elastic stenosis with good long-term patency. Ko et al[26] reported a series of patients following living donor LT with early occurrence of PVS that were treated with transhepatic primary stent placement and showed good patency of the stents after a mean follow up of 66 mo in six out of nine patients. In the same paper, three post-procedural major complications were reported, two cases of hemoperitoneum and one case of intrahepatic pseudoaneurysm. In our practice, when a transhepatic approach is preferred, the procedure is performed under monitored anesthesia care. Transhepatic puncture of the portal vein is performed under ultrasound guide with a 21-Ga needle. An Accustik system (Boston Scientific) is advanced in the portal branch, over a nitinol wire, and then exchanged, over a 0.035 inch wire, for a 6F or 7F vascular sheath. The hemodynamic trans-stenotic pressure gradient measurement is performed using a 5F hydrophilic catheter. Before the dilatation, a bolus of heparin is administered (2000 UI) to reduce the risk of thrombosis during the balloon occlusion. The dilatation can be performed with balloon catheters up to 10 mm in diameter or more, according to the size of the vessel. Technical success is considered the resolution of the stenosis in a final portogram and a significant reduction of the trans-stenotic pressure gradient. In our practice, we embolize the transhepatic tracks with a coil to reduce the risk of bleeding, but in other series[25], transhepatic track embolization is not performed routinely without evidence of perihepatic bleeding. In patients with severe coagulopathy and/or ascites, the transjugular approach can be chosen, reducing the risk of bleeding[2728]. The procedure is performed with monitored anesthesia care from the right internal jugular vein approach using the standard Colapinto set. Balloon dilatation and/or metallic stent placement are performed with the same technique as the transhepatic approach. Note that when the stenosis is very near the intrahepatic branches bifurcation, it is mandatory to use a non-covered metallic stent because the use of a covered stent, such as a Viatorr, would cause the occlusion of one branch (Figure 3).
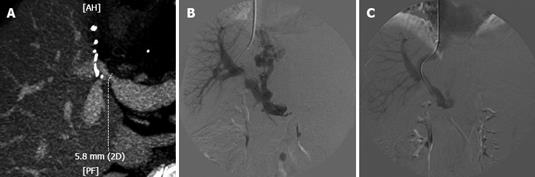
Figure 3 Status post right lobe LRLT in 60-year-old female.
A: MDCT, MIP reconstruction showed a stenosis in portal vein anastomosis. The stenosis is very near the bifurcation of anterior and posterior branches; B: DSA, portogram performed from transjugular approach confirmed the PVS, 15 mmHg trans-stenotic pressure gradient was measured; note large patent coronary vein with filling gastro-oesophageal varices; C: DSA, final portogram performed after the deployement of a 10-mm diameter WallStent; note good filling of intrahepatic branches and no more evidence of the coronary vein, trans-stenotic pressure gradient reduced to 6 mmHg. Patient currently in good general condition with 1 year of follow-up.
IVC stenosis (IVCS)
IVC anastomosis stenosis after orthotopic LT is a rare complication occurring in approximately 1% of patients but more frequently in the superior anastomosis of IVC[2930]. Clinical manifestations are usually refractory ascites and/or pleural effusion associated with renal insufficiency, lower extremities edema and alterations of liver function tests. IVCs is usually related to technical problems during surgery or fibrous scar development at the anastomosis, and concomitant stenosis at hepatic vein anastomosis can be present. For this reason, hepatic vein catheterization is recommended during the same procedure. Donor-recipient size mismatch can also be responsible for IVCS. Transluminal angioplasty is the first-choice treatment for this complication[31] (Figure 4). Trans-stenotic metallic stent deployment is reserved for resistant stenoses or those with elastic recoil not responsive to angioplasty[32]. A transfemoral approach is preferred for a diagnostic cavogram, as is trans-stenotic pressure gradient measurement. Filling of collateral peri-caval vessels is possible in case of hemodynamically significant stenosis. Balloon dilatation is performed with large-sized catheters. Due to the large size of the IVC, simultaneous inflation of multiple balloons has been described[1]. In our practice, during the procedure, radial or femoral artery pressure measurement is performed to continuously monitor changes in systemic hemodynamics during balloon dilatation, and consequent IVC occlusion, deflating the balloon before an excessive drop of systemic pressure. Repeat dilatations may be necessary for long-term patency. In patients with IVCS recurrence and severe renal insufficiency, trans-anastomotic pressure gradient measurement and balloon dilatation can be easily performed without injection of iodinate contrast.
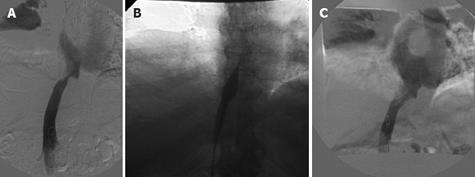
Figure 4 Status post deceased donor LT.
Patient with refractory ascites in association with renal insufficiency and lower extremities edema 4 years after the transplant. A: DSA, cavogram showed a stenosis of IVC upper anastomosis, 12 mmHg trans-stenotic pressure gradient was measured. In the same session hepatic veins catheterization was performed showing no concomitant stenoses in hepatic vein anastomosis; B: DSA percutaneous transluminal angioplasty performed by 16-mm diameter balloon catheter; C: DSA final cavogram showed good patency of the caval anastomosis with trans-stenotic pressure gradient reduced to 2 mmHg. From 2001 to 2007, four other trans-luminal caval angioplasties were performed in the same patient. The patient is currently in good general condition after 15 mo and has avoided re-transplantation.
Hepatic vein stenosis (HVS)
HVS, inducing outflow insufficiency, is a major postoperative complication of LT, especially in patients with partial liver graft transplantation producing graft failure with a reported incidence of 1%-4%[33–35]. Hepatic congestion can cause refractory ascites, refractory hydrothorax and alteration of liver function tests. HVS usually occurs at the anastomosis site; less frequent is the presence of an intrahepatic stenosis in the HV, likely due to previous venous injury during surgery or during previous percutaneous procedures, such as biopsy or biliary catheter placement. If a clinical and/or imaging suspicion of HVS is present, selective catheterization of all the HVs is mandatory to confirm the stenosis and measure the trans-stenotic pressure gradient (Figure 5). A pressure gradient greater than 3 mmHg between the HV and right atrium has been reported to be pathological[35]. Transjugular or transfemoral angioplasty or metallic stent placement is usually performed, as a first choice, to treat this complication[3133–35]. In our experience, balloon dilatation is considered the preferred treatment choice because long-term patency of metallic stents is still unknown and metallic stent placement is reserved for persistent HVS not responsive to multiple angioplasties. Good technical and clinical success rates for percutaneous interventions are reported[33–36]. Long-term patency may require repeated interventions, especially if only trans-luminal angioplasty is performed. Better long-term patency results are reported in cases of stent deployment[36]. The primary percutaneous transhepatic approach for HVS treatment, has been reported[35] to have an easier negotiation through the stenosis, leading to shorter procedure time and ionizing radiation. In our experience, we use the percutaneous transhepatic approach only when the transjugular or the transfemoral approach fails. For the transhepatic approach, previous drainage of ascites and the embolization of the transhepatic tracks at the end of the procedure are, in our opinion, mandatory to reduce the risk of bleeding.
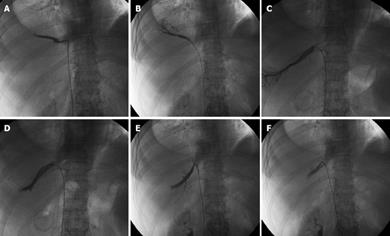
Figure 5 Status post deceased donor right lobe split LT in 65-year-old female.
Three separates HV anastomoses were performed. One month after LT, patient developed refractory ascites and right pleural effusion with worsening of liver function tests. Doppler US was suspicious of HVs. A cavogram performed from the femoral approach showed widely patent IVC anastomosis. Selective catheterization of the three HVs (A, C and E) showed stenosis in the three anastomoses with trans-stenotic pressure gradient of 15, 20 and 16 mmHg, respectively. Balloon angioplasties were performed with balloon catheters ranging from 7 to 10 mm in diameter. Final venogram showed patent anastomoses (B, D and F). Trans-anastomotic pressure gradient reduced to 4, 2.5 and 8 mmHg. Eight months of follow-up without recurrence of refractory ascites and/or hydrothorax.
TREATMENT OF BILIARY COMPLICATIONS
Biliary complications occur in 10%-40% of patients after LT[37–40], with major incidence in patients with partial LT[4142]. Complications include BSs, bile leakage (BL), biliary stones and bilomas. The majority of biliary complications develop during the first 3 mo, but strictures and stones may develop months or years after LT. The preferred methods for biliary tract reconstruction in LT are the duct-to-duct anastomosis between the donor and recipient common ducts and, less frequently, Roux-en-Y choledocojejunostomy. In right lobe split LT (from deceased or living related donor), a duct-to-duct anastomosis is usually performed between the donor right biliary duct and recipient bile duct, but due to possible anatomical variants of the donor biliary tree, two different biliary anastomoses, or less frequently three anastomoses, are performed with the recipient bile duct and the recipient cystic duct or with a Roux-en-Y limb, in up to 40% of patients[4142]. Patients with multiple biliary reconstructions have a higher incidence of biliary complications[42]. Clinical presentation of biliary complications is often indistinguishable from other post-transplantation complications (vascular complications, rejection, graft dysfunction, infections). Ultrasonography is commonly used as screening test; however, due to the high rate of false-negative results, a negative test cannot exclude the presence of biliary complications.
Endoscopic retrograde cholangiopancreatography (ERCP) is usually the initial method of choice to treat post LT biliary complications in patients with duct-to-duct anastomosis. Percutaneous transhepatic cholangiography (PTC) is the method to manage biliary complications in patients with choledochojejunostomy, in presence of intraepatic strictures and when endoscopic management fails.
Biliary strictures (BS)
BS is a common problem after LT with a reported incidence of 10%-35%[37–40]. Anastomotic BS is usually related to scar tissue and retraction at the suture site [4142]. Intrahepatic BS is usually related to chronic rejection or arterial insufficiency due to HAS, thrombosis or ABO blood group incompatibility or infections. Single focal strictures and multiple/combined intrahepatic and anastomotic strictures can be present. Untreated BS is associated with high rate of morbidity and mortality. Endoscopic intervention is the preferred approach in patients with duct-to-duct anastomosis. PTC with biliary drainage placement and consequent percutaneous balloon dilatation is performed in patients with the Roux-en-Y reconstruction or in cases of endoscopic failure. In patients with partial LT, knowledge of the number of the anastomoses performed is mandatory before a possible percutaneous treatment. Possible complications of PTC are hemobilia, drop in hematocrit, intra or extrahepatic hematoma, fever with bacteremia, with a reported incidence of 3%-26% of cases[4344]. Severe injury to intrahepatic arteries with massive hemobilia and possible formation of intrahepatic aneurysm is reported in 2% of cases. In those cases, emergency arterial embolization is required[43]. Suspicion of BS is based on one or more findings: clinical picture (fever, cholangitis), biochemistry (elevation of alkaline phosphatase, direct bilirubin, and transaminases), ultrasound and/or CT scan and/or MR (biliary duct dilatation), and liver biopsy (with histology consistent for cholestasis due to biliary obstruction). BS can also be present in cases of non-dilated biliary ducts. An ultrasound sensitivity of 38% in the detection of biliary obstruction has been reported in transplanted patients[45]. Better results, with sensitivity in detecting biliary obstructions ranging from 80% to 100%, are reported with the use of magnetic resonance cholangiography (MRCP)[46]. Percutaneous treatment of BS is considered safe and effective, avoiding in most cases the need for surgical revision of the anastomosis. Multiple treatments are often necessary. Long-term patency of percutaneous bilioplasty in adult recipients is reported from 50% to 60% at 5 years[4748]. Prolonged cold ischemic and operative times, multiple or peripheral strictures and the presence of hepatic artery disease, predispose to treatment failure or a lower patency of anastomotic BS after balloon dilation[4849]. The use of cutting balloon catheters or combined cutting and conventional balloon protocol has been proposed in patients with refractory anastomotic stricture[5051]. Metallic stent deployment is reserved for patients who are refractory to repetitive balloon dilation of BS and who are poor surgical candidates[52]. In our practice, PTC is performed in an angiographic suite under monitored anesthesia care, with spontaneous respiration and additional local anesthesia. The patient is monitored continuously by electrocardiography, a pulse oximeter, and automatic blood pressure and pulse recordings. Intravenous antibiotic prophylaxis is administered before all procedures. Patients with coagulation defects (platelets < 50 000 mm3 and/or prothrombin activity < 50%) receive infusions of platelets and/or fresh frozen plasma. PTC is generally performed through an intercostal approach, with an ultrasound- and fluoroscopy-guided 20-Ga needle inserted in a peripheral bile duct. If the cholangiography shows a stricture, the biliary tree is catheterized using an Accustick Introducer System (Boston Scientific, Natik, USA) over a Cope wire (Cook, Bjaeverskov, Denmark), the stricture crossed when possible with 0.035 or 0.038 inch hydrophilic guide wires, and a trans-anastomotic biliary catheter ranging from 6F to 8.5F with side holes placed above and below the stricture. The catheter is left to external gravity drainage for at least 1 d. If the patient has no fever and/or cholangitis the day after the procedure, the catheter is clamped for internal drainage. If, after the diagnostic cholangiogram, a guide wire cannot be passed through the stricture, an external drainage catheter is placed to allow for biliary decompression and to reduce the stricture’s possible inflammatory component. A second attempt to cross the stricture is usually performed after 7 d. The first BS balloon dilatation session is never performed on the same day as the diagnostic cholangiogram, so as to reduce the risk of sepsis. It is generally performed after 1 wk, following a cholangiography performed with a 6F or 7F sheath and a balloon size ranging from 6 to 10 mm. Every dilatation session consists of three trans-anastomotic dilatations of 10 min each. Trans-anastomotic biliary catheters with sizes ranging from 6F to 14F according to the diameter of the anastomosis are placed after every dilatation session. An antibiotic infusion is re-administered 6 h after every procedure. At each dilatation session, the size of the balloon catheter is increased by 1 mm, up to a maximum diameter of 12 mm. Catheters are removed when the cholangiography performed through the sheath shows evidence of stricture resolution or of minimal residual stenosis of less than 20% of the expected lumen caliber. In all cases, catheters are removed only upon evidence of complete contrast transition from the bile ducts into the bowel loop within 3 min after the cholangiography (Figure 6), and if a significant reduction of cholestasis serum liver enzymes is achieved after bilioplasty. Our protocol envisages three BS dilatation sessions performed every 4-8 wk, followed by a cholangiographic evaluation 4 wk after the last session. If the stricture is resolved, the catheter is removed; if the BS persists, a supplementary balloon dilatation and follow up cholangiogram is performed, followed by potential catheter removal or balloon dilatation after 4-6 wk. In our experience, when a BS is crossed and a biliary catheter placed, percutaneous balloon dilatation gives good results, although multiple sessions over several months are necessary to obtain stricture resolution.
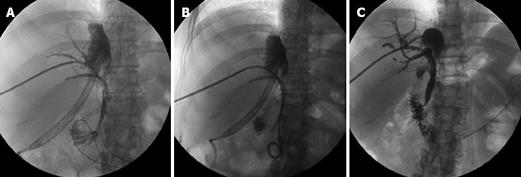
Figure 6 Status post deceased donor LT in 40-year-old man.
Hepatico-jejunostomy was performed. A: PTC shows moderate biliary duct dilatation and subocclusive biliary anastomosis stricture; B: The stricture was crossed and trans-anastomotic. 6F Ring biliary catheter was placed; C: cholangiogram performed after four sessions of percutaneous bilioplasty shows no bile duct dilatation and patent anastomosis; D: Evidence of complete contrast transition from the bile ducts into the bowel loop within 2 min after the cholangiography. Three years of follow-up without clinical evidence of stricture recurrency.
Bile leakage (BL)
Post-operative BL is a complication of LT that usually occurs within a few weeks from the transplant in 5%-20% of patients[37–3953]. BL can arise from bile duct anastomosis of the resection margin in partial LT. Another possible site of leakage is the T-tube insertion in patients with choledocho-choledocho anastomosis. Small leakages usually close spontaneously, while large BLs are a serious complication and need to be treated because of possible associated complications such as fever, abdominal pain, fluid electrolyte depletion, fat malabsorption, possible sepsis or bleeding for hilar vascular erosion. The initial management should be non-operative. Percutaneous drainages, placed with a sonographic guide, are used to drain the bile collection. Endoscopic or percutaneous transhepatic management, with placement of large-size biliary catheters, allows achievement of good results in the treatment of large BL in adult patients, avoiding surgical repair in many cases[54–58]. In selected patients, when prior endoscopic or percutaneous transhepatic attempts to stent the biliary tree have failed, the combined transhepatic-endoscopic approach (rendezvous technique) can be successfully used to place large-size biliary catheters[5960]. When minimally invasive treatments fail, operative intervention is mandatory. In our practice, in patients with anastomotic BL who undergo percutaneous trans-hepatic treatment, we do not use biliary catheters with standard side holes, but we prefer to modify multipurpose large-size drainages by adding holes only in the intrahepatic bile ducts and in the distal bile duct, so as to reduce bile contact in the duct lesion to favor the repair process (Figure 7).
Figure 7 Status post LRLT (right lobe) in 56-year-old woman.
Two separate biliary anastomoses were performed. Approximately 300 mL of bile was drained every day from the existing perihepatic drainage catheter (JP) placed during the transplantation. ERCP was performed, revealing a BL from the anastomotic region. An endoscopic stent was deployed in the posterior duct anastomosis but endoscopy failed to place a stent in the anterior duct anastomosis. A: PTC of the anterior duct showed no bile ducts dilatation and a BL arising from the anastomosis; B: 12F external internal catheter without side holes was placed in the leak region. The bile output from the JP progressively reduced and stopped a few days later. The JP and the endoscopic stent were removed 1 mo later; C: Final cholangiogram performed 4 mo later showed no BL and patent biliary anastomosis. The catheter was removed. The patient is still in good condition after 12 mo of follow-up.
CONCLUSION
LT, in patients with end-stage liver diseases, has become an accepted treatment. Advances in the field of percutaneous, radiological, minimally invasive techniques have increased the importance of interventional radiology in the management of patients after LT. Interventional radiology procedures are used in the treatment of vascular and non-vascular complications, improving graft and patient survival and avoiding, in the majority of cases, surgical revision and/or re-transplantation.