Published online Dec 28, 2009. doi: 10.3748/wjg.15.6091
Revised: August 24, 2009
Accepted: August 31, 2009
Published online: December 28, 2009
AIM: To evaluate the efficacy and complications of stereotactic body radiotherapy in localized paraaortic lymph node recurrence from colorectal cancer.
METHODS: From 2003 to 2009, 7 patients with paraaortic lymph node recurrence (1-3 lesions) from colorectal cancer were treated with stereotactic body radiotherapy. Total gross tumor volumes ranged from 4 to 40 mL. The doses were escalated from 36 Gy/patient to 51 Gy/patient and were delivered in 3 fractions.
RESULTS: One and 3 year overall survival rates were 100% and 71.4%, respectively, and median survival was 37 mo. Grade IV intestinal obstruction was reported in 1 of 7 patients. This patient received 48 Gy in 3 fractions with a maximum point dose to the intestine of 53 Gy and V45Gy = 3.6 mL. However, 6 patients received an intestinal maximum point dose of < 51 Gy and V45Gy of < 1 mL, and did not develop any severe complications.
CONCLUSION: This pilot study suggests selected paraaortic lymph node recurrence (1-3 closed lesions) that failed to respond to chemotherapy can be potentially salvaged by stereotactic body radiotherapy.
- Citation: Kim MS, Cho CK, Yang KM, Lee DH, Moon SM, Shin YJ. Stereotactic body radiotherapy for isolated paraaortic lymph node recurrence from colorectal cancer. World J Gastroenterol 2009; 15(48): 6091-6095
- URL: https://www.wjgnet.com/1007-9327/full/v15/i48/6091.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.6091
Metastatic hepatic and pulmonary lesions from colorectal cancer (CRC) are commonly resected, and if tumorectomy for liver and lung recurrence from CRC is performed successfully, about 20% of patents are expected to achieve a long-term cure. On the other hand, isolated paraaortic lymph node (PALN) recurrences are rarely encountered from CRC. Moreover, surgical treatment in these cases is not widely accepted even when lesions are localized, due to their relative rarity, high postoperative morbidity, and poor prognosis. However, if such patients are left untreated, the survival rates are 31% at 1 year, 7.9% at 2 years, and 0.9% at 4 years[1,2]. Therefore, this patient subset is considered for various chemotherapy regimens. However, despite optimal treatment and initial response, overall survival approaches only 20 mo[3]. Furthermore, radiotherapy usually provides only temporary symptom relief in most cases.
On the other hand, stereotactic body radiotherapy (SBRT) is an emerging technology in the radiation oncology field. This technique utilizes stereotactic principles for localization and delivers multiple beams to well defined targets in a few fractions, and therefore, SBRT can deliver higher doses to tumors due to reduced mechanical error margins, and thus, causes less normal tissue damage. To our knowledge, no previous report has described the role of radiation, including conventional RT, intensity-modulated RT, and SBRT, for PALN recurrence from CRC, although several reports have been issued on the treatment of cervical cancer with isolated PALN recurrence, which responds well to both chemo- and radiation therapy[4-6]. Therefore, the aim of this study was to evaluate the feasibility and efficacy and the complications associated with SBRT in patients with isolated PALN recurrence from CRC.
From May 2003 to March 2009, we reviewed retrospectively 7 patients with isolated PALN recurrence from rectal cancer after curative resection who were treated with SBRT using a CyberKnife (Accuray Inc., Sunnyvale, CA). Isolated PALN recurrence was initially detected by computed tomography (CT) or by an elevated serum carcinoembryonic antigen (CEA) level during a routine check-up, and was confirmed by elevated standardized uptake values of paraaortic lesions by positron emission tomography (PET) or PET/CT. According to our hospital’s protocol, patient eligibility criteria for curative SBRT for paraaortic lymph node recurrence from CRC were as follows: (1) resection of CRC after diagnosis; (2) PALN recurrence after primary cancer resection; (3) progression after chemotherapy for recurrence; (4) a single conglomerate recurrent node or 2-3 recurrent nodes in close proximity (< 1 cm); (5) a greatest tumor diameter of < 8 cm; and (6) an Eastern Cooperative Oncology Group (ECOG) performance score of 1 or 2. Exclusion criteria were as follows: (1) a tumor attached to the stomach or intestine by CT; (2) extra-lymphatic active lesion by CT or PET/CT; (3) more than three separate lymph nodes affected (4) time from primary operation to recurrence of < 6 mo; or (5) previous radiation therapy applied to the treatment site. Patients’ characteristics are summarized in Table 1. Ages ranged from 47 to 73 years (median 59 years) and the male:female ratio was 5:2. All patients underwent primary tumor resection. Liver resection was also performed in 2 patients with liver metastasis. Adjuvant chemotherapy was performed in all patients. Initial pathologic stages were stage II in 2, stage III in 3, and stage IV in 2. Pathologic diagnoses were adenocarcinoma in all 7. Times between operation and first relapse ranged from 7 to 44 mo (median 21 mo). Three patients had a conglomerated LN and the other four had 2 or 3 separate enlarged lymph nodes on a paraaortic lesion. Greatest tumor diameters and heights were calculated using measurements taken from CT scans during planning, and are itemized in Table 1. Total gross tumor volumes (GTV) ranged from 4 to 40 mL (median 22 mL). After recurrence had been detected, all patients received chemotherapy based on 5-FU before SBRT. Chemotherapy regimens were variable because of the different initial adjuvant chemotherapy regimens used. All 7 patients demonstrated disease progression despite chemotherapy, and thus, were defined as non-responders. After performing SBRT, patients were followed up every 2 or 3 mo. When recurrence was detected after SBRT, salvage or palliative treatment was performed according to the status of recurrence. All patients provided written informed consent for SBRT. This study was approved by Institutional Review Board-approved protocol for SBRT at the Korea Institute of Radiological and Medical Sciences (KIRAMS).
| Patient No. | Age (yr)/Sex | Latent time (mo) | GTV (mL) | Dose (Gy) | Prescribed isodose (%) | Dmax of intestine (Gy) | V45 (mL) | Failure pattern | F/U (mo) | Final status |
| 1 | 63/M | 9 | 20 | 36 | 83 | 33 | 0 | Lt SCLN (23) | 70 | AWD |
| Lung (32) | ||||||||||
| 2 | 64/F | 44 | 4 | 41 | 82 | 38 | 0 | - | 37 | Died due to lymphoma |
| 3 | 52/F | 29 | 24 | 45 | 78 | 48 | 0.2 | Spine (26) | 41 | DOD |
| 4 | 59/M | 21 | 22 | 48 | 80 | 51 | 0.7 | Lung (7) | 22 | DOD |
| 5 | 56/M | 10 | 9 | 48 | 80 | 40 | 0 | PALN(9) | 21 | AWD |
| 6 | 47/M | 18 | 40 | 48 | 76 | 53 | 3.6 | Rectum (7) | 25 | DOD |
| Lt SCLN (7) | ||||||||||
| Peritoneal seeding (25) | ||||||||||
| Local recur (13) | ||||||||||
| 7 | 73/M | 7 | 29 | 51 | 78 | 50 | 0.9 | - | 26 | CDF |
In accord with our hospital protocol, gold fiducials (4 mm long and 0.8 mm in diameter) were used as markers for tumor localization. Six fiducials were placed percutaneously on transverse processes of the spine located nearest tumors using an 18 gauge spinal needle under fluoroscopic guidance. Patients were immobilized using an Alpha Cradle (Smithers Medical products, North Canton, OH) 5-7 d after fiducial placement. Panning CT scans were performed with patients in the planned treatment position, and these images were then processed for the CyberKnife planning system. GTV were determined based on CT tumor visualizations. To better delineate tumor volumes, PET/CT images were used as a reference. Clinical target volumes (CTV) were considered to be identical to GTV. Planning target volumes (PTV) were CTV plus a 2-3 mm margin. Radiation doses were prescribed to the 76%-83% isodose line of the maximum dose covering the PTV (Table 1). Critical structures, such as the esophagus and spinal cord, were contoured. Treatment plans involved the use of hundreds of pencil beams shaped using a single 20, 25, or 30 mm diameter circular collimator. The method used to increase SBRT dose is described in detail in our previous report[7]. Briefly, the protocol adopted was follows; if at least 5 patients who received SBRT due to PALN from variable primary tumors (cervical cancer, CRC, or gastric cancer) did not develop Grade IV or V complications for 3-4 mo after radiation was administered, escalations of 1 Gy/fraction (to a total increase of 3 Gy/fraction) were administered for the next cohort. According to this protocol, total SBRT doses ranged from 36 to 51 Gy (median 48 Gy), and were delivered in 3 fractions. Maximum point dose limits were applied for critical organs, i.e. 18 Gy for the spinal cord and 24 Gy for the esophagus. No constraints were applied to limit intestinal or colon exposure. Table 1 summarizes SBRT dosage details.
Overall survival was calculated from the commencement of SBRT using the Kaplan-Meier method. Disease progression free survival was also measured from the commencement of SBRT to the date of local progression, distant metastasis, or both. All statistical calculations were performed using SPSS, version 13.0 (SPSS, Inc., Chicago, IL).
Tumor response during follow up was assessed using Response Evaluation and Criteria for Solid Tumors (RECIST)[8]. Local progression was defined as an increase in tumor size vs the previous CT image or the development of a new lesion in the radiation field. Regional failure was defined as the development of a new lesion in the PALN region.
Acute and late toxicities were defined as symptoms that developed within or after 3 mo of treatment completion, respectively. Toxicities were graded using the National Cancer Institute Common Toxicity Criteria version 3.0[9]. To identify factors related to complications, total CTV, intestinal maximum point dose (Dmax) and intestinal volume administered ≥ 45 Gy (V45 ) were calculated retrospectively (Table 1). Total CTV was defined as sum of the CTV of affected lymph node as determined by the CyberKnife planning system. Vpre was defined as the total volume administered the prescribed dose or more.
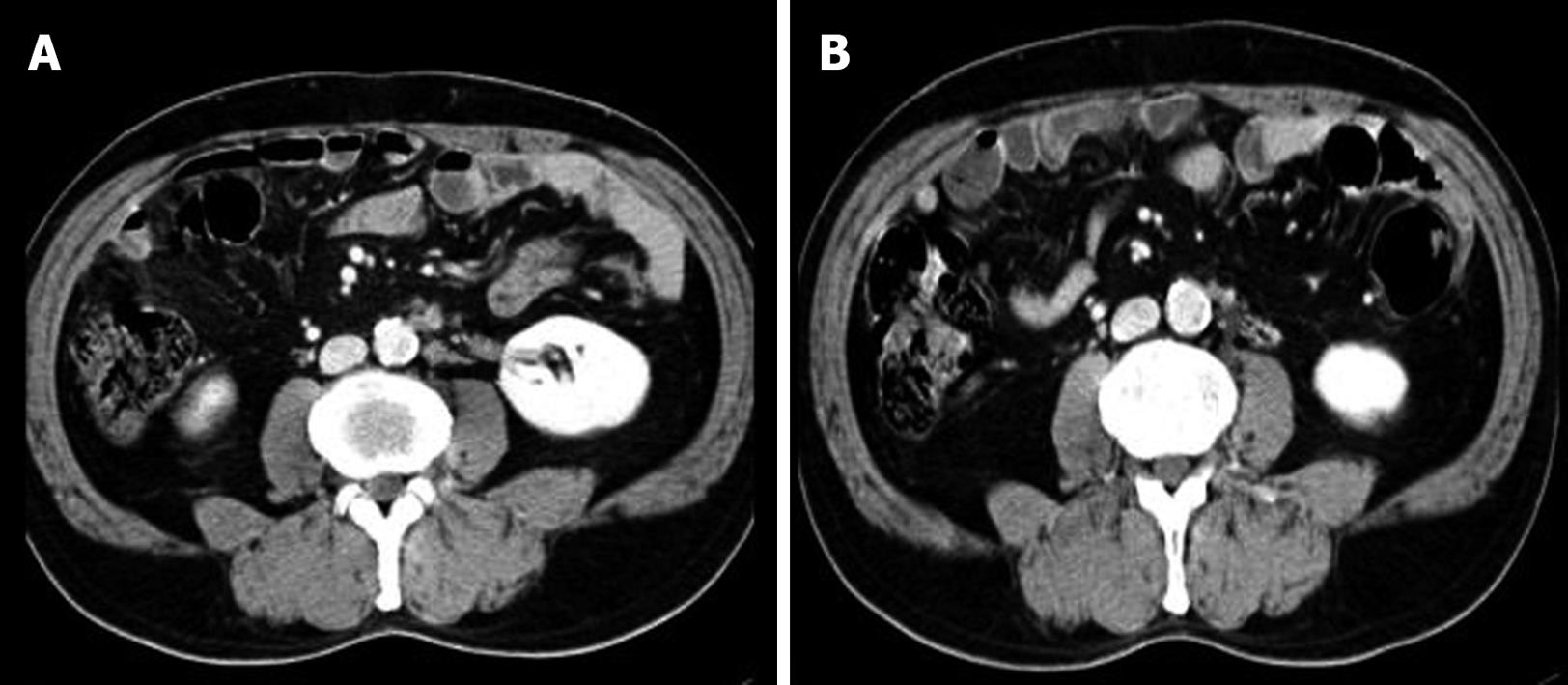
Follow up durations ranged from 15 to 70 mo (median; 26 mo). Final outcomes were as follows: 1 patient remained alive without evidence of disease; 2 patients remained alive with disease; 3 patients died of disease; and 1 patient died of an unrelated disease without recurrence (Table 1). During follow-up, 3 patients achieved a complete response and 4 patients achieved a partial response (Figure 1). One- and 3-year overall survival rates were 100% and 71.4%, respectively, and median survival was 37 mo. Local recurrence was observed at 13 mo after SBRT in patient No 6 (Table 1). Regional recurrence in the PALN region was observed in patient No. 5. However, 4 patients experienced distant failure with/without primary rectal recurrence (Table 1).
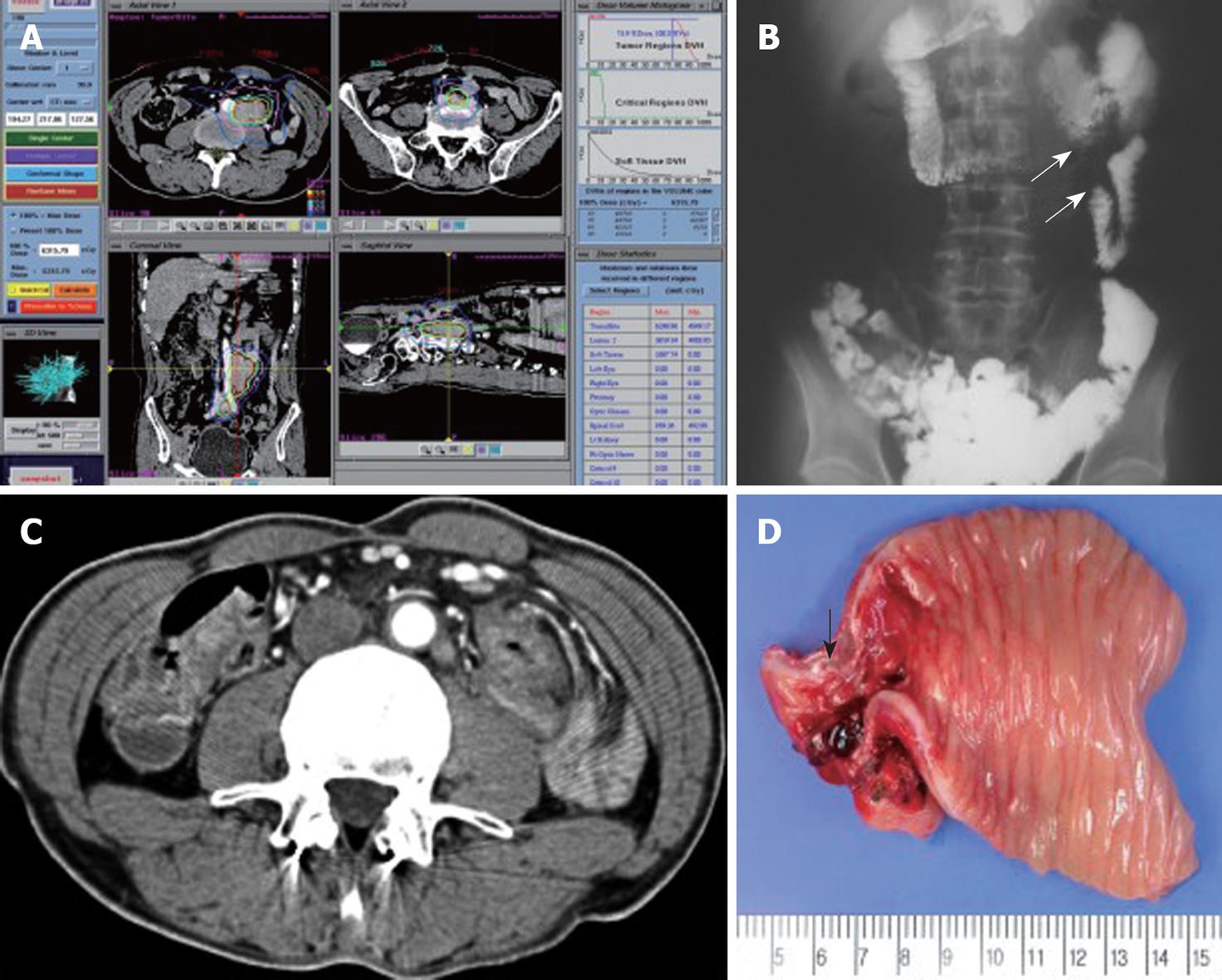
Grade I acute toxicity (nausea and vomiting) occurred in 2 patients at first date of SBRT, and these resolved spontaneously without medication. Grade 4 toxicity occurred in 1 patient (patient No. 6; Table 1). This patient had one conglomerated lymph node and another lymph node and had received 48 Gy in 3 fractions. Abdominal pain developed at 4 mo post-SBRT, and at 5 mo an obstruction was observed during a colon study. He underwent bypass surgery and recovered completely. A photograph of the excised obstructive lesion is shown in Figure 2. This patient had a larger CTV than CTV of the other patients. In this patient, the maximum point intestine dose was 53 Gy and V45Gy was 3.6 mL. No late complications occurred in any patients.
The management of patients with locally recurrent rectal cancer is challenging. For technically resectable recurrent rumors, complete resection can be achieved by limited surgery and outcomes are relatively favorable. Vassilopoulos et al[10] and Pihl et al[11] reported 5-year survival rates of 49% and 42%, respectively, after resection for anastomotic recurrence. However, the treatment of isolated PALN recurrence from CRC is not well established. Recently, Min et al[12] categorized PALN recurrence as a retroperitoneal recurrence, which is a type of locoregional recurrence. Furthermore, several studies[13-16] have investigated the therapeutic efficacies of surgery for retroperitoneal, intraabdominal, and PALN recurrences, and several reported outstanding survival rates, which appear to have resulted from the selection of patients with a resectable mass at time of recurrence. In these studies, reported 5-year survival rates approached a maximum of 56% after complete resection, whereas they ranged from 0% to 7% after incomplete resection (Table 2). Because radical surgery is rarely feasible for PALN recurrence, traditionally, those affected have been considered for chemotherapy. However, despite optimal treatment and the achievement of initial response, patients invariably become non-responsive and achieve overall survivals approaching 20 mo. On the other hand, conventional radiotherapy has played a limited palliative role in the treatment of recurrent CRC involving locoregional recurrence, especially PALN recurrence. The proximities of involved lymph nodes and critical organs, such as the spinal cord, intestine, and colon, often prevent the delivery of sufficient radiation to achieve local control when conventional radiation modalities are used. However, SBRT can deliver higher doses to tumor and cause less tissue damage. Furthermore, it can have three times the biological effect of fractionated radiation therapy. However, the SBRT field is usually directed at the tumor burden, and thus, the prophylactic effect of SBRT in peritumoral regions is limited which in turn means the incidence of regional failure after SBRT seems higher than that after conventional radiation therapy. Fortunately, in the present study, only 1 regional failure pattern was observed.
| Study | Failure site | Treatment | n | Median survival (mo) | Survival rate (yr) |
| Gwin et al[15], 1993 | Non-hepatic intra-abdominal | R01 | 15 | 25.5 | 60 (2), 0 (3) |
| R12 | 6 | 8 | 34 (2), 34 (3) | ||
| R23 | 7 | 3.5 | 15 (2) | ||
| Shibata et al[13], 2002 | Retroperitoneum | R0 | 15 | 81 | 56 (5) |
| R1 | 5 | 29 | 0 (5) | ||
| R2 | 4 | 3 | 0 (5) | ||
| Bowne et al[14], 2005 | Retroperitoneum | R0 | 8 | 44 | NA |
| R1 | 8 | ||||
| Min et al[12], 2008 | PALN | R0 | 6 | 34 | 80 (3), 0 (5) |
| Chemotherapy4 | 33 | 12 | 18 (3), 7 (5) | ||
| Present study | PALN | SBRT | 7 | 41 | 71.4 (3) |
At our institute, SBRT has been utilized for isolated PALN from gastric or cervical cancer, and 3-year survival rates of 43%[17] and 63%[18] have been achieved, respectively. In addition, an excellent 3-year overall survival rate of 71.4% was achieved in the present study. Accordingly, the findings of studies on PALN recurrence from variable primary cancers treated by SBRT appear to support our hypothesis that a subset of isolated PALN recurrence cases exist that are likely to pursue an indolent disease course and be salvaged by adjustable treatments.
Theoretically, rectal cancer is classified as a slow-growing tumor that is likely to respond better to hypofractionation. However, no recommended optimal doses, fraction numbers, or planning constraints for SBRT of PALN recurrence are available in the literature. SBRT doses and fractions for PALN recurrence from variable tumors were started at our hospital from 33 Gy in 3 fractions. In the present study, converted 58 Gy in normalized total doses of 2 Gy was used, and escalated step by step[18]. Although, 1 of our patients developed grade 4 toxicity due to an intestinal obstruction and required surgery, this patient recovered after surgery. Specifically, this patient received 48 Gy in 3 fractions, and had a larger GTV than the other 6 patients. Based on our experience, we consider the factors that most contribute to severe complications are maximum point dose delivered to normal tissue and the volume of normal tissue administered a high dose. Therefore, we tentatively consider maximum intestinal point dose of 51 Gy or V45Gy < 1 mL as constraints of the intestine for SBRT.
Summarizing, the findings of this preliminary study suggest that selected isolated PALN recurrence patients that fail to respond to chemotherapy with 1-3 closely located attached lymph nodes may be salvaged by SBRT. However, a further larger-scale study is required to define optimal dose, intestinal constraints, and adequate indications for SBRT in recurrent CRC.
Isolated paraaortic lymph node (PALN) recurrences are rarely encountered from colorectal cancer (CRC). Moreover, surgical treatment in these cases is not widely accepted even when lesions are localized, due to their relative rarity, high postoperative morbidity, and poor prognosis. This patient subset is considered for various chemotherapy regimens. However, despite optimal treatment and initial response, overall survival approaches only 20 mo.
Stereotactic body radiotherapy (SBRT) is an emerging technology in the radiation oncology field. This technique utilizes stereotactic principles for localization and delivers multiple beams to well defined targets in a few fractions. SBRT can deliver higher doses to tumors due to reduced mechanical error margins, and thus, causes less normal tissue damage.
Delivery of a therapeutic dose of radiation to the PALNs is limited by the sensitivity of the surrounding normal tissues, such as those in the gastrointestinal tract, liver, spinal cord, and kidneys. However recent technologies such as intensity-modulated RT, image-guided RT, and SBRT have allowed higher doses to be delivered to tumor and caused less normal tissue damage. SBRT could lead to better local control through delivery of a higher radiation dose to the tumor and this could ultimately translate into survival gain. Furthermore, SBRT requires complex planning and relatively long treatment times (30-45 min) but generally is completed in 3-5 treatments. SBRT is associated with few side effects because the treatment field is generally very small and treatment is precisely delivered.
This pilot study suggests selected paraaortic lymph node recurrence (1-3 closed lesions) that failed to respond to chemotherapy can be potentially salvaged by stereotactic body radiotherapy.
Isolated PALN metastasis is defined as metastasis only to the PALNs. SBRT is an image-guided radiation method. SBRT is directed to extremely well-defined targets within the body. SBRT has evolved from the intracranial experience of stereotactic radiosurgery (single fraction treatment) or stereotactic radiotherapy (multiple fractions of treatment).
The authors evaluate efficacy and complications of stereotactic body radiotherapy in localized paraaortic lymph node recurrence from colorectal cancer. it is well written.
Peer reviewer: Luis Bujanda, PhD, Professor, Departament of Gastroenterology, CIBEREHD, University of Country Basque, Donostia Hospital, Paseo Dr. Beguiristain s/n, 20014 San Sebastián, Spain
S- Editor Tian L L- Editor O'Neill M E- Editor Lin YP
| 1. | Biasco G, Derenzini E, Grazi G, Ercolani G, Ravaioli M, Pantaleo MA, Brandi G. Treatment of hepatic metastases from colorectal cancer: many doubts, some certainties. Cancer Treat Rev. 2006;32:214-228. |
| 2. | Saltz LB. Metastatic colorectal cancer: is there one standard approach? Oncology (Williston Park). 2005;19:1147-1154; discussion 1154, 1157-1158, 1160. |
| 3. | Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476-487. |
| 4. | Singh AK, Grigsby PW, Rader JS, Mutch DG, Powell MA. Cervix carcinoma, concurrent chemoradiotherapy, and salvage of isolated paraaortic lymph node recurrence. Int J Radiat Oncol Biol Phys. 2005;61:450-455. |
| 5. | Chou HH, Wang CC, Lai CH, Hong JH, Ng KK, Chang TC, Tseng CJ, Tsai CS, Chang JT. Isolated paraaortic lymph node recurrence after definitive irradiation for cervical carcinoma. Int J Radiat Oncol Biol Phys. 2001;51:442-448. |
| 6. | Kim JS, Kim JS, Kim SY, Kim KH, Cho MJ. Hyperfractionated radiotherapy with concurrent chemotherapy for para-aortic lymph node recurrence in carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2003;55:1247-1253. |
| 7. | Kim MS, Choi C, Yoo S, Cho C, Seo Y, Ji Y, Lee D, Hwang D, Moon S, Kim MS. Stereotactic body radiation therapy in patients with pelvic recurrence from rectal carcinoma. Jpn J Clin Oncol. 2008;38:695-700. |
| 8. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. |
| 9. | Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. |
| 10. | Vassilopoulos PP, Yoon JM, Ledesma EJ, Mittelman A. Treatment of recurrence of adenocarcinoma of the colon and rectum at the anastomotic site. Surg Gynecol Obstet. 1981;152:777-780. |
| 11. | Pihl E, Hughes ES, McDermott FT, Price AB. Recurrence of carcinoma of the colon and rectum at the anastomotic suture line. Surg Gynecol Obstet. 1981;153:495-496. |
| 12. | Min BS, Kim NK, Sohn SK, Cho CH, Lee KY, Baik SH. Isolated paraaortic lymph-node recurrence after the curative resection of colorectal carcinoma. J Surg Oncol. 2008;97:136-140. |
| 13. | Shibata D, Paty PB, Guillem JG, Wong WD, Cohen AM. Surgical management of isolated retroperitoneal recurrences of colorectal carcinoma. Dis Colon Rectum. 2002;45:795-801. |
| 14. | Bowne WB, Lee B, Wong WD, Ben-Porat L, Shia J, Cohen AM, Enker WE, Guillem JG, Paty PB, Weiser MR. Operative salvage for locoregional recurrent colon cancer after curative resection: an analysis of 100 cases. Dis Colon Rectum. 2005;48:897-909. |
| 15. | Gwin JL, Hoffman JP, Eisenberg BL. Surgical management of nonhepatic intra-abdominal recurrence of carcinoma of the colon. Dis Colon Rectum. 1993;36:540-544. |
| 16. | Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol. 2005;23:4553-4560. |
| 17. | Kim MS, Yoo SY, Cho CK, Yoo HJ, Yang KM, Kang JK, Lee DH, Lee JI, Bang HY, Kim MS. Stereotactic body radiotherapy for isolated para-aortic lymph node recurrence after curative resection in gastric cancer. J Korean Med Sci. 2009;24:488-492. |
| 18. | Choi CW, Cho CK, Yoo SY, Kim MS, Yang KM, Yoo HJ, Seo YS, Kang JK, Lee DH, Lee KH. Image-guided stereotactic body radiation therapy in patients with isolated para-aortic lymph node metastases from uterine cervical and corpus cancer. Int J Radiat Oncol Biol Phys. 2009;74:147-153. |