Published online Dec 21, 2009. doi: 10.3748/wjg.15.5898
Revised: November 10, 2009
Accepted: November 17, 2009
Published online: December 21, 2009
Tumor-host interaction at the invasive front of colorectal cancer represents a critical interface encompassing a dynamic process of de-differentiation of colorectal carcinoma cells known as epithelial mesenchymal transition (EMT). EMT can be identified histologically by the presence of “tumor budding”, a feature which can be highly specific for tumors showing an infiltrating tumor growth pattern. Importantly, tumor budding and tumor border configuration have generated considerable interest as additional prognostic factors and are also recognized as such by the International Union Against Cancer. Evidence seems to suggest that the presence of tumor budding or an infiltrating growth pattern is inversely correlated with the presence of immune and inflammatory responses at the invasive tumor front. In fact, several tumor-associated antigens such as CD3, CD4, CD8, CD20, Granzyme B, FOXP3 and other immunological or inflammatory cell types have been identified as potentially prognostic in patients with this disease. Evidence seems to suggest that the balance between pro-tumor (including budding and infiltrating growth pattern) and anti-tumor (immune response or certain inflammatory cell types) factors at the invasive front of colorectal cancer may be decisive in determining tumor progression and the clinical outcome of patients with colorectal cancer. On one hand, the infiltrating tumor border configuration and tumor budding promote progression and dissemination of tumor cells by penetrating the vascular and lymphatic vessels. On the other, the host attempts to fend off this attack by mounting an immune response to protect vascular and lymphatic channels from invasion by tumor buds. Whereas standard pathology reporting of breast and prostate cancer involves additional prognostic features, such as the BRE and Gleason scores, the ratio of pro- and anti-tumor factors could be a promising approach for the future development of a prognostic score for patients with colorectal cancer which could complement tumor node metastasis staging to improve the clinical management of patients with this disease.
- Citation: Zlobec I, Lugli A. Invasive front of colorectal cancer: Dynamic interface of pro-/anti-tumor factors. World J Gastroenterol 2009; 15(47): 5898-5906
- URL: https://www.wjgnet.com/1007-9327/full/v15/i47/5898.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5898
The tumor node metastasis (TNM) staging system from the American Joint Committee on Cancer/International Union Against Cancer (UICC) remains the most reliable prognostic indicator for patients with colorectal cancer[1]. Overall 5-year survival rates are reported at 65% and correspond closely to disease progression; patients with stage I disease have more favourable prognoses with 5-year survival rates exceeding 80%-90%. In contrast, patients with stage II, III and IV disease experience progressively worse outcomes with varying 5-year survival rates of 70%-85%, 44%-80% and < 10%, respectively[2]. It is recognized, however, that patients with tumors of the same TNM stage may be variable both in terms of prognosis and response to therapy.
A range of other histomorphological, molecular and protein biomarkers have additionally been investigated for their prognostic value independently of TNM stage. These tumor-related factors such as venous and lymphatic invasion, tumor grade, perineural invasion, histological type, loss of heterozygosity at 18q, mutation in p53, tumor expression of vascular endothelial growth factor and thymidylate synthase are recognized as essential, additional or new and promising prognostic factors by the UICC[3,4]. In particular, microsatellite instability (MSI) status has revealed itself not only as a significant prognostic factor but also as an attribute categorizing colorectal carcinogenesis into two major pathways: the chromosomal instability (or microsatellite stable; MSS) and MSI pathways, the latter including both sporadic and hereditary Lynch syndrome [Hereditary non-polyposis colorectal cancer (HNPCC)] patients both demonstrating mismatch repair deficiencies and high level MSI (MSI-H)[5].
Tumor-host interaction at the invasive front of colorectal cancer represents a critical interface where tumor progression and tumor cell dissemination ensue. The invasive tumor front encompasses a dynamic process of de-differentiation of colorectal carcinoma cells, a process known as epithelial mesenchymal transition (EMT)[6]. EMT can be identified histologically by the presence of “tumor budding”, a feature which is specific for tumors showing an infiltrating growth pattern[7]. Importantly, tumor budding and tumor border configuration have generated considerable interest as additional prognostic factors and are also recognized as such by the UICC[3,4]. Evidence seems to suggest that the presence of tumor budding or an infiltrating growth pattern is inversely correlated with the presence of immune and inflammatory responses at the invasive tumor front[8,9]. In fact, several tumor-associated antigens such as CD3, CD4, CD8, CD20, Granzyme B, FOXP3 and other immunological or inflammatory cell types have been identified as potentially prognostic in patients with this disease[10-16].
Together, evidence seems to suggest that the balance between pro-tumor (including budding and infiltrating growth pattern) and anti-tumor (immune response or certain inflammatory cell types) factors at the invasive front of colorectal cancer may be decisive in determining tumor progression and the clinical outcome of patients with colorectal cancer.
The aim of this review is to outline the evidence supporting a pro-/anti-tumor factor model of colorectal cancer progression, one which highlights the dynamic interface between tumor and host-related factors at the invasive front of colorectal cancer.
In 1985, a study of colonic adenocarcinoma in rats reported a peculiar feature at the invasive border of differentiated tumors[17,18]. Using both light and electron microscopy, Gabbert et al[18] observed neoplastic glands irregularly arranged into small strands and cords. In addition, they noted discontinuous small aggregates or single tumor cells which ultrastructurally did not possess junctional complexes, often had incomplete desmosomes, missing or rudimentary basement membranes and absent or incomplete brush borders. They determined that at the invasive tumor front of colorectal cancer, differentiated tumors could acquire an undifferentiated phenotype. Their observation is credited for pioneering the concept known as de-differentiation at the invasive margin, which today is commonly referred to as “tumor budding”.
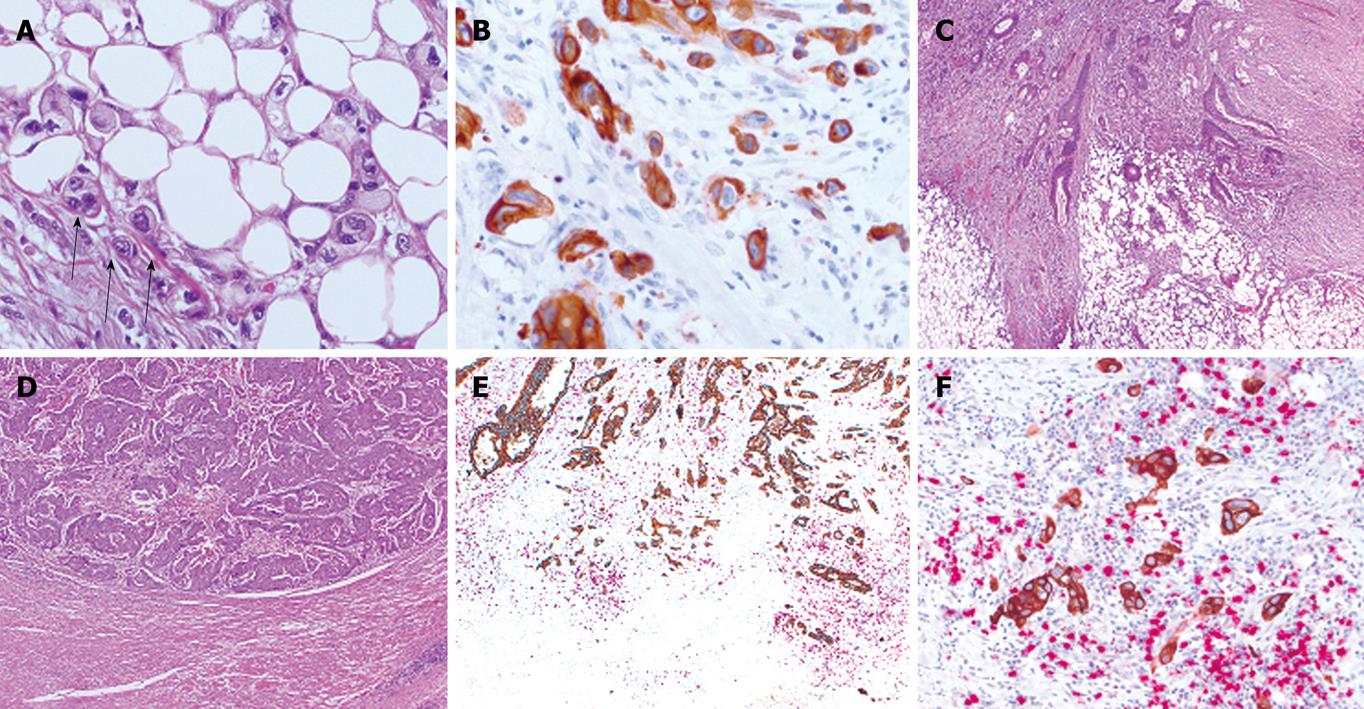
Tumor budding is a histological feature diagnosed at high magnification and is defined as single cells or clusters of up to four or five cells at the invasive tumor front[19]. Budding cells can be spotted using standard HE-stained tissue slides and their visualization is further facilitated using pan-cytokeratin stains (Figure 1A and B)[7]. The process of tumor budding is likened to EMT observed during gastrulation in embryonic development[7]. In the early phase of EMT, epithelial cells reduce intercellular contacts and cell-matrix contacts and reorganize the cytoskeleton to form cell membrane ruffles (lamellipodia) or cytoplasmic protrusions. Migration ensues and new cell-matrix contacts are formed providing cells with an anchorage for the contraction of the cell body. In colorectal cancer, tumor budding is a highly dynamic process giving temporal heterogeneity to the tumor.
Budding cells have been credited with the properties of malignant stem cells including the potential for redifferentiation both locally and at sites of distant metastasis and marking, what appears to be, the first histological event in tumor cell migration and invasion. Supporting this hypothesis further is the presence of “pseudopodia-like” cytoplasmic protrusions in tumor buds which have been identified by both electron microscopy and recently by immunohistochemistry with pan-cytokeratins[17,20]. These podia appear to be in direct contact with the adjacent interstitial tissue suggesting their formation occurs during tumor cell migration. Moreover, Shinto et al[21] recently suggested that cytoplasmic pseudo-fragments could be used as a marker for an activated budding phenotype that is associated with cell motility and increased invasiveness independent of the extent of budding. Not surprisingly, tumor buds have been shown to over-express proteins involved in extracellular matrix degradation and to under-express adhesion molecules. Previous studies on EMT and events occurring at the invasive tumor front implicate, in particular, the Wingless-INT (WNT) signalling pathway in the process of tumor budding evidenced by increased β-catenin immunohistochemical staining in tumor buds, a concomitant loss of E-cadherin and over-expression of laminin5γ2 along with activation of transcriptional repressors SLUG, and ZEB1[22,23]. Over-expression of urokinase plasminogen activator receptor (uPAR), matrix metalloproteinase-7 and -9 (MMP7, MMP9), matrilysin, CD44, Ep-CAM, and extensive staining of β(III)-tubulin, a major constituent of microtubules, have all been reported[20,23-30] suggestive of the invasion and migration potential of tumor buds. Tumor buds seem to over-express CXCL12, a stromal cell-derived factor whose receptor CXC4 is involved in chemotaxis and angiogenesis[31]. In addition, we recently documented the over-expression of the putative colorectal CSC marker ABCG5 within tumor buds leading to a poorer outcome of patients including those with node-negative disease (Hostettler, World Journal of Gastroenterology, in press). Whether a sub-population of tumor buds may in fact represent malignant stem cells is still an open question which necessitates further investigation.
Since tumor budding appears to play a critical role in the initiation of metastasis, several authors have investigated the potential of this feature to predict dissemination of tumor cells to regional lymph nodes. A significant association between tumor budding and lymph node positivity has been consistently demonstrated correlating with tumor aggressiveness and more advanced TNM stage[32-43]. Tumor budding is frequently associated with poorly differentiated tumors, and with the presence of vascular and lymphatic invasion independently of disease extent[44-48]. Local tumor recurrence and distant metastasis to the lung and liver are also more commonly observed in patients with tumor budding[36,39,48-50] and additionally represent a reproducible prognostic factor in stage II patients[51]. Recently, Suzuki et al[52] found that tumor budding and venous invasion were significant predictors of local and distant metastases in patients with T1 stage colorectal cancers. Xu et al[53] demonstrated an increased rate of tumor budding in colorectal carcinomas with the aggressive micropapillary component. The presence of tumor budding has repeatedly been linked to poor clinical outcome, underlined by the adverse effect on overall survival independently of TNM stage[47,51,54].
Tumor budding is closely linked to tumor growth pattern, a feature described by Jass et al[55] in 1987 which led to the proposal of an alternative prognostic classification system for rectal cancers[55,56]. The diagnosis of either a pushing (or expanding) or infiltrating tumor border configuration can be made at low magnification and is reproducible among pathologists thereby underlining its usefulness as a prognostic indicator (Figure 1C and D)[7]. The pushing tumor border is one in which margins are reasonably well-circumscribed and often associated with a well-developed inflammatory lamina. In contrast, the infiltrative tumor border is characterized by widespread dissection of normal tissue structures with loss of a clear boundary between tumor and host tissues.
Several studies have confirmed that an infiltrative tumor border configuration has a significant adverse prognostic impact in colorectal cancer and may predict local recurrence[57,58]. Our study group has also recently provided evidence for the improved stratification of stage II colorectal cancer patients based on the diagnosis of tumor border configuration. In particular, the 5-year survival rates for patients with stage II tumors decreased substantially from 80% in those with a pushing margin to 62.7% in patients with an infiltrating growth pattern, a survival rate similarly found in patients with stage III disease[59]. Considering that patients with stage III tumors are generally considered for adjuvant therapy[60], the implications of these findings suggest that stage II patients with an infiltrating tumor margin should perhaps be considered for post-operative therapy. The addition of tumor border configuration to TNM stage improved the prognostic classification of colorectal cancer patients by 17.9%. Since the presence of tumor budding can be strongly specific for an infiltrating, rather than a pushing/expanding growth pattern, it is not surprising that loss of cell-adhesion molecule E-cadherin and apoptosis activating factor-1, a pro-apoptotic molecule and over-expression of uPA and uPAR have all been reported as significant predictors of an infiltrating tumor border configuration in colorectal cancer[9,61].
Immunotherapy for patients with colorectal cancer represents a realistic alternative approach to treatment of this disease[62-64]. The last 20 years has seen a wide range of publications on tumor immunity and the prognostic impact of immune and inflammatory cell types in the microenvironment of colorectal tumors demonstrating promising results both in vitro and in vivo.
The presence of conspicuous peritumoral lymphocytic (PTL) inflammation, viewed as a distinctive “encapsulating” connective tissue mantle cap at the invasive front of colorectal cancer, is inversely correlated with the presence of tumor budding and positively associated with improved survival[19,65,66]. Jass[8] demonstrated that PTL infiltration in rectal cancer decreased with more advanced Dukes’ stage to 53%, 28% and 13% with Dukes’ A, B and C cases, respectively. In addition, the significantly worsened prognosis in patients lacking PTL inflammation at the tumor border was highlighted, while patients with moderate or pronounced infiltration performed significantly better independently of disease stage. The results have also been confirmed by other study groups[67]. However, the presence of PTL inflammation at the invasive front does not appear to be an independent prognostic factor in patients with this disease[59]. Nonetheless, PTL inflammation seems to be intimately linked with abundant CD8+ tumor infiltrating T-lymphocytes, further implicating tumor immunity in the defense against colorectal cancer.
Most studies to date confirm that a high rate of tumor infiltrating lymphocytes (TILs), in particular those located intra-epithelially characterized by CD4+ and CD8+ tumor-associated antigens are beneficial for patient outcome[68,69]. An abundant TIL count appears to be linked to earlier Dukes’ stage, decreased local recurrence rate following curative surgery and improved overall and disease-free survival time both in non-metastatic and metastatic patients undergoing hepatic resection[10,68-73].
Galon et al[13] evaluated by gene expression profiling and immunohistochemistry, the type, density and location (whether at the invasive margin or the tumor centre) of TILs in a large number of cases. They evaluated CD3, CD8, granzyme B and memory CD45RO T cells and demonstrated a significant independent and positive effect of TILs on both recurrence and survival. Pages et al[15] performed a comprehensive analysis of TILs focusing on early metastatic invasion. They found, by RT-PCR on 75 cases that mRNA levels of CD8, granzyme B and granulysin were significantly greater in patients without vascular emboli, lymphatic and perineural invasion (collectively known as VELIPI) compared to those with these features and that CD45RO+ cells had independent prognostic value[15]. Diederichsen et al[74] showed that a low CD4/CD8 ratio by flow cytometry was an independent prognostic factor for prolonged survival. In addition, Milasiene et al[75] evaluated inter-epithelial CD3, CD4, CD8, CD20 and CD16 and found that increased levels of all these markers, particularly of the natural killer cell marker CD16 led to significantly improved overall outcome[11,75]. Moreover, regulatory T-cells expressing FOXP3+ has been shown to correlate with improved outcome independently of TNM stage[16,76].
In addition to T cells in colorectal cancer, a growing number of studies have demonstrated the clinical impact of dendritic cells, mast cells, macrophages and neutrophils on survival. An improved survival time and a preventative effect of mast cells on local recurrence and distant metastasis in patients with rectal tumors with high mast cell counts have been identified[77-79]. Further, the significant benefit of mast cell number on tumor progression in colorectal cancer was highlighted by Gounaris et al[80] who reported that depletion of mast cells whether by pharmacological means or through generation of chimeric mice with genetic lesions in mast cell development led to remission of existing polyps. Moreover, Halazun et al[81] found that an elevated neutrophil/lymphocyte ratio led to a poorer survival time and higher rate of recurrence in colorectal cancer patients undergoing surgery for liver metastasis.
Dendritic cells are the most potent antigen-presenting cells and as such are now one of the many important tools for tumor immunotherapy. Evidence is accumulating which suggests that the presence of dendritic cells may be of significant benefit to patients with colorectal cancer[82]. Using immunohistochemistry for CD83, Suzuki et al[83] described the presence of mature dendritic cells at the invasive margin of cancer stroma and demonstrated by light and electron microscopy their formation into clusters with lymphocytes, the majority of which were CD45RO+ T cells. They conclude that mature CD83+ dendritic cells at the invasive margin promote T-cell activation for the generation of tumor specific immunity. Using electron microscopy, tumor-infiltrating dendritic cells were found to make contacts among themselves, with TILs and tumor cells. The presence of dendritic cells was found predominantly in early compared to later disease stages and mostly located in tumor surrounding tissue[84]. Dadabayev et al[12] demonstrated that dendritic cells were significantly correlated with intra-epithelial CD4+ and CD8+ lymphocytes. Recently, HLA-DR expressed constitutively on antigen-presenting cells such as dendritic cells and macrophages has also been found to correlate with the presence of TILs and PTLs as well as improved patient outcome[85].
Works by Banerjea et al[86] clearly show that MSI status (MSS; sporadic MSI-H and hereditary Lynch syndrome-associated colorectal cancers) should be taken into consideration when discussing tumor immunity in colorectal cancer. Compared to MSS tumors, both sporadic and hereditary MSI-H cancers from patients with Lynch syndrome (hereditary non-polyposis coli; HNPCC) are characterized by prolonged survival time, significantly more frequent PTL inflammation at the invasive front and by an inherent abundance of intra-epithelial TILs[87-94]. In contrast to MSS tumors which primarily arise following disruption of WNT signalling, sporadic MSI-H tumors are linked to mutations in TGFβRII[95,96]. Baker et al[97] hypothesized that retention of TILs in MSI-H cancers may be a consequence of refractoriness to normal TGF-β signalling. In a subsequent study, these authors show in more than 1000 MSS and 223 MSI tumors that an abundant CD8+ TIL infiltrate has a beneficial effect on survival time in MSS, but not MSI cancers[71]. Other authors confirm the abundance of CD8+ TILs and granzyme-positive cells as well as improved clinical outcome in patients with MSI-H compared to MSS colorectal cancers[98-100]. In addition, a positive correlation between apoptosis rates and higher TIL number has been described, a finding which could perhaps help to explain the improved prognosis seen in patients with MSI-H compared to MSS tumors[98,101]. Studies on T-regs such as FOXP3 and CD25+ have recently been undertaken[102]. Drescher et al[102], evaluating both MSS and MSI-H cancers found that in contrast to CD8+ T-cells which may be involved in actively preventing growth and/or metastatic in MSI-H tumors, CD25+ cell counts were similar between MSS and MSI-H tumors suggesting no active immunosuppressive mechanisms in MSS cancers. Finally, the upregulation in MSI-H cancers of a large number of genes involved in immune response and increased levels of pro-inflammatory cytokines and cytotoxic mediators indicate an activated anti-tumor immune response in these tumors[86].
Several observations have led to the hypothesis that tumor progression and prognosis in patients with colorectal cancer is not based solely on the presence of pro-tumor or absence of anti-tumor factors but rather on the balance between the two. First, the presence of tumor buds is inversely correlated with the presence of PTL inflammation and intra-epithelial CD8+ TILs[9,21]. In MSI-H cancers, where intra-epithelial TILs are abundant, PTL inflammation “encapsulating” the tumor at the invasive front and pushing tumor border are commonly seen, tumor budding is virtually absent[20]. When it occurs, tumor budding in the MSI-H lacks the full budding immunophenotype and the cytoplasmic podia which give budding cells a temporal dimension[20]. In a previous study on MSS colorectal cancers, we hypothesized that an intense peritumoral inflammatory reaction at the invasive front could be “nipping colorectal cancer in the bud” by specifically targeting budding cells for destruction[9]. We recently investigated CD8+ lymphocytes directly positioned in the microenvironment of the tumor buds. We could demonstrate that the ratio of CD8+ lymphocytes to tumor buds (CD8+/tumor budding index) out-performed both tumor budding or CD8+ lymphocytes alone as independent prognostic factors in two independent cohorts[103]. Using double immunostaining for CD8+ antibody and pan-cytokeratin, a ratio of 3:1 for CD8+ lymphocytes to tumor buds was a highly favourable phenotypic constellation associated with earlier pT stage, lymph node negativity, low tumor grade and absence of vascular and lymphatic invasion in addition to conferring a prolonged clinical outcome in both stage II and stage III colorectal cancer (Figure 1E and F). Although we cannot allude to the direct functional interaction between CD8+ lymphocytes and tumor buds themselves, the strong circumstantial relationship between the ratio of tumor budding and CD8+ lymphocytes in the microenvironment of budding cells appears, nonetheless, to be a reproducible and independent prognostic factor in colorectal cancer.
The invasive front of colorectal cancer represents a dynamic interface between pro- and anti-tumor factors, which can be visualized as a balance between “attackers” (pro-tumor) and “defenders” (anti-tumor). On the one hand, the infiltrating tumor border configuration and its “cavalry” tumor budding promote progression and dissemination of tumor cells by penetrating the vascular and lymphatic vessels. On the other, the host attempts to fend off this attack by mounting an immune response using its “infantry”, in particular cytotoxic T lymphocytes, to protect vascular and lymphatic channels from invasion by tumor buds. Although evidence shows that both pro- and anti-tumor factors contribute prognostic information in a TNM-independent manner, the ratio of attackers and defenders may better capture the interaction at the invasive front which could translate into more powerful prognostic indicators.
Whereas standard pathology reporting of breast and prostate cancer involves additional prognostic features, such as the BRE and Gleason scores, the ratio of attackers/defenders could be a promising approach for the future development of a prognostic score for patients with colorectal cancer which could complement TNM stage to improve the clinical management of patients with this disease.
Peer reviewer: Dr. Marek Bebenek, MD, PhD, Department of Surgical Oncology, Regional Comprehensive Cancer Center, pl. Hirszfelda 12, 53-413 Wroclaw, Poland
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH
| 1. | Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295-308. |
| 2. | O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420-1425. |
| 3. | Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739-1757. |
| 4. | Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979-994. |
| 5. | Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113-130. |
| 6. | Natalwala A, Spychal R, Tselepis C. Epithelial-mesenchymal transition mediated tumourigenesis in the gastrointestinal tract. World J Gastroenterol. 2008;14:3792-3797. |
| 7. | Prall F. Tumour budding in colorectal carcinoma. Histopathology. 2007;50:151-162. |
| 8. | Jass JR. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986;39:585-589. |
| 9. | Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol. 2007;212:260-268. |
| 10. | Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, McArdle CS. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92:651-654. |
| 11. | Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Menegaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520-529. |
| 12. | Dadabayev AR, Sandel MH, Menon AG, Morreau H, Melief CJ, Offringa R, van der Burg SH, Janssen-van Rhijn C, Ensink NG, Tollenaar RA. Dendritic cells in colorectal cancer correlate with other tumor-infiltrating immune cells. Cancer Immunol Immunother. 2004;53:978-986. |
| 13. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. |
| 14. | Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877-884. |
| 15. | Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654-2666. |
| 16. | Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186-192. |
| 17. | Gabbert H. Mechanisms of tumor invasion: evidence from in vivo observations. Cancer Metastasis Rev. 1985;4:293-309. |
| 18. | Gabbert H, Wagner R, Moll R, Gerharz CD. Tumor dedifferentiation: an important step in tumor invasion. Clin Exp Metastasis. 1985;3:257-279. |
| 19. | Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40:127-132. |
| 20. | Shinto E, Jass JR, Tsuda H, Sato T, Ueno H, Hase K, Mochizuki H, Matsubara O. Differential prognostic significance of morphologic invasive markers in colorectal cancer: tumor budding and cytoplasmic podia. Dis Colon Rectum. 2006;49:1422-1430. |
| 21. | Shinto E, Mochizuki H, Ueno H, Matsubara O, Jass JR. A novel classification of tumour budding in colorectal cancer based on the presence of cytoplasmic pseudo-fragments around budding foci. Histopathology. 2005;47:25-31. |
| 22. | Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. J Pathol. 2009;217:307-317. |
| 23. | Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356-10361. |
| 24. | Baldus SE, Monig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, Schneider PM, Thiele J, Holscher AH, Dienes HP. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res. 2004;10:2790-2796. |
| 25. | Gosens MJ, van Kempen LC, van de Velde CJ, van Krieken JH, Nagtegaal ID. Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod Pathol. 2007;20:221-232. |
| 26. | Guzinska-Ustymowicz K. MMP-9 and cathepsin B expression in tumor budding as an indicator of a more aggressive phenotype of colorectal cancer (CRC). Anticancer Res. 2006;26:1589-1594. |
| 27. | Horkko TT, Klintrup K, Makinen JM, Napankangas JB, Tuominen HJ, Makela J, Karttunen TJ, Makinen MJ. Budding invasive margin and prognosis in colorectal cancer--no direct association with beta-catenin expression. Eur J Cancer. 2006;42:964-971. |
| 28. | Masaki T, Sugiyama M, Matsuoka H, Abe N, Izumisato Y, Sakamoto A, Atomi Y. Coexpression of matrilysin and laminin-5 gamma2 chain may contribute to tumor cell migration in colorectal carcinomas. Dig Dis Sci. 2003;48:1262-1267. |
| 29. | Sordat I, Rousselle P, Chaubert P, Petermann O, Aberdam D, Bosman FT, Sordat B. Tumor cell budding and laminin-5 expression in colorectal carcinoma can be modulated by the tissue micro-environment. Int J Cancer. 2000;88:708-717. |
| 30. | Portyanko A, Kovalev P, Gorgun J, Cherstvoy E. beta(III)-tubulin at the invasive margin of colorectal cancer: possible link to invasion. Virchows Arch. 2009;454:541-548. |
| 31. | Akishima-Fukasawa Y, Nakanishi Y, Ino Y, Moriya Y, Kanai Y, Hirohashi S. Prognostic significance of CXCL12 expression in patients with colorectal carcinoma. Am J Clin Pathol. 2009;132:202-210; quiz 307. |
| 32. | Hori H, Fujimori T, Fujii S, Ichikawa K, Ohkura Y, Tomita S, Ono Y, Imura J, Kuroda Y. Evaluation of tumor cell dissociation as a predictive marker of lymph node metastasis in submucosal invasive colorectal carcinoma. Dis Colon Rectum. 2005;48:938-945. |
| 33. | Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Yokoo T, Ishii T. Histopathologic determinants of regional lymph node metastasis in early colorectal cancer. Cancer. 2008;112:924-933. |
| 34. | Kanazawa H, Mitomi H, Nishiyama Y, Kishimoto I, Fukui N, Nakamura T, Watanabe M. Tumour budding at invasive margins and outcome in colorectal cancer. Colorectal Dis. 2008;10:41-47. |
| 35. | Masaki T, Matsuoka H, Sugiyama M, Abe N, Sakamoto A, Watanabe T, Nagawa H, Atomi Y. Tumor budding and evidence-based treatment of T2 rectal carcinomas. J Surg Oncol. 2005;92:59-63. |
| 36. | Okuyama T, Oya M, Ishikawa H. Budding as a useful prognostic marker in pT3 well- or moderately-differentiated rectal adenocarcinoma. J Surg Oncol. 2003;83:42-47. |
| 37. | Prall F, Nizze H, Barten M. Tumour budding as prognostic factor in stage I/II colorectal carcinoma. Histopathology. 2005;47:17-24. |
| 38. | Sohn DK, Chang HJ, Park JW, Choi DH, Han KS, Hong CW, Jung KH, Kim DY, Lim SB, Choi HS. Histopathological risk factors for lymph node metastasis in submucosal invasive colorectal carcinoma of pedunculated or semipedunculated type. J Clin Pathol. 2007;60:912-915. |
| 39. | Ueno H, Mochizuki H, Hashiguchi Y, Hatsuse K, Fujimoto H, Hase K. Predictors of extrahepatic recurrence after resection of colorectal liver metastases. Br J Surg. 2004;91:327-333. |
| 40. | Choi DH, Sohn DK, Chang HJ, Lim SB, Choi HS, Jeong SY. Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: a prospective cohort study. Dis Colon Rectum. 2009;52:438-445. |
| 41. | Ogawa T, Yoshida T, Tsuruta T, Tokuyama W, Adachi S, Kikuchi M, Mikami T, Saigenji K, Okayasu I. Tumor budding is predictive of lymphatic involvement and lymph node metastases in submucosal invasive colorectal adenocarcinomas and in non-polypoid compared with polypoid growths. Scand J Gastroenterol. 2009;44:605-614. |
| 42. | Ohtsuki K, Koyama F, Tamura T, Enomoto Y, Fujii H, Mukogawa T, Nakagawa T, Uchimoto K, Nakamura S, Nonomura A. Prognostic value of immunohistochemical analysis of tumor budding in colorectal carcinoma. Anticancer Res. 2008;28:1831-1836. |
| 43. | Yamauchi H, Togashi K, Kawamura YJ, Horie H, Sasaki J, Tsujinaka S, Yasuda Y, Konishi F. Pathological predictors for lymph node metastasis in T1 colorectal cancer. Surg Today. 2008;38:905-910. |
| 44. | Bayar S, Saxena R, Emir B, Salem RR. Venous invasion may predict lymph node metastasis in early rectal cancer. Eur J Surg Oncol. 2002;28:413-417. |
| 45. | Kazama S, Watanabe T, Ajioka Y, Kanazawa T, Nagawa H. Tumour budding at the deepest invasive margin correlates with lymph node metastasis in submucosal colorectal cancer detected by anticytokeratin antibody CAM5.2. Br J Cancer. 2006;94:293-298. |
| 46. | Park KJ, Choi HJ, Roh MS, Kwon HC, Kim C. Intensity of tumor budding and its prognostic implications in invasive colon carcinoma. Dis Colon Rectum. 2005;48:1597-1602. |
| 47. | Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H, Ozawa K. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385-394. |
| 48. | Yasuda K, Inomata M, Shiromizu A, Shiraishi N, Higashi H, Kitano S. Risk factors for occult lymph node metastasis of colorectal cancer invading the submucosa and indications for endoscopic mucosal resection. Dis Colon Rectum. 2007;50:1370-1376. |
| 49. | Choi HJ, Park KJ, Shin JS, Roh MS, Kwon HC, Lee HS. Tumor budding as a prognostic marker in stage-III rectal carcinoma. Int J Colorectal Dis. 2007;22:863-868. |
| 50. | Tanaka M, Hashiguchi Y, Ueno H, Hase K, Mochizuki H. Tumor budding at the invasive margin can predict patients at high risk of recurrence after curative surgery for stage II, T3 colon cancer. Dis Colon Rectum. 2003;46:1054-1059. |
| 51. | Wang LM, Kevans D, Mulcahy H, O’Sullivan J, Fennelly D, Hyland J, O’Donoghue D, Sheahan K. Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol. 2009;33:134-141. |
| 52. | Suzuki A, Togashi K, Nokubi M, Koinuma K, Miyakura Y, Horie H, Lefor AT, Yasuda Y. Evaluation of venous invasion by Elastica van Gieson stain and tumor budding predicts local and distant metastases in patients with T1 stage colorectal cancer. Am J Surg Pathol. 2009;33:1601-1607. |
| 53. | Xu F, Xu J, Lou Z, Di M, Wang F, Hu H, Lai M. Micropapillary component in colorectal carcinoma is associated with lymph node metastasis in T1 and T2 Stages and decreased survival time in TNM stages I and II. Am J Surg Pathol. 2009;33:1287-1292. |
| 54. | Hase K, Shatney C, Johnson D, Trollope M, Vierra M. Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum. 1993;36:627-635. |
| 55. | Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987;1:1303-1306. |
| 56. | Jass JR, Atkin WS, Cuzick J, Bussey HJ, Morson BC, Northover JM, Todd IP. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology. 1986;10:437-459. |
| 57. | Turner RR, Li C, Compton CC. Newer pathologic assessment techniques for colorectal carcinoma. Clin Cancer Res. 2007;13:6871s-6876s. |
| 58. | Zlobec I, Terracciano LM, Lugli A. Local recurrence in mismatch repair-proficient colon cancer predicted by an infiltrative tumor border and lack of CD8+ tumor-infiltrating lymphocytes. Clin Cancer Res. 2008;14:3792-3797. |
| 59. | Zlobec I, Baker K, Minoo P, Hayashi S, Terracciano L, Lugli A. Tumor border configuration added to TNM staging better stratifies stage II colorectal cancer patients into prognostic subgroups. Cancer. 2009;115:4021-4029. |
| 60. | Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408-3419. |
| 61. | Minoo P, Baker K, Baumhoer D, Terracciano L, Lugli A, Zlobec I. Urokinase-type plasminogen activator is a marker of aggressive phenotype and an independent prognostic factor in mismatch repair-proficient colorectal cancer. Hum Pathol. 2010;41:70-78. |
| 62. | Mazzolini G, Murillo O, Atorrasagasti C, Dubrot J, Tirapu I, Rizzo M, Arina A, Alfaro C, Azpilicueta A, Berasain C. Immunotherapy and immunoescape in colorectal cancer. World J Gastroenterol. 2007;13:5822-5831. |
| 63. | Nizar S, Copier J, Meyer B, Bodman-Smith M, Galustian C, Kumar D, Dalgleish A. T-regulatory cell modulation: the future of cancer immunotherapy? Br J Cancer. 2009;100:1697-1703. |
| 64. | Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. |
| 65. | Kaneko I, Tanaka S, Oka S, Kawamura T, Hiyama T, Ito M, Yoshihara M, Shimamoto F, Chayama K. Lymphatic vessel density at the site of deepest penetration as a predictor of lymph node metastasis in submucosal colorectal cancer. Dis Colon Rectum. 2007;50:13-21. |
| 66. | Wang HS, Liang WY, Lin TC, Chen WS, Jiang JK, Yang SH, Chang SC, Lin JK. Curative resection of T1 colorectal carcinoma: risk of lymph node metastasis and long-term prognosis. Dis Colon Rectum. 2005;48:1182-1192. |
| 67. | Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg. 2009;249:788-793. |
| 68. | Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711-1717. |
| 69. | Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491-3494. |
| 70. | Ali AA, McMillan DC, Matalka II, McNicol AM, McArdle CS. Tumour T-lymphocyte subset infiltration and tumour recurrence following curative resection for colorectal cancer. Eur J Surg Oncol. 2004;30:292-295. |
| 71. | Baker K, Zlobec I, Tornillo L, Terracciano L, Jass JR, Lugli A. Differential significance of tumour infiltrating lymphocytes in sporadic mismatch repair deficient versus proficient colorectal cancers: a potential role for dysregulation of the transforming growth factor-beta pathway. Eur J Cancer. 2007;43:624-631. |
| 72. | Katz SC, Pillarisetty V, Bamboat ZM, Shia J, Hedvat C, Gonen M, Jarnagin W, Fong Y, Blumgart L, D’Angelica M. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:2524-2530. |
| 73. | Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318-324. |
| 74. | Diederichsen AC, Hjelmborg JB, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52:423-428. |
| 75. | Milasiene V, Stratilatovas E, Norkiene V. The importance of T-lymphocyte subsets on overall survival of colorectal and gastric cancer patients. Medicina (Kaunas). 2007;43:548-554. |
| 76. | Frey DM, Droese RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3+ regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2009;Epub ahead of print. |
| 77. | Nagtegaal ID, Marijnen CA, Kranenbarg EK, Mulder-Stapel A, Hermans J, van de Velde CJ, van Krieken JH. Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect--a histopathological and immunohistochemical study. BMC Cancer. 2001;1:7. |
| 78. | Gulubova M, Vlaykova T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol. 2009;24:1265-1275. |
| 79. | Sandel MH, Dadabayev AR, Menon AG, Morreau H, Melief CJ, Offringa R, van der Burg SH, Janssen-van Rhijn CM, Ensink NG, Tollenaar RA. Prognostic value of tumor-infiltrating dendritic cells in colorectal cancer: role of maturation status and intratumoral localization. Clin Cancer Res. 2005;11:2576-2582. |
| 80. | Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977-19982. |
| 81. | Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, Lodge JP. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55-60. |
| 82. | Sandel MH, Speetjens FM, Menon AG, Albertsson PA, Basse PH, Hokland M, Nagelkerke JF, Tollenaar RA, van de Velde CJ, Kuppen PJ. Natural killer cells infiltrating colorectal cancer and MHC class I expression. Mol Immunol. 2005;42:541-546. |
| 83. | Suzuki A, Masuda A, Nagata H, Kameoka S, Kikawada Y, Yamakawa M, Kasajima T. Mature dendritic cells make clusters with T cells in the invasive margin of colorectal carcinoma. J Pathol. 2002;196:37-43. |
| 84. | Xie ZJ, Jia LM, He YC, Gao JT. Morphological observation of tumor infiltrating immunocytes in human rectal cancer. World J Gastroenterol. 2006;12:1757-1760. |
| 85. | Walsh MD, Dent OF, Young JP, Wright CM, Barker MA, Leggett BA, Bokey L, Chapuis PH, Jass JR, Macdonald GA. HLA-DR expression is associated with better prognosis in sporadic Australian clinicopathological Stage C colorectal cancers. Int J Cancer. 2009;125:1231-1237. |
| 86. | Banerjea A, Ahmed S, Hands RE, Huang F, Han X, Shaw PM, Feakins R, Bustin SA, Dorudi S. Colorectal cancers with microsatellite instability display mRNA expression signatures characteristic of increased immunogenicity. Mol Cancer. 2004;3:21. |
| 87. | Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527-535. |
| 88. | Gafa R, Maestri I, Matteuzzi M, Santini A, Ferretti S, Cavazzini L, Lanza G. Sporadic colorectal adenocarcinomas with high-frequency microsatellite instability. Cancer. 2000;89:2025-2037. |
| 89. | Greenson JK, Bonner JD, Ben-Yzhak O, Cohen HI, Miselevich I, Resnick MB, Trougouboff P, Tomsho LD, Kim E, Low M. Phenotype of microsatellite unstable colorectal carcinomas: Well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol. 2003;27:563-570. |
| 90. | Jass JR, Do KA, Simms LA, Iino H, Wynter C, Pillay SP, Searle J, Radford-Smith G, Young J, Leggett B. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42:673-679. |
| 91. | Jenkins MA, Hayashi S, O’Shea AM, Burgart LJ, Smyrk TC, Shimizu D, Waring PM, Ruszkiewicz AR, Pollett AF, Redston M. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007;133:48-56. |
| 92. | Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994;145:148-156. |
| 93. | Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91:2417-2422. |
| 94. | Shia J, Ellis NA, Paty PB, Nash GM, Qin J, Offit K, Zhang XM, Markowitz AJ, Nafa K, Guillem JG. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003;27:1407-1417. |
| 95. | Baker K, Raut P, Jass JR. Microsatellite unstable colorectal cancer cell lines with truncating TGFbetaRII mutations remain sensitive to endogenous TGFbeta. J Pathol. 2007;213:257-265. |
| 96. | Malesci A, Laghi L, Bianchi P, Delconte G, Randolph A, Torri V, Carnaghi C, Doci R, Rosati R, Montorsi M. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13:3831-3839. |
| 97. | Baker K, Chong G, Foulkes WD, Jass JR. Transforming growth factor-beta pathway disruption and infiltration of colorectal cancers by intraepithelial lymphocytes. Histopathology. 2006;49:371-380. |
| 98. | Michael-Robinson JM, Biemer-Huttmann A, Purdie DM, Walsh MD, Simms LA, Biden KG, Young JP, Leggett BA, Jass JR, Radford-Smith GL. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. 2001;48:360-366. |
| 99. | Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. 2004;91:469-475. |
| 100. | Prall F, Duhrkop T, Weirich V, Ostwald C, Lenz P, Nizze H, Barten M. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808-816. |
| 101. | Houston AM, Michael-Robinson JM, Walsh MD, Cummings MC, Ryan AE, Lincoln D, Pandeya N, Jass JR, Radford-Smith GL, O’Connell J. The “Fas counterattack” is not an active mode of tumor immune evasion in colorectal cancer with high-level microsatellite instability. Hum Pathol. 2008;39:243-250. |
| 102. | Drescher KM, Sharma P, Watson P, Gatalica Z, Thibodeau SN, Lynch HT. Lymphocyte recruitment into the tumor site is altered in patients with MSI-H colon cancer. Fam Cancer. 2009;8:231-239. |
| 103. | Lugli A, Karamitopoulou E, Panayiotides I, Karakitsos P, Rallis G, Peros G, Iezzi G, Spagnoli G, Bihl M, Terracciano L. CD8+ lymphocytes/ tumour-budding index: an independent prognostic factor representing a ‘pro-/anti-tumour’ approach to tumour host interaction in colorectal cancer. Br J Cancer. 2009;101:1382-1392. |