Published online Dec 7, 2009. doi: 10.3748/wjg.15.5751
Revised: October 22, 2009
Accepted: October 29, 2009
Published online: December 7, 2009
Intragastric balloon therapy, as a part of a multidisciplinary weight management program, is an effective short-term intervention for weight loss. Although the insertion procedure is easy and generally well tolerated by patients, a few complications can occur. We report here a heavy smoker with intragastric balloon insertion complicated by colonization with opportunistic organisms. The 27-year-old female, body mass index 35.5 kg/m2, had a BioEnterics® Intragastric Balloon inserted under conscious sedation without any perioperative complications. Six months later, when the standard removal time arrived, the balloon was seen to be covered with a necrotic white-gray material. Microbiological examination revealed Enterobacter cloacae and Candida species yeast colonies. We recommend that asymptomatic fungal and/or bacterial colonization should be considered among the complications of the intragastric balloon procedure, despite its rarity.
- Citation: Coskun H, Bozkurt S. A case of asymptomatic fungal and bacterial colonization of an intragastric balloon. World J Gastroenterol 2009; 15(45): 5751-5753
- URL: https://www.wjgnet.com/1007-9327/full/v15/i45/5751.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5751
Obesity will be one of the 21st century’s most important problems and will become a worldwide epidemic. In 2015 approximately 2.3 billion people will be overweight and 700 million people will be reported to be obese[1]. Obesity treatment options consist of a calorie-restricted diet, lifestyle modification, medical treatment, endoscopic intragastric balloon application and bariatric surgery[2]. In 1982, the intragastric balloon was used for the first time by Nieben et al[3], who observed that long-lasting and well-tolerated gastric bezoars can result in significant weight loss.
Intragastric balloon therapy, as a part of a multidisciplinary weight management program is an effective short-term intervention for weight loss. In experienced hands the application and removal of the intragastric balloon is easy and has relatively low morbidity and mortality compared to bariatric surgery[4,5].
The major complications of the intragastric balloon are intolerance, gastric ulcers, gastric erosion and esophagitis, spontaneous deflation of the balloon, ongoing vomiting for 1-3 wk or more, abdominal pain and gastroesophageal reflux. There are also a few reported cases of gastric perforation, dilatation and small intestinal obstruction[6,7].
We present here an asymptomatic intragastric balloon infected by Candida and Enterobacter species which has not been encountered previously in the literature, and we discuss possible and probable causes.
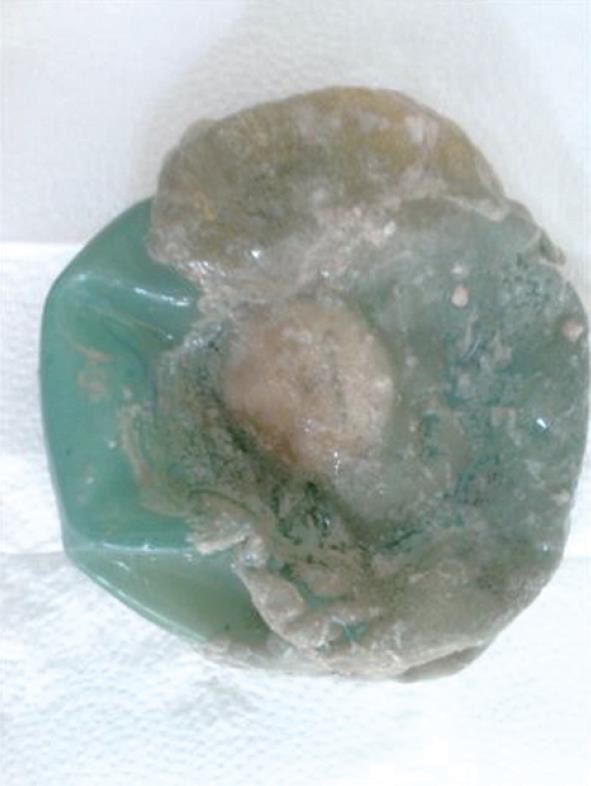
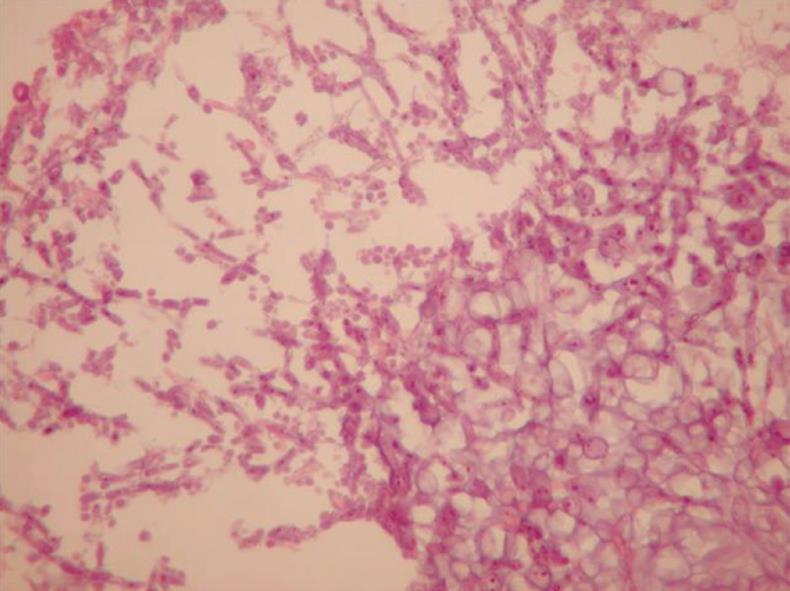
A 27-year-old female, heavily smoking patient, 91 kg in weight and body mass index (BMI) 35.5 kg/m2 had a BioEnterics® Intragastric Balloon (BIB; BioEnterics Corporation, California, USA, S/N: 123456789) inserted. The patient was premedicated with 0.05 mg/kg iv midazolam (Dormicum Roche, Turkey) for 10 min before the insertion and an additional 0.5-0.75 mg/kg propofol (Diprivan, Astra Zeneca, Turkey) was given iv during the application to attain deeper sedation[8]. Under video-endoscopic view, the balloon was positioned in the stomach and inflated with 550 mL NaCl 0.09% mixed with 10 mL methylene blue solution. The upper gastrointestinal endoscopic findings were normal. As is common, the patient experienced intense nausea, vomiting and cramping abdominal pain in the first 48 h. With appropriate medical support these standard complaints subsided significantly by the fifth day after the procedure. In the following days the patient was given a 1200 kcal/d diet. The balloon was removed according to protocol on the 180th d after placement. On the 180th d, the patient’s weight was 69 kg, with BMI 26.9 kg/m2, excess weight loss 62.8%, and excess BMI loss 81.4%. During the removal of the balloon, endoscopy revealed normal esophageal and gastric mucosal surfaces except slight gastritis. However, the surface of the silicon balloon was covered with a necrotic white-gray layer (Figure 1). This necrotic material covered almost 80% of the total surface of the balloon. The balloon had been removed endoscopically with no problems. Histopathologic examination revealed a few fungal hyphae, polymorphonuclear infiltration of leukocytes and mononuclear cells (Figure 2), with active and chronic inflammation. Gram staining showed epithelial cells, leukocytes, gram-negative bacilli and yeast cells. With Ziehl-Nielsen staining, acid-resistant bacilli were not seen. Microbiologic examination revealed Enterobacter cloacae and Candida species yeast colonies. The patient was discharged with appropriate non-specific supportive treatment without any clinical problems. The patient was evaluated endoscopically after a 3-mo clinical follow-up and no pathology was found.
Intragastric balloon application has relatively low morbidity and mortality compared to other bariatric procedures. In particular it is the primary choice for excessively morbidly obese patients who are prepared to undergo surgical bariatric procedures.
The complication rate is extremely variable between studies. This is because minor complications can easily be managed by phone calls or during scheduled visits. The major complications of the intragastric balloon procedure are balloon intolerance (7%), gastric ulcer (0.4%), gastric erosion and esophagitis (18.2%), spontaneous deflation of the balloon (3%-23%), ongoing vomiting for 1-3 wk or more (0%-15.9%), abdominal pain (5%), and gastroesophageal reflux (1.8%). There are also a few reported cases of gastric perforation (0.1%-0.21%), dilatation and small intestinal obstructions (0.17%-0.8%)[5-7]. Infection arising from intragastric balloon insertion is not a common issue. There is no such published case in the literature.
The isolated microorganism, Enterobacter cloacae is a gram-negative bacillus. Frequently, it causes nosocomial infections in patients in whom respiratory support instruments or catheterizations are applied, or it appears as opportunistic infections in patients with immunodeficient and debilitating diseases[9].
Candida is a yeast and the most common cause of opportunistic mycoses worldwide. It is also a frequent colonizer of human skin and mucous membranes. Candida is a constituent of the normal flora of the skin, mouth, vagina, and stool[10].
Colonization of soft lining materials with microorganisms, particularly Candida species, is a common clinical problem. The intragastric balloon has a silicon elastomer structure and da Silveira et al[11] mentioned in their paper that silicone-based soft lining materials are more susceptible to Candida adhesion.
Candidiasis of the gastrointestinal system mostly affects the esophagus and the infection is seen as patch plaques on the mucosa[12]. Although a typical fungal infection was not detected in the esophagus of our patient, such contamination can not be excluded and may have occurred during endoscopic intervention, i.e. the balloon passage through the oral cavity or during its application.
Of the predisposing factors causing gastric candidiasis, delayed gastric emptying and gastric stasis have been proposed. It is a well known issue that one of the mechanisms of action of the intragastric balloon is to delay gastric emptying[13]. Thus the slowing down of normal peristalsis of the stomach by the intragastric balloon may allow opportunistic organisms to colonize more readily.
The patients in whom an intragastric balloon is inserted should take proton pump inhibitors during application of the balloon because of gastric hyperacidity. These proton pump inhibitors result in a hypochlorhydric gastric medium which makes opportunistic infections more likely[14-17].
The important feature identified in the present case was that she was a heavy smoker. It is known that cigarette smoke has many effects on the gastric mucosa. These can result in progression from gastritis to gastric ulcers and even carcinoma. Smokers appear to be at higher risk of becoming infected with Helicobacter pylori (H pylori) and this increased risk may result from the adverse effects of smoking on antioxidants or the immune system which may interfere with normal protection against H pylori[18-20].
Because the infection detected on the balloon was a colonization rather than a systemic infection we recommended appropriate non-specific supportive treatment for the patient. However, we recommend systemic antifungal and antibacterial treatment for patients in whom the gastrointestinal integrity is damaged, who are immunocompromised or who are scheduled for bariatric surgery.
We present here a heavy smoker with an intragastric balloon infection. In our series of 201 endoscopic removals of 263 intragastric balloons, this case was the only one with such an infection. The rare incidence of colonization leads us to believe that this kind of infection may be multifactorial, and no single cause can be highlighted. In patients who receive an intragastric balloon, predisposing factors for opportunistic infections such as gastric stasis, smoking and antacid medications should be taken into consideration and patients monitored for these infections. Any asymptomatic balloon infection, especially opportunistic pathogens, should be treated with appropriate medications if the integrity of the gastrointestinal system is damaged.
Peer reviewers: Jeroen Maljaars, MD, Department of Internal Medicine, Division of Gastroenterology & Hepatology, University Hospital Maastricht, PO Box 5800, 6202 AZ Maastricht, The Netherlands; Andrada Seicean, MD, PhD, University of Medicine and Pharmacy Cluj Napoca, Romania, Third Medical Clinic Cluj Napoca, 15 Closca Street, 400039, Cluj-Napoca, Romania
S- Editor Wang YR L- Editor Cant MR E- Editor Zheng XM
| 1. | Deitel M. Overweight and obesity worldwide now estimated to involve 1.7 billion people. Obes Surg. 2003;13:329-330. |
| 2. | Escudero Sanchis A, Catalán Serra I, Gonzalvo Sorribes J, Bixquert Jiménez M, Navarro López L, Herrera García L, Durbán Serrano L, Monforte Albalat A. [Effectiveness, safety, and tolerability of intragastric balloon in association with low-calorie diet for the treatment of obese patients]. Rev Esp Enferm Dig. 2008;100:349-354. |
| 3. | Nieben OG, Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet. 1982;1:198-199. |
| 4. | Melissas J. IFSO guidelines for safety, quality, and excellence in bariatric surgery. Obes Surg. 2008;18:497-500. |
| 5. | Imaz I, Martínez-Cervell C, García-Alvarez EE, Sendra-Gutiérrez JM, González-Enríquez J. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg. 2008;18:841-846. |
| 6. | Gostout CJ, Rajan E. Endoscopic treatments for obesity: past, present and future. Gastroenterol Clin North Am. 2005;34:143-150. |
| 7. | Dumonceau JM. Evidence-based review of the Bioenterics intragastric balloon for weight loss. Obes Surg. 2008;18:1611-1617. |
| 8. | Coskun H, Aksakal C. Experience with sedation technique for intragastric balloon placement and removal. Obes Surg. 2007;17:995-996. |
| 9. | The enterobactericeae. In: Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC Jr, editors. Color atlas and textbook of diagnostic microbiology. 5th ed. Philadelphia: Lippincott-Raven 1997; 171-252. |
| 10. | The enterobactericeae; Mycology. In: Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC Jr, editors. Color atlas and textbook of diagnostic microbiology. 5th ed. Philadelphia: Lippincott-Raven 1997; 983-1069. |
| 11. | da Silveira LC, Charone S, Maia LC, Soares RM, Portela MB. Biofilm formation by Candida species on silicone surfaces and latex pacifier nipples: an in vitro study. J Clin Pediatr Dent. 2009;33:235-240. |
| 12. | Kliemann DA, Pasqualotto AC, Falavigna M, Giaretta T, Severo LC. Candida esophagitis: species distribution and risk factors for infection. Rev Inst Med Trop Sao Paulo. 2008;50:261-263. |
| 13. | Rajablou M, Ganz RA, Batts KP. Candida infection presenting as multiple ulcerated masses. Gastrointest Endosc. 2007;65:164-166. |
| 14. | Larner AJ, Hamilton MI. Review article: infective complications of therapeutic gastric acid inhibition. Aliment Pharmacol Ther. 1994;8:579-584. |
| 15. | Pasqualotto AC, Nedel WL, Machado TS, Severo LC. A comparative study of risk factors and outcome among outpatient-acquired and nosocomial candidaemia. J Hosp Infect. 2005;60:129-134. |
| 16. | Brzozowski T, Zwolinska-Wcislo M, Konturek PC, Kwiecien S, Drozdowicz D, Konturek SJ, Stachura J, Budak A, Bogdal J, Pawlik WW. Influence of gastric colonization with Candida albicans on ulcer healing in rats: effect of ranitidine, aspirin and probiotic therapy. Scand J Gastroenterol. 2005;40:286-296. |
| 17. | Wang K, Lin HJ, Perng CL, Tseng GY, Yu KW, Chang FY, Lee SD. The effect of H2-receptor antagonist and proton pump inhibitor on microbial proliferation in the stomach. Hepatogastroenterology. 2004;51:1540-1543. |
| 18. | Koivisto TT, Voutilainen ME, Färkkilä MA. Effect of smoking on gastric histology in Helicobacter pylori-positive gastritis. Scand J Gastroenterol. 2008;43:1177-1183. |
| 19. | Parasher G, Eastwood GL. Smoking and peptic ulcer in the Helicobacter pylori era. Eur J Gastroenterol Hepatol. 2000;12:843-853. |