Published online Nov 28, 2009. doi: 10.3748/wjg.15.5525
Revised: September 14, 2009
Accepted: September 21, 2009
Published online: November 28, 2009
AIM: To investigate the diagnostic accuracy of acoustic radiation force impulse (ARFI) imaging as a noninvasive method for the assessment of liver fibrosis in chronic hepatitis C (CHC) patients.
METHODS: We performed a prospective blind comparison of ARFI elastography, APRI index and FibroMax in a consecutive series of patients who underwent liver biopsy for CHC in University Hospital Bucharest. Histopathological staging of liver fibrosis according to the METAVIR scoring system served as the reference. A total of 74 patients underwent ARFI elastography, APRI index, FibroMax and successful liver biopsy.
RESULTS: The noninvasive tests had a good correlation with the liver biopsy results. The most powerful test in predicting fibrosis was ARFI elastography. The diagnostic accuracy of ARFI elastography, expressed as area under receiver operating characteristic curve (AUROC) had a validity of 90.2% (95% CI AUROC = 0.831-0.972, P < 0.001) for the diagnosis of significant fibrosis (F ≥ 2). ARFI sonoelastography predicted even better F3 or F4 fibrosis (AUROC = 0.993, 95% CI = 0.979-1).
CONCLUSION: ARFI elastography had very good accuracy for the assessment of liver fibrosis and was superior to other noninvasive methods (APRI Index, FibroMax) for staging liver fibrosis.
- Citation: Fierbinteanu-Braticevici C, Andronescu D, Usvat R, Cretoiu D, Baicus C, Marinoschi G. Acoustic radiation force imaging sonoelastography for noninvasive staging of liver fibrosis. World J Gastroenterol 2009; 15(44): 5525-5532
- URL: https://www.wjgnet.com/1007-9327/full/v15/i44/5525.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5525
The prognosis and clinical management of chronic liver diseases are highly dependent on the extent of liver fibrosis. This is particularly true in patients with chronic hepatitis C (CHC), which is the leading cause of cirrhosis in many countries. CHC progresses to cirrhosis through a sequence of morphological changes that reflect increasing fibrosis and architectural distortion[1]. Staging systems of varying complexity based on these morphological changes have been used in patient management as a guide to prognosis, and in clinical investigations as an estimate of natural history and response to therapy.
Liver biopsy is the current reference examination for the assessment of liver fibrosis. It is important to remember that a needle biopsy is merely a sample of the entire liver, and that scarring in chronic liver disease is typically irregularly distributed in the liver. Needle liver biopsy removes only about 1/50 000th of the liver and carries substantial interpreter errors[2]. Liver biopsy is an invasive procedure with certain unavoidable risks and complications. Therefore, the development of noninvasive tests to assess hepatic inflammation and fibrosis has been an active area of research. Furthermore, as new antifibrotic therapies are studied, there is a growing need to develop noninvasive tests for fibrosis for use in clinical trials.
Several noninvasive methods have been proposed to stage liver fibrosis, including biochemical tests and imaging techniques. The biochemical tests consist of sophisticated indices and scores, or a large number of serological markers of liver fibrosis. However, the value of these diagnostic methods remains debated.
Among the imaging methods, transient elastography is a new technique that rapidly and noninvasively measures mean tissue stiffness. It is largely accepted that hepatic stiffness is related to the degree of fibrosis. Most clinical studies have been performed with FibroScan. Recently, acoustic radiation force impulse (ARFI) imaging sonoelastography has been proposed as an alternative method to assess liver elasticity. To the best of our knowledge, no study has compared ARFI sonoelastography and biochemical tests (APRI index, FibroMax) for the assessment of liver fibrosis.
The aim of our study was to evaluate the value of ARFI elastography for the diagnosis of liver fibrosis and to compare the accuracy of ARFI elastography, APRI index and FibroMax for staging liver fibrosis in patients who underwent liver biopsy for CHC.
The study was a single-centered, prospective, blind comparison of ARFI sonoelastography, APRI index and FibroMax in 100 patients with CHC who underwent liver biopsy at the University Hospital Bucharest between January 2007 and June 2008. Patients with CHC were selected out of 215 patients who attended the University Hospital Bucharest for hepatic cytolysis.
Inclusion criteria were the presence of HCV RNA in serum without previous antiviral treatment and hepatic cytolysis [alanine aminotransferase (ALT) > 1.5 × normal]. Patients with other forms of chronic liver disease and those with ascites were excluded from the study.
Hepatitis C virus (HCV) infection was defined by the presence in the serum of anti-HCV antibodies using the third generation ELISA. HCV infection was confirmed by performing the COBAS TaqMan HCV test (Roche Molecular Systems, Inc., NJ, USA). This test is an in vitro amplification of the HCV nucleic acid, which uses the High Pure System Viral nucleic acid kit (Roche Diagnostics Corp, IN, USA) for manual preparation and the COBAS TaqMan 48 analyzer (Roche Diagnostics Corp, IN, USA) for automatic amplification and detection. The RNA titer was expressed in IU/mL. The detection limit was > 15 IU/mL with a positive rate of 95%.
Patients who fulfilled the inclusion criteria were enrolled and underwent blood tests for APRI, FibroMax measurement, and ultrasound elastography on the day before the liver biopsy.
The study was approved by the local ethics committees and all individuals provided written informed consent prior to enrollment in the study.
Liver function tests were performed prior to the liver biopsy. Blood samples were obtained under fasting conditions and routine liver function tests [ALT and aspartate transaminase (AST), total proteins, serum albumin and γ globulins, γ-glutamyl transferase (GGT), total bilirubin, alkaline phosphatase, international normalized ratio of prothrombin time] were performed. All were measured using Dade Behring reactants and the Dimension RXL analyzer (Dade Behring, FL, USA).
AST levels and platelet counts were measured with a Dimension RXL analyzer and a Hematology System analyzer (Beckman-Coulter, Inc., CA, USA). The APRI index was calculated as follows: AST (/Upper Limit of Normal) × 100/platelet count (109/L). The upper normal limit of AST was considered as 38 U/L.
FibroMax (Biopredictive, France) is a new noninvasive panel of markers reported to predict advanced fibrosis. It contains five tests: FibroTest (to assess the degree of fibrosis); ActiTest (to assess the degree of activity, inflammation and necrosis); SteatoTest (to diagnose hepatic steatosis); NashTest (to diagnose nonalcoholic steatohepatitis); and AshTest (to diagnose severe alcoholic steatohepatitis). FibroMax combines the measurement of 10 indirect parameters adjusted for age, sex, weight and height: α2-macroglobulin, haptoglobin, apolipoprotein A1, total bilirubin, GGT, ALT, AST, fasting glucose, triglycerides and total cholesterol.
FibroMax serum samples were taken on the day before biopsy from patients in the fasting state. Biochemical parameters were measured using Dade Behring reactants and the Dimension RXL analyzer. Specific proteins, α 2-macroglobulin, haptoglobin and apolipoprotein A1, were measured by immunonephelometry using the BN Pro-Spect Dade Behring system.Using FibroMax, fibrosis was staged on a scale of 0-4 with respect to the METAVIR fibrosis staging. For FibroTest, 0-0.21 fibrosis was staged as F0, 0.22-0.27 as F0-F1, 0.28-0.31 as F1, 0.32-0.48 as F1-F2, 0.49-0.58 as F2, 0.59-0.72 as F3, 0.73-0.74 as F3-F4, and 0.75-1 as F4.
ARFI imaging is a new tissue strain imaging technology that utilizes sound waves to interrogate the mechanical stiffness properties of tissues. Virtual Touch tissue imaging and Virtual Touch tissue quantification (Siemens AG, Germany) are the first available applications to implement this technology. Unlike conventional B-mode sonography, which provides anatomical details based on differences in acoustic impedance, Virtual Touch imaging describes relative physical tissue stiffness properties. In complement, Virtual Touch™ tissue quantification provides accurate numerical measurements related to tissue stiffness at user-defined anatomical locations.
Conventional elastography, however, requires manual compression of the tissues in order to provide relative displacement between the mass and the surrounding structures and therefore an assessment of strain. This can be difficult to apply to abdominal structures because of the presence of the ribs and abdominal wall. ARFI technology quantifies stiffness without manual compression. Using the Virtual Touch application, the tissue is compressed by acoustic energy. Virtual Touch tissue quantification is a quantitative assessment of tissue stiffness, through measurement of shear wave speed. Shear waves are generated by displacement of tissue and attenuate approximately 10 000 times more rapidly than conventional ultrasound waves.
Prior to liver biopsy, the patients underwent liver ultrasonographic elastography, under fasting conditions, using ARFI imaging [Acuson S2000 (Siemens AG, Germany] with Virtual Touch tissue quantification software). The system uses a standard ultrasonographic probe and offers elastography with a flexible metering box of 1 cm at variable depths. An acoustic push pulse transmitted by the transducer (3.5 MHz) toward the tissue induces an elastic shear wave that propagates through the tissue. The propagation of the shear wave is followed by detection pulses that are used to measure the velocity of shear wave propagation, which is directly related to tissue stiffness: speed increases with stiffness.
Liver stiffness was assessed by the same physician blinded to clinical and biological data. The measurements were performed on the right lobe of the liver through the intercostal spaces, with the patient lying in the decubitus dorsal position, with the right hand under the head and the head turned toward the left. The tip of the probe was covered with coupling gel and placed on the skin between the ribs at the level of the right lobe of the liver. The operator positioned the probe over the area of interest: segment eight of the right lobe, away from motion and portal/hepatic vessels, about 2 cm from the liver capsula, at a depth between 3.8 and 5.5 cm. When the target area had been located after optimizing the B-mode image, the operator pressed the button Virtual Touch tissue quantification, and asked the patient to stop breathing for a moment. Then, we observed the velocity of shear wave on screen. We used this protocol because we saw that, when the right lobe was scanned through the intercostal spaces with normal breathing, the variance of measurement was low. A total of 12 valid measurements per patient were performed. In difficult patients, to obtain better access to the liver without excessive pushing or breath holding, the measurements were performed on patients lying in the left lateral decubitus position, or using a subcostal approach to the left lobe. The results of ARFI ultrasonographic elastography were expressed as liver propagation velocity (m/s). The success rate of liver elasticity measurements was calculated as the ratio between validated and total measurements. Results were expressed as the median value of the total measurements (12 measurements per patient) in m/s, with values ranging from 0.8 to 3.7 m/s. Only procedures with a success rate of at least 60%, and with the interquartile range of all validated measurements < 30% of the median value, were considered reliable.
Percutaneous liver biopsy was performed by senior operators using the Menghini technique with a 1.4-mm-diameter needle (Hepafix; B Braun Melsungen AG, Germany). After biopsy, the liver samples were fixed in formalin, paraffin embedded, and stained with hematoxylin-eosin and Masson’s trichrome. All biopsy specimens were analyzed by an expert pathologist blinded to the biological and clinical data and to the results of ultrasound elastography. The length of each liver biopsy was established in millimeters and the number of portal tracts was counted.
Liver fibrosis and necroinflammatory activity were evaluated by the METAVIR scoring system. Fibrosis was staged on a four-point scale according to this score: F0 represented no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis and a few septa; F3, numerous septa without cirrhosis; and F4, cirrhosis. Necroinflammatory activity was also graded on a four point scale: A0, none; A1, mild; A2, moderate; A3, severe.
Steatosis was assessed according to the number of hepatocytes with degeneration, as follows: 0, none; 1, steatosis in 1%-10% of hepatocytes; 2, steatosis in 11%-33% of hepatocytes; 3, steatosis in 34%-66% and 4, 67%-100% of hepatocytes[3].
Results are presented as the mean ± SD, counts and percentages. The results are illustrated as the median and 25th- to 75th-percentile values. The correlation between the noninvasive tests and liver biopsy was tested using the non-parametric Spearman’s correlation coefficient. The overall validity was measured using area under the receiver operating characteristic curve (AUROC) with 95% CI. Cutoff values that defined prediction regions for each fibrosis stage were defined by a common optimization step that maximized the sum of the sensitivities in predicting the single stages. Finally, sensitivity, specificity, and positive (PPV) and negative predictive value (NPV) were calculated. The optimal cutoff was chosen at the highest left point on the curve. For all tests, significance was achieved at P < 0.05.
A total of 100 patients underwent liver biopsy for CHC. The patients were selected out of 215 patients who attended the University Hospital Bucharest for hepatic cytolisis. Ten patients refused to participate and six were excluded: three had hepatitis B virus coinfection, and three had a current daily alcohol intake of at least 60 g/d. In 10 patients, liver biopsy was unsuitable for fibrosis staging with the length of sample < 15 mm.
Seventy-four patients underwent ARFI elastography, APRI index, FibroMax and successful liver biopsy (Figure 1). Demographic, clinical and biochemical characteristics of the patients included in the study are shown in Table 1.
| Characteristics | Patients included (n = 74) |
| Sex (male/female) | 32/42 |
| Age (yr) | 55.32 ± 9.6 (31-72) |
| BMI (kg/m2) | 25.59 ± 4.03 (17.23-33.29) |
| AST (IU/L) | 50.06 ± 32.36 (18-188) |
| ALT (IU/L) | 67.06 ± 26.79 (20-147) |
| INR | 1.12 ± 0.15 (0.9-1.74) |
| Bilirubin (mg/dL) | 0.69 ± 0.32 (0.29-2.08) |
| Albumin (g/dL) | 3.9 ± 0.55 (2.6-4.9) |
| Cholesterol (mg/dL) | 192.36 ± 46.58 (120-311) |
| Triglycerides (mg/dL) | 123.24 ± 73.33 (48-467) |
The median biopsy length was 20 mm (range: 15-25 mm); the number of portal tracts was 12 ± 4 (range: 8-16). Patient distribution according to METAVIR fibrosis stage and activity grade and steatosis are presented in Table 2.
| Fibrosis | Activity | Steatosis | |||
| Stage | n (%) | Grade | n (%) | Grade | n (%) |
| 0 | 1 (1.3) | 0 | 1 (1.3) | 0 | 24 (32.4) |
| 1 | 9 (12.1) | 1 | 32 (43.2) | 1 | 23 (31.08) |
| 2 | 25 (33.7) | 2 | 29 (39.1) | 2 | 12 (16.2) |
| 3 | 19 (25.6) | 3 | 12 (16.2) | 3 | 11 (14.8) |
| 4 | 20 (27.02) | 4 | 4 (5.40) | ||
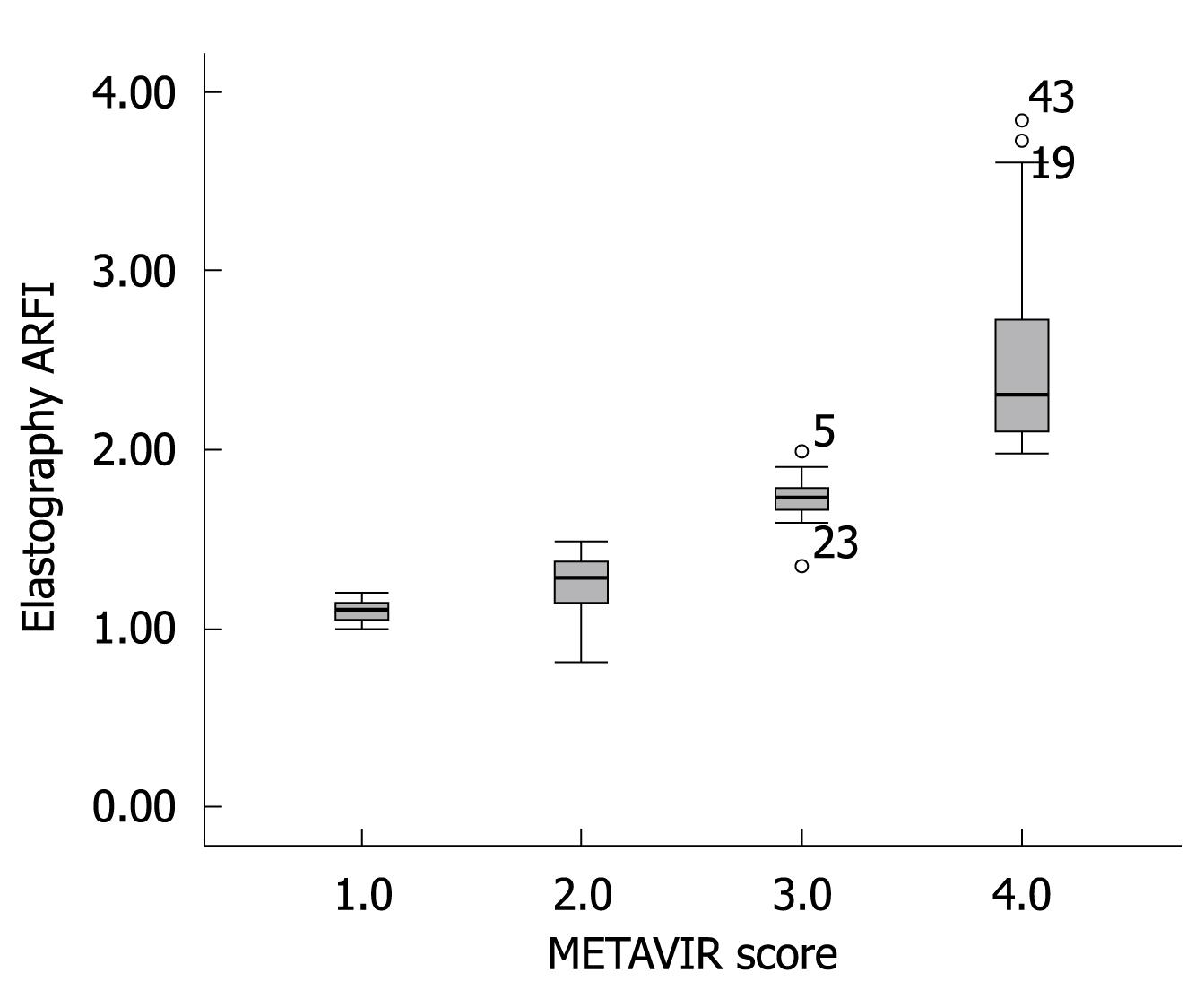
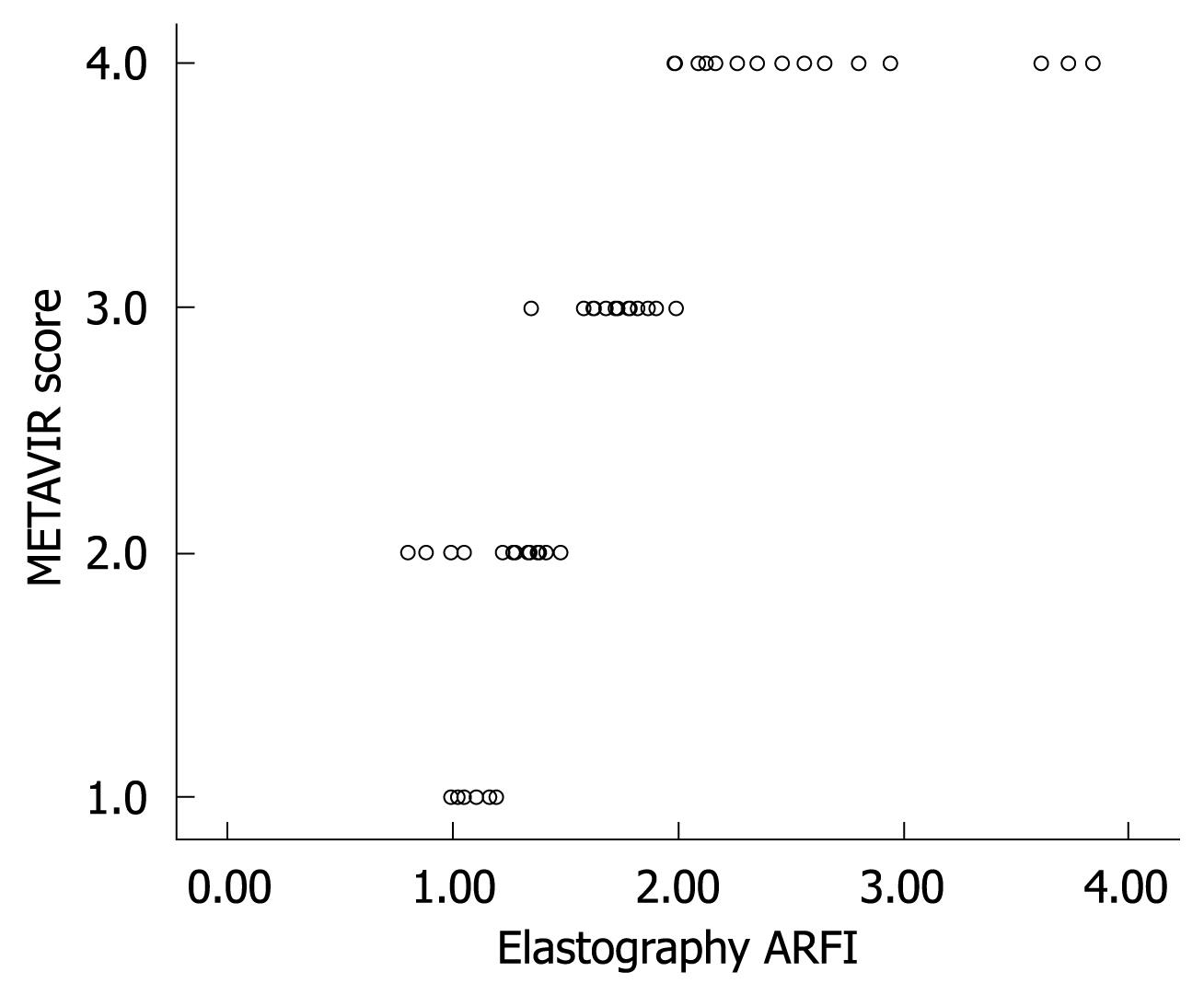
Figure 2 shows the median value (95% CI) of liver elasticity compared with fibrosis stages according to METAVIR score. A high correlation of increasing liver stiffness with increasing stage of fibrosis was observed. The Spearman’s correlation coefficient between the liver stiffness and the histological fibrosis stages was highly significant with a value of 0.919 (P < 0.001). Steatosis is a frequent occurrence in CHC[4]. According to ANOVA test, no influence of the degree of activity or steatosis on elasticity values was observed in this study.
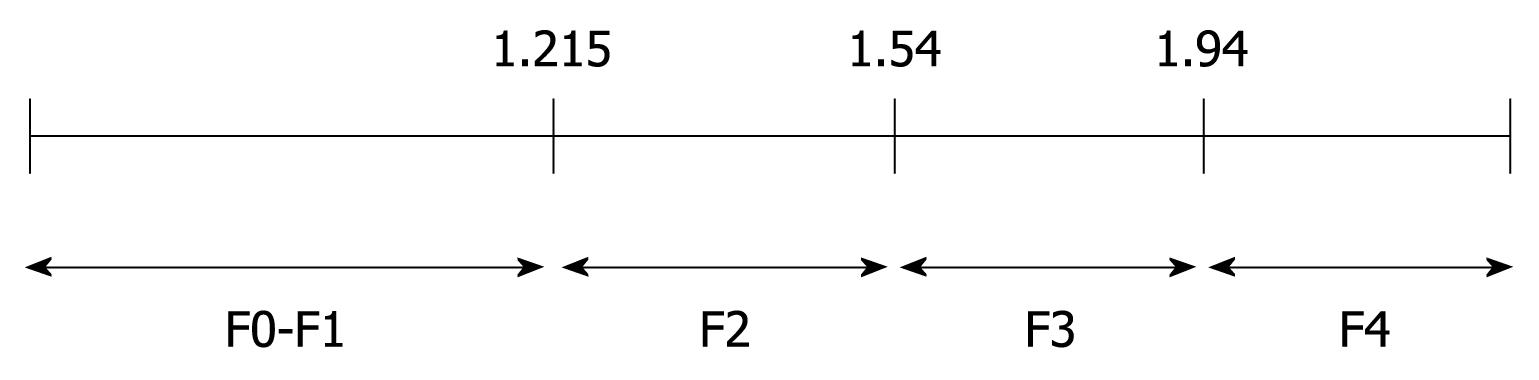
To determine the cutoffs that predicted each degree of fibrosis, we used the ROC curve method (Figure 3). ARFI elastography could predict fibrosis F2 or more, with a validity of 90.2% (95% CI AUROC = 0.831-0.972, P < 0.001). The optimal cutoff point between F1 and F2 was 1.215. At this value, ARFI elastography had a sensitivity of 89.4% and a specificity of 100%.
ARFI sonoelastography predicted even better F3 or F4 fibrosis (AUROC = 99.3%, 95% CI = 0.979-1). The optimal cutoff between F3 and F4 was 1.54, with sensitivity and specificity of 97% and 100%, respectively. The specificity of 100% shows that all study patients who presented with a value of ≥ 1.54 upon sonoelastography had liver fibrosis F3 or more (confirmed by liver biopsy).
The same high validity was maintained as in predicting cirrhosis: (AUROC = 99.3%, 95% CI = 0.989-1).
The optimal cutoff in predicting cirrhosis was 1.94 with a sensitivity of 100% and a specificity of 98.1%. In other words, every patient in this study that had a sonoelastography result with a value < 1.94 did not have cirrhosis.
The most discriminative cutoff values of ARFI elastography are presented in Figure 4.
The corresponding sensitivity, specificity, PPV and NPV are shown in Table 3.
| ≥F1 | ≥F2 | ≥F3 | F4 | |
| Cutoff (m/s) | 1.185 | 1.215 | 1.54 | 1.94 |
| Sensitivity (95% CI) | 89% (79-95) | 100% (91-100) | 97% (86-100) | 100% (83-100) |
| Specificity (95% CI) | 87% (47-99) | 71% (53-85) | 100% (90-100) | 98% (90-99) |
| PPV (95% CI) | 98% (91-99) | 79% (65-89) | 100% (90-100) | 95% (76-100) |
| NPV (95% CI) | 50% (23-76) | 100% (86-100) | 97% (85-100) | 100% (93-100) |
| Positive likelihood ratio | 7.1 | 3.5 | 3.5 | 54 |
A high correlation of increasing noninvasive measurements with increasing stage of fibrosis was observed. The Spearman’s correlation coefficient between APRI index and FibroMax and the histological fibrosis stages was highly significant: 0.712 for APRI index and 0.674 for FibroMax. Table 4 shows the correlation between noninvasive tests and liver histology.
| Noninvasive test | Correlation (Spearman coefficient) | 95% CI | P | |
| Liver biopsy | Elastography ARFI | 0.919 | 0.872-0.949 | < 0.001 |
| APRI index | 0.712 | 0.572-0.811 | < 0.001 | |
| FibroMax | 0.674 | 0.521-0.784 | < 0.001 |
The correlation between two noninvasive tests should be better than with histological assessments for a correct prediction of liver fibrosis stage. The inter-test correlation coefficients, ARFI elastography/APRI index, and ARFI elastography/FibroMax were 0.712 and 0.718, respectively. All noninvasive tests used (Elastography ARFI, APRI index and FibroMax) were highly correlated with each other.
In chronic viral hepatitis, the knowledge of the stage of liver fibrosis is important for prognosis and for decisions about antiviral treatment[5]. Significant fibrosis (≥ F2) in these patients is an indicator for antiviral treatment, hence the great therapeutic value of a highly accurate diagnostic test. At present, liver biopsy is used as the reference standard for the assessment of liver fibrosis[2]. However, liver biopsy is becoming increasingly useless in the management of HCV-related chronic liver disease because of large sampling error, consistent inter-observer disagreement, unavoidable risks and complications, and the fact that it is a snapshot of a process that is everything but a static one. Replacement of liver biopsy in the assessment of chronic liver disease is the goal of any noninvasive technique. Therefore, a test with diagnostic performance for precise estimation of the degree of liver fibrosis is of great therapeutic value. Previous studies have reported that transient elastography (FibroScan) can detect liver fibrosis and accurately predict significant fibrosis[5-11]. In the present study, we showed that APRI elastography should be taken into consideration as a noninvasive sonographical method for evaluation of liver fibrosis. A significant positive correlation between liver stiffness and the stages of fibrosis was noticed in patients with CHC. Significant AUROC for fibrosis F2 or more had a validity of 90.2% (95% CI AUROC = 0.831-0.972, P < 0.001).
ARFI proved to be an even better predictor of F3 or F4 fibrosis (AUROC = 99.3%, 95% CI = 0.979-1). Early and rapid detection of significant fibrosis using this noninvasive procedure is essential since patients with significant fibrosis are at a high risk of developing complications, such as portal hypertension or hepatocellular carcinoma, and consequently need specific follow-up.
We considered ascites as one of the exclusion criteria from our study since ARFI, similar to Fibro-Scan, is performed in close contact with the liver. Nevertheless, ascites indicates the presence of cirrhosis, which makes noninvasive staging of fibrosis unnecessary[5].
Liver elastometry with FibroScan is unsuccessful in patients with narrow intercostal spaces and in those with morbid obesity[7,8]. These are the major limitations of FibroScan measurements[12]. In the present study, patients with a body mass index (BMI) of up to 30 were enrolled, and ARFI elastography was performed successfully in all included patients. For the majority of the patients, the measurements were performed through a right intercostal space, and patients did not need to hold their breath during the examination. In difficult patients with narrow intercostal spaces, the examination was improved because of the subcostal approach to the left liver lobe. Elastography of the left liver lobe is especially helpful in obese patients. Rifai et al[13] have found recently that ARFI results of the right and left liver lobe were comparable (n = 26, r = 0.44, P < 0.03). Furthermore, ARFI elastography is less time consuming than FibroScan. The new software allows one to do 12 measurements in under 1 min, as compared to FibroScan that does 10 measurements in under at least 4 min[9].
The current study confirmed that, in CHC, no influence of steatosis or activity grade on correlation between elasticity values and fibrosis stages was observed. According to ANOVA test, the elasticity values were not affected by steatosis or activity grade. Similar results have been reported from studies analyzing transient elastography with FibroScan[7-9].
Three noninvasive tests were performed. The tests had a good correlation with the liver biopsy results. The most powerful test in predicting fibrosis was ARFI elastography, with a non-parametric correlation coefficient of 0.919 (95% CI = 0.872-0.949, P < 0.001). Other correlation coefficients were: 0.712 (95% CI = 0.572-0.811, P < 0.001) for APRI index, and 0.674 (95% CI = 0.521-0.784, P < 0.001).
In the present study, we found highly significant positive correlations between liver propagation velocity obtained with ARFI elastography and the METAVIR fibrosis stage. The correlation coefficients showed a linear relationship between ARFI elastography and liver biopsy fibrosis, but to determine the cutoffs that predicted each degree of fibrosis, we used the ROC curve method.
An AUC value of 0.902 (0.831-0.972) was obtained using ARFI elastography for the diagnosis of significant fibrosis (≥ F2), with a cutoff value of 1.215 m/s. In comparison, three recent studies that analyzed the noninvasive assessment of liver fibrosis with FibroScan have revealed AUCs between 0.75 and 0.84 for the diagnosis of significant fibrosis (≥ F2)[7-9]. The ARFI sonoelastography predicted even better F3 or F4 fibrosis (AUROC = 99.3%, 95% CI = 0.979-1).
Considering the increased values of sensitivity and specificity, ARFI sonoelastography was shown to be a valuable investigative technique that could be of great help in the evaluation and follow-up of liver fibrosis.
At present, it is difficult to determine the real impact of ARFI elastometry in the early diagnosis of fibrosis in patients with CHC. Figure 5 clearly indicates an overlap observed between F0-F1 and F2. The increase in liver propagation velocity was more important between stages F2 (1.21 m/s) and F3 (1.54 m/s) than between stages F1 (1.18 m/s) and F2 (1.21 m/s). This is consistent with the fact that the increase in fibrous tissue is more important between stages F2 and F3 than between stages F1 and F2.
This limit of ARFI elastography (reduced discriminatory value between F1 and F2) was overcome by the fact that significant fibrosis (≥ F2) is considered a hallmark of progressive liver disease. Studies have shown that antiviral treatment of patients with CHC prolongs life, improves quality of life, and is cost-effective[14,15]. However, treatment may be associated with severe side effects and the decision for treatment needs to be made on an individual basis. Patients with fibrosis stage F2 are at increased risk for developing cirrhosis with its complications (ascites, encephalopathy, or portal hypertension). Therefore, patients with fibrosis stage ≥ F2 have a stronger indication for treatment as compared with patients with no or mild fibrosis (F1)[16,17].
Compared with fibrosis biomarkers, the disadvantage of liver elastometry is the absence of a large control group to assess the limit of normal values[14-18]. In studies using liver biopsy as a reference method, as in our study, the number of patients without fibrosis (F0) is very small.
Liver biopsy was considered the gold standard in our study because it is the only reference method available at present[1,19]. However, this technique is an invasive procedure that is known to have serious limitations. Various studies have shown that a single-needle liver biopsy can miss the diagnosis of cirrhosis in 20%-50% of patients[19]. To maximize the diagnostic yield of liver biopsy, we selected only the patients with a biopsy length > 15 mm and histological sections with at least eight portal tracts. The major advantage of ARFI liver elastography compared with liver biopsy is that it is painless, rapid, has no risk of complications, is very well tolerated and it is more representative of the entire liver parenchyma than is liver biopsy (1/50 000 of the total liver mass)[2].
To assess the performance of ARFI elastography, we have compared the liver propagation velocity with predictive blood tests (FibroMax) and APRI index in the same population. Our results showed that the most powerful test in predicting fibrosis was ARFI elastography. The high correlation between these three noninvasive tests suggests a sequential combination of them in algorithms used for staging liver fibrosis in patients with CHC.
In conclusion, our study showed that ARFI sonoelastography stands out on account of its very good correlation with liver biopsy (gold standard for evaluating liver fibrosis), good sensitivity and excellent specificity. It has proved to be a noninvasive imaging method, which is far superior to other methods investigated (APRI and FibroMax), for staging liver fibrosis. Further investigations on a larger number of patients are necessary to validate ARFI elastography as a noninvasive method of diagnosing liver fibrosis.
Liver biopsy is an invasive procedure with certain unavoidable risks and complications. Therefore, the development of noninvasive tests to assess hepatic inflammation and fibrosis has been an active area of research. Several noninvasive methods have been proposed to stage liver fibrosis, including biochemical tests and imaging techniques. The biochemical tests consist of sophisticated indices and scores, or a large number of serological markers of liver fibrosis. However, the value of these diagnostic methods remains debatable. Among the imaging methods, transient elastography, based on ultrasound, is a new technique that rapidly and noninvasively measures mean tissue stiffness.
Transient elastography is a noninvasive method with performance equivalent to that of serum markers for the diagnosis of significant fibrosis in patients with hepatitis C. Combining transient elastography with serum markers as first-line assessment could avoid liver biopsy in the majority of these patients. There is now a general consensus that liver fibrosis is potentially reversible. Follow-up biopsy is too insensitive for real-time monitoring of progression and regression of fibrosis. When considering anti-fibrotic therapy, it is important to recognize that fibrosis is a dynamic process. Clinical proof and monitoring of anti-fibrotic drug effects requires better noninvasive tests for fibrosis. Incorporation of noninvasive tests into large studies and therapeutic trials should be a priority in the next few years.
Acoustic radiation force impulse (ARFI) imaging sonoelastography has been proposed as an alternative method to assess liver elasticity. No comparison of ARFI sonoelastography and biochemical tests (APRI and FibroMax) has been reported for the assessment of liver fibrosis.
ARFI imaging could be applied to the noninvasive assessment of hepatic fibrosis initially, or after treatment. This study evaluated ARFI imaging in patients with chronic hepatitis C, but probably it could be used for other liver diseases that lead to fibrosis (we need further studies).
Elastography is a noninvasive method by which stiffness of soft tissue is evaluated.
The present study provides readers with a reliable, relatively inexpensive tool to measure liver fibrosis. It is a well designed, conducted and reported study.
Peer reviewers: Giovanni Tarantino, MD, Professor, Department of Clinical and Experimental Medicine, Federico II University Medical School, Via S. Pansini, 5, Naples 80131, Italy; Dr. Ching-Chung Lin, Gastroenterology Division, Mackay Memorial Hospital, 5F, No 28, Lane 286, Shidong Road, 11154 Taipei, Taiwan, China
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
| 1. | Goodman ZD. Fibrosis in chronic hepatits C - assessment by liver biopsy. In: AASLD Postgraduate Course: Liver Diseases: Pathophysiologic Basis for the Therapy of Liver Disease. Boston, USA 2007; 86-91. |
| 2. | Afdhal NH. Non-biopsy methods to determine hepatic fibrosis. In: AASLD Postgraduate Course: Liver Diseases: Pathophysiologic Basis for the Therapy of Liver Disease. Boston, USA 2007; 91-95. |
| 3. | Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, Sarrazin C. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758-764. |
| 4. | Fierbinţeanu-Braticevici C, Mohora M, Creţoiu D, Creţoiu S, Petrişor A, Usvat R, Ion DA. Role of oxidative stress in the pathogenesis of chronic hepatitis C (CHC). Rom J Morphol Embryol. 2009;50:407-412. |
| 5. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. |
| 6. | Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, de Lédinghen V. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46:628-634. |
| 7. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. |
| 8. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. |
| 9. | Chang J, Tan HH, Yew BS. Transient elastography (FibroScan®) to assess hepatic fibrosis in Chinese with chronic hepatitis B. 17th Asian Pacific Association for the Study of Liver Conference. In: AASLD Postgraduate Course: Liver Diseases: Pathophysiologic Basis for the Therapy of Liver Disease. Boston, USA 2007; . |
| 10. | Kim KM, Choi WB, Park SH, Yu E, Lee SG, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. Diagnosis of hepatic steatosis and fibrosis by transient elastography in asymptomatic healthy individuals: a prospective study of living related potential liver donors. J Gastroenterol. 2007;42:382-388. |
| 11. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. |
| 12. | Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48:606-613. |
| 13. | Rifai K, Bahr MJ, Mederacke I, Bantel H, Bayer D, Boozari B, Wedemeyer H, Manns MP, Gebel M. Acoustic Radiation Force Imaging (ARFI) as a new method of Ultrasonographic elastography allows accurate and flexible assessment of liver stiffness. Copenhagen: Poster presentation EASL 2009; . |
| 14. | Poynard T, Halfon P, Castera L, Charlotte F, Le Bail B, Munteanu M, Messous D, Ratziu V, Benhamou Y, Bourlière M. Variability of the area under the receiver operating characteristic curves in the diagnostic evaluation of liver fibrosis markers: impact of biopsy length and fragmentation. Aliment Pharmacol Ther. 2007;25:733-739. |
| 15. | Poynard T, Halfon P, Castera L, Munteanu M, Imbert-Bismut F, Ratziu V, Benhamou Y, Bourlière M, de Ledinghen V. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem. 2007;53:1615-1622. |
| 16. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. |
| 17. | Poynard T, Munteanu M, Imbert-Bismut F, Charlotte F, Thabut D, Le Calvez S, Messous D, Thibault V, Benhamou Y, Moussalli J. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344-1355. |
| 18. | Ngo Y, Munteanu M, Messous D, Charlotte F, Imbert-Bismut F, Thabut D, Lebray P, Thibault V, Benhamou Y, Moussalli J. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896. |
| 19. | Crockett SD, Kaltenbach T, Keeffe EB. Do we still need a liver biopsy? Are the serum fibrosis tests ready for prime time? Clin Liver Dis. 2006;10:513-534, viii. |