Published online Nov 21, 2009. doi: 10.3748/wjg.15.5481
Revised: October 12, 2009
Accepted: October 19, 2009
Published online: November 21, 2009
AIM: To clarify the benefit of surgical excision for patients with extrahepatic metastases of hepatocellular carcinoma (HCC).
METHODS: We retrospectively reviewed the medical records of 140 patients with pathologically proven extrahepatic metastases of HCC and evaluated the outcomes of those who had undergone surgical resection (SR) for extrahepatic metastatic lesions. Prognoses made on the basis of extrahepatic metastatic sites were also examined.
RESULTS: The survival rates of patients who underwent SR of extrahepatic metastases were significantly better than those of patients who did not receive SR. For the SR group, 1- and 3-year survival rates were 24% and 7%, respectively, while for the non-resection group, the survival rates were 8% and 0%, respectively (P < 0.0001). Survival rates related to metastatic sites were also significantly superior after SR of extrahepatic metastases: median survivals were 32 mo with lung metastasis, 10 mo with bone metastasis, 6.1 mo with brain metastasis.
CONCLUSION: SR can provide survival benefits for patients with 1 or 2 isolated extrahepatic metastases and who concurrently exhibit good hepatic functional reserve and general performance status as well as successful treatment of intrahepatic HCC.
- Citation: Chan KM, Yu MC, Wu TJ, Lee CF, Chen TC, Lee WC, Chen MF. Efficacy of surgical resection in management of isolated extrahepatic metastases of hepatocellular carcinoma. World J Gastroenterol 2009; 15(43): 5481-5488
- URL: https://www.wjgnet.com/1007-9327/full/v15/i43/5481.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5481
Hepatocellular carcinoma (HCC) is an aggressive malignant tumor that occurs throughout the world. It is one of the leading causes of cancer-related death among people in East Asian countries. Recent progress in diagnostic tools has led to an improved prognosis for HCC patients because of enhanced detection of early and small HCC and better identification of those patients who qualify for hepatic resection (HR)[1-5]. Moreover, treatment trends towards aggressive multimodal therapy in patients with intra-hepatic HCC have also improved long-term outcomes[6-9]. Nevertheless, extrahepatic metastasis of primary HCC is still considered terminal-stage cancer, and the prognosis for patients at this stage continues to be poor due to limited effective treatment. Despite recognition of this situation, there appears to be no consensus among the medical community regarding treatment strategies for HCC patients with extrahepatic metastasis. Therefore, to improve the overall long-term survival of patients with HCC, more active treatment of extrahepatic metastases is necessary. Several studies have recently demonstrated that surgical resection (SR) of extrahepatic metastases is able to provide benefits for selected patients with HCC[10-13]. However, the role of SR in HCC patients with extrahepatic metastases required further clarification. In this study, we present our experience with patients with extrahepatic metastases of primary HCC and evaluate the benefits of surgical removal of extrahepatic lesions.
A total of 2245 patients with primary HCC were treated at the Department of Surgery, Chang Gung Memorial Hospital-Linkou Medical Center in Taiwan, during the study period between June 1988 and June 2008. Among them, medical records of 151 patients with pathologically proven extrahepatic metastases from primary HCC were retrospectively reviewed. Metastasis of HCC was confirmed for all patients by histological examination of specimens derived from either biopsy or excision of extrahepatic tumor lesions. Of the 151 patients, 11 were lost to follow-up; thus, the remaining 140 patients, including 108 men and 32 women, were enrolled in the present study.
Intrahepatic primary or recurrent HCC was diagnosed by dynamic liver computed tomography (CT) scans and hepatic angiography, which shows hyperattenuation in the arterial phase and hypoattenuation in the late phase indicating hypervascular tumor mass. Ultrasound-guided biopsy was performed only when considered necessary. α-fetoprotein was also routinely checked as a parameter of diagnosis.
Treatment of intrahepatic HCC consisted of multimodal therapeutic procedures including hepatic resection (HR), radiofrequency ablation, percutaneous chemical reagent injection, transcatheter arterial chemoembolization (TACE), or a combination of these treatment modalities. Treatment methods were selected for hospitalized HCC patients based on the following principles. HR was considered a priority for patients with intrahepatic HCC whenever the tumor was determined to be resectable. An alternative approach, such as local ablation or TACE, was performed as indicated if patients had systemic multiple tumors, a difficult tumor location, unsuitable conditions for HR, or refused surgical treatment. Patients might also have received concurrent or sequential multimodal treatments if conditions warranted.
Detection of extrahepatic HCC and determination of tumor location was accomplished through X-ray, CT, magnetic resonance imaging (MRI), or bone scintigraphy as indicated and under two conditions: namely, recurrent and concurrent. Recurrent extrahepatic HCC was noted during post-operative follow-up after treatment for primary HCC. Concurrent extrahepatic HCC was observed at the initial diagnosis stage of intrahepatic HCC. In order to ensure the significance and validity of our research, only those patients with pathologically proven extrahepatic metastasis of HCC were included in our study.
Treatment of extrahepatic metastasis varied according to the individual clinical characteristics of each patient. SR was selected for patients whose extrahepatic HCC was solitary, isolated, and considered resectable, and for whom intrahepatic tumor recurrence was not observed after previous treatment or for whom the intrahepatic tumor was completely treated by either HR or other treatment modalities. Systemic chemotherapy was reserved for those patients deemed unsuitable for SR. Radiotherapy was performed to relieve patients’ symptoms as indicated.
All patients were closely followed up at regular intervals until death or until the writing of this article. The duration of follow-up after first diagnosis of HCC ranged from 1 to 138 mo (median, 16 mo). Follow-up after diagnosis of extrahepatic metastasis ranged from 0.8 to 96 mo (median, 6.1 mo).
All data were analyzed using the statistical software Prism 5.0 (GraphPad Software, San Diego, CA, USA). Survival rates were calculated by the Kaplan-Meier method, and the log-rank test was used to compare survival rates. Patients who died from surgical complications were excluded from the survival curve. A value of P < 0.05 was considered statistically significant.
The clinical characteristics of patients at initial diagnosis of extrahepatic metastases are summarized in Table 1. The average age of these patients was 55.8 years (range, 13 to 81 years). Predominant clinical elements were male gender (77%, 108/140) and a positive test for the hepatitis B virus (47%, 66/140). These findings were similar to those of our previous reports on primary HCC[14,15]. Further, 66 patients (47%) had previously undergone HR for primary HCC. Among these patients, 7 had received multiple HRs for intrahepatic HCC and 2 had undergone liver transplantation. Extrahepatic metastases had occurred mainly at bone, brain, lung, and soft tissue locations. Patients categorized into the soft tissue group had extrahepatic metastases to intra-abdominal soft tissue (6 patients), intra-abdominal organs (4 patients), abdominal wall (16 patients), breast (1 patient), and skin plus subcutaneous tissue (8 patients).
| Characteristics | n (%) |
| Age (yr) | 55.8 ± 14.4 |
| Male:Female | 108:32 |
| Hepatitis B virus surface antigen | 66 (47) |
| Hepatitis C virus antibody | 30 (21.4) |
| Previous hepatic resection | |
| Yes | 66 (47) |
| Single hepatic resection | 57 |
| Multiple hepatic resection | 7 |
| Liver transplantation | 2 |
| No | 74 (53) |
| Location of metastatic tumor | |
| Lung | 25 (17.8) |
| Bone | 47 (33.6) |
| Brain | 28 (20) |
| Soft tissue | 35 (25) |
| Heart | 2 (1.4) |
SR of extrahepatic metastases of HCC was performed on 86 patients (64 male and 22 female: Table 2). Further, 59 out of 86 patients had intrahepatic tumors at the time of extrahepatic metastasis detection. Among these patients, 54 were also treated by TACE, local ablation, or a combination of both therapies for their intrahepatic lesion; thus, most patients received more than one course of treatment prior to resection of the extrahepatic lesion. Five patients underwent simultaneous HR for intrahepatic HCC and SR of extrahepatic metastases.
| Characteristics | n (%) |
| Age (yr) | 54.1 ± 14.9 |
| Male:Female | 64:22 |
| Hepatitis B virus surface antigen | 46 (53) |
| Hepatitis C virus antibody | 22 (21.4) |
| Concurrent treated intrahepatic lesion | |
| Yes | 59 (69) |
| No | 27 (31) |
| Location of metastatic tumor | |
| Lung | 11 (12.8) |
| Bone | 24 (27.9) |
| Brain | 25 (29.1) |
| Soft tissue | 24 (27.9) |
| Heart | 2 (2.3) |
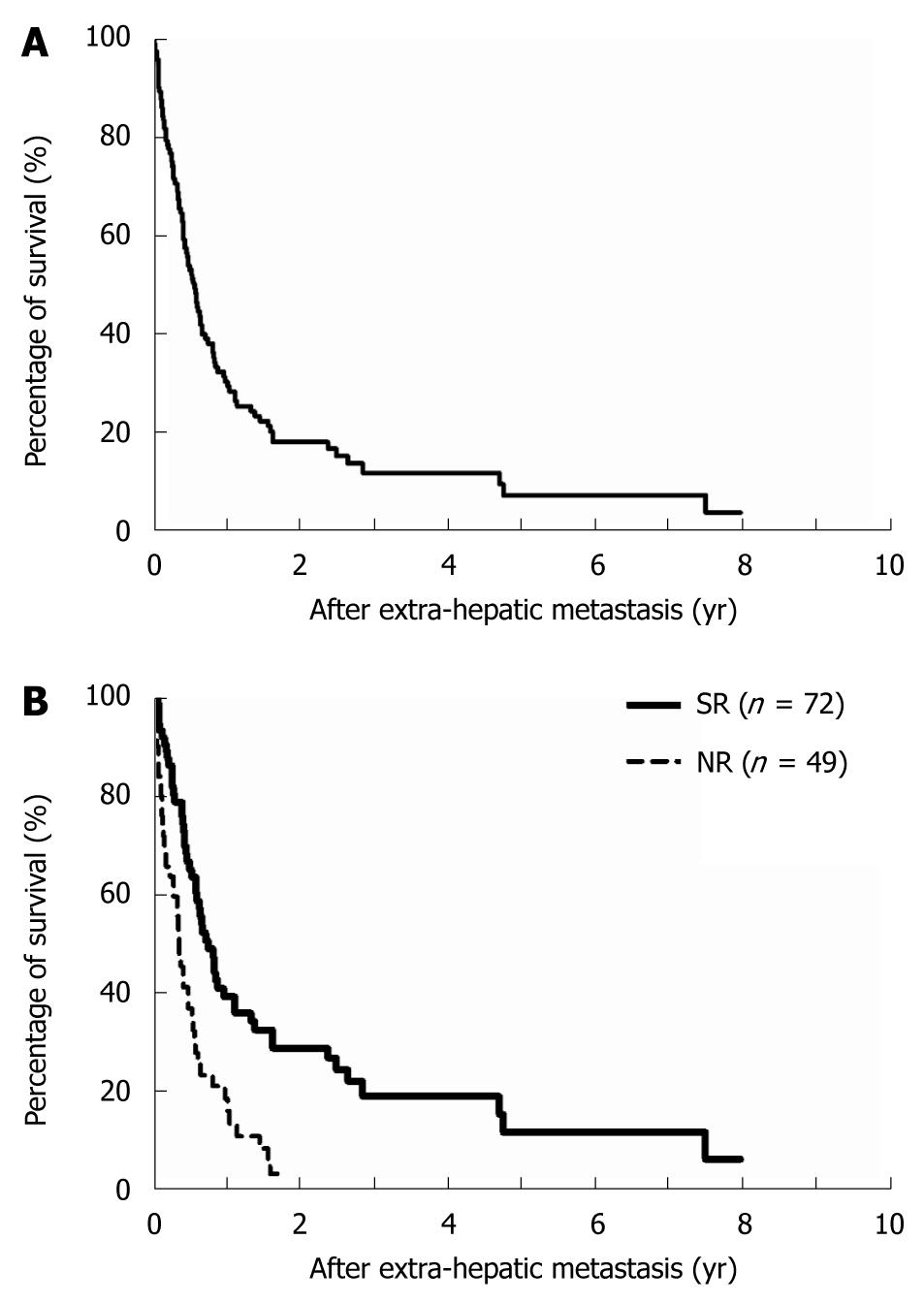
The median length of patient survival was 6.9 mo (range, 0.8 to 96 mo), and the overall 1-, 3-, and 5-year survival rates were 31%, 7%, and 4%, respectively, for all patients with extrahepatic metastases of HCC (Figure 1A). Long-term overall survival was significantly better in patients who underwent SR of extrahepatic metastases than in those who did not [non-resection (NR) patients]. The 1-year/3-year survival rates were 24%/7% for the SR group as compared with the 1-year/3-year survival rates of 8%/0% for the NR group (Figure 1B, P < 0.0001).
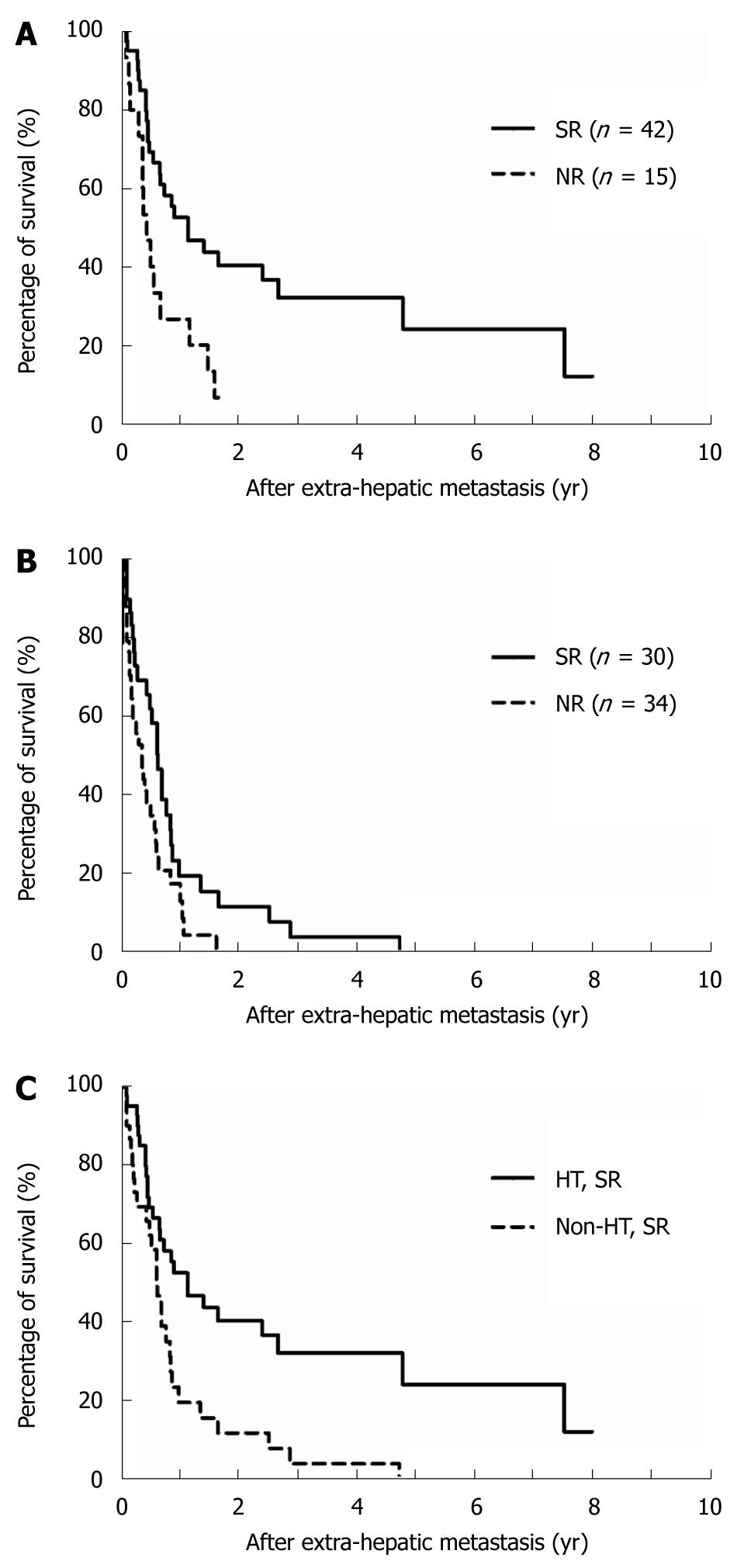
We further analyzed survival rates according to whether HR had been performed. Sixty-six patients had previously undergone HR (Table 1), including 5 patients who underwent simultaneous resection of intrahepatic and extrahepatic tumors at initial diagnosis and 61 patients who underwent resection of intrahepatic tumors followed by subsequent resection of extrahepatic tumor recurrence. The outcome for patients who underwent SR was significantly better than that of the NR patients (Figure 2A and B), and the survival rate of SR patients in the HR group (1-year/3-year, 52.5%/32.1%) was considerably better than that of the non-HR group (1-year/3-year, 19.4%/3.9%, P = 0.0020; Figure 2C).
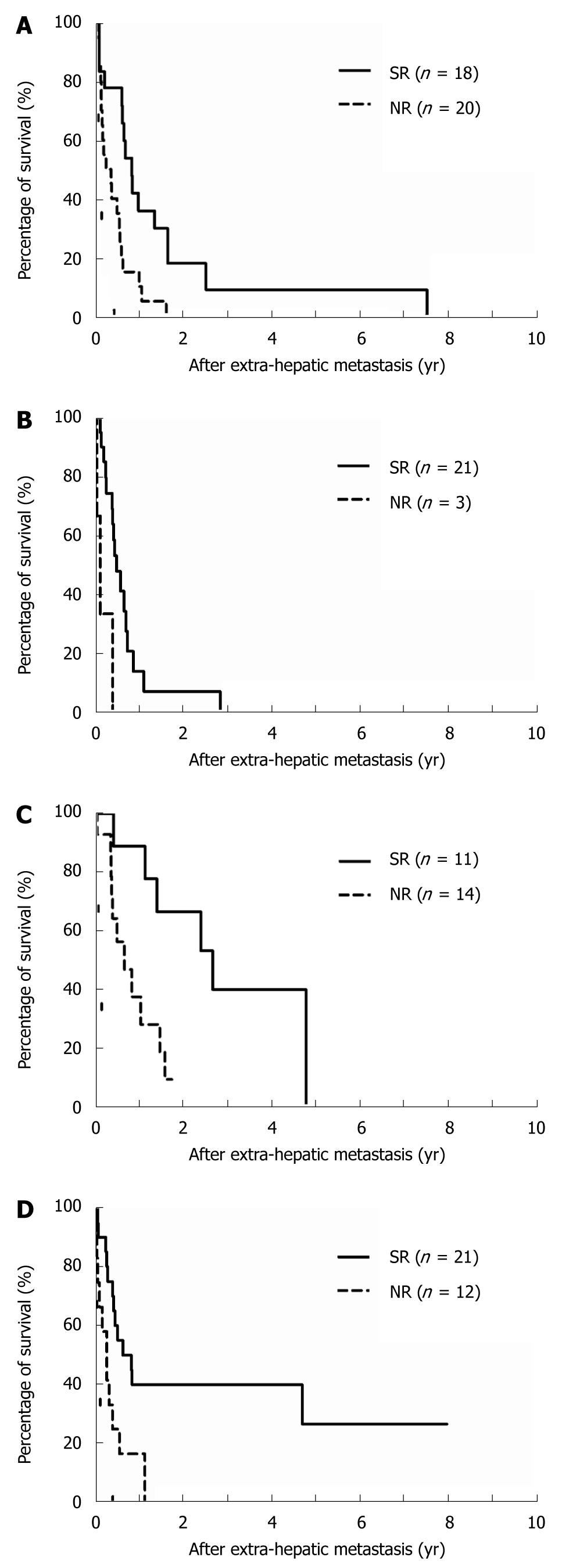
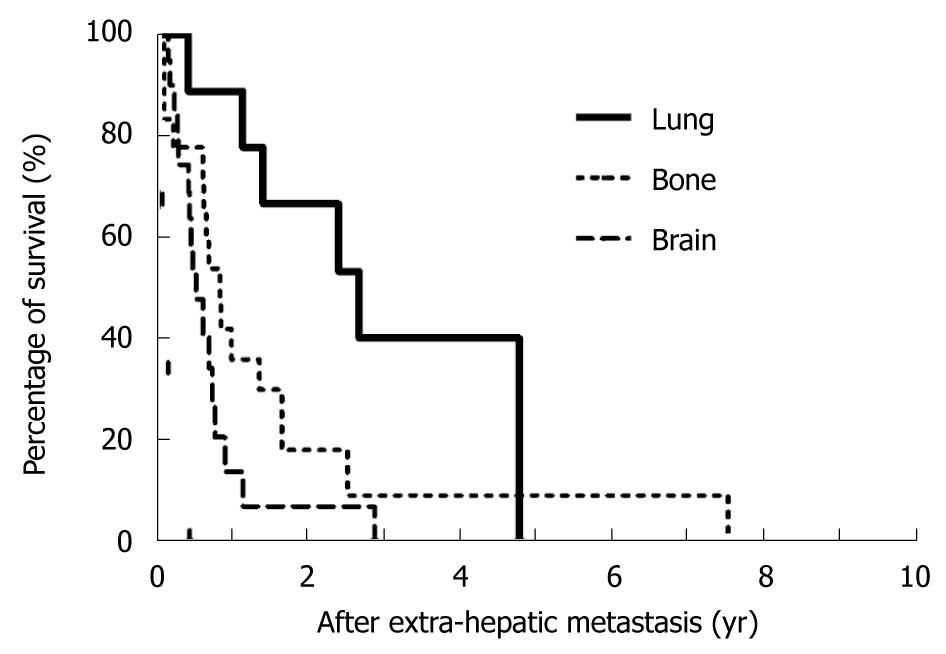
The cumulative 1- and 3-year survival rates after extrahepatic metastases were 22.1%/4.1% for bony metastases, 11.8%/0% for brain metastases, 59.4%/20.8% for lung metastases, and 31.2%/27.3% for soft tissue metastases, respectively. The comparison of survival curves in relation to metastatic sites is depicted in Figure 3 and indicates that patients who received SR of extrahepatic HCC had a significantly better prognosis than patients in the NR group. The 1- and 3-year survival rates of the SR group vs the NR group by metastatic site were, respectively, 35.9%/9.0% (median survival, 10 mo) vs 10%/0% (median survival, 3.6 mo) for bony metastases (Figure 3A, P = 0.0022); 13.6%/0% (median survival, 6.1 mo) vs 0%/0% (median survival, 1.7 mo) for brain metastases (Figure 3B, P = 0.0055); 89%/40% (median survival, 32 mo) vs 37.5%/9.4% (median survival, 7.9 mo) for lung metastases (Figure 3C, P = 0.0063); and 40.2%/40.2% (median survival, 10.1 mo) vs 16.7%/0% (median survival, 3.5 mo) for soft tissue metastases (Figure 3D, P = 0.0080). Among those patients who had undergone SR, resection of lung metastases demonstrated most favorable outcomes when compared with the other metastatic sites (Figure 4, P < 0.05).
The clinical features of patients with favorable outcomes who survived more than 3 years after extrahepatic metastases diagnosis are listed in Table 3. Four patients had undergone HR and one had received TACE for their primary HCC. All patients had experienced intrahepatic recurrence and were treated by either TACE or local ablation after initial treatment. A solitary metastatic lesion at the 12th thoracic spine was noted in patient 2 (Table 3) 16 mo after hepatic resection, and another episode of isolated pulmonary metastasis was encountered in the subsequent 32 mo. Both events were successfully treated by SR, but the patient died 89 mo after initial detection of extrahepatic metastasis due to systemic metastases. Three patients were still living at the end of this study; follow-up of patient 4 has been ongoing for 96 mo after SR of his intra-abdominal metastatic lesion.
| No. ofpatient | Age (yr)/Sex | Treatments for intrahepatic HCC | Extrahepatic metastases | Follow-up after metastases (mo) | Outcome | |
| Duration to metastases (mo) | Location/Management | |||||
| 1 | 44/Male | Hepatic resection: 1 time | 7 | Lung/wedge resection | 40 | Alive |
| TACE: 1 time | ||||||
| 2 | 36/Female | Hepatic resection: 1 time | 16 | T-spine/resection of tumor | 89 | Dead |
| TACE: 1 time | Lung/wedge resection | |||||
| 3 | 31/Female | Hepatic resection: 1 time | 11 | Transverse colon and peritoneal soft tissue/surgical resection | 67 | Alive |
| TACE: 3 times | ||||||
| 4 | 51/Male | Hepatic resection: 1 time | 32 | Peritoneal soft tissue/surgical resection | 96 | Alive |
| TACE: 2 times | ||||||
| PEI: 1 time | ||||||
| RFA:1 time | ||||||
| 5 | 67/Male | TACE: 11 times | 7 | Abdominal wall/wide excision | 57 | Dead |
| PEI: 8 times | ||||||
HCC is an aggressive malignant neoplasm with dismal prognosis because of the high incidence of intrahepatic recurrence after initial treatment. However, recent trends towards aggressive treatment of intrahepatic recurrence with multimodal therapy have significantly improved the overall outcome of patients with HCC[6,9,16]. This trend, along with better treatment of intrahepatic HCC, may not only prolong a patient’s survival but may also retard anticipated extrahepatic metastases. Because the prognosis of patients with extrahepatic metastases is considered very poor and because there is limited information regarding treatment strategies for these patients, it is necessary to actively develop more aggressive treatment methods in order to further improve the long-term survival of HCC patients with extrahepatic metastases.
HCC is a hypervascular tumor and is believed to spread mainly through a hematogenous route, causing extrahepatic metastases. It has been reported that extrahepatic metastases occur in 13.5%-41.7% of HCC patients[17-20]; however, actual prevalence may be higher than that reported. Although all HCC patients are followed up at regular intervals for intrahepatic recurrence using a variety of diagnostic tools, not all patients receive complete examination for extrahepatic metastases. Moreover, the majority of patients with extrahepatic metastases experience no specific symptoms, and it is possible to overlook extrahepatic metastases upon examination. Therefore, increased detection of extrahepatic metastases suitable for aggressive treatment may provide great benefit to patients and improve overall survival of those with HCC.
Increasing evidence shows that SR of metastatic lesions with curative intent has become standard practice for the management of several malignancies. SR of isolated metastatic lesions from colorectal cancer, gastrointestinal stromal tumors, neuroendocrine cancers, renal-cell carcinoma and sarcoma is associated with favorable outcomes[21-24]. Furthermore, improvements in patient safety during complex surgeries have also lowered the threshold for more aggressive surgical intervention. Recently, several reports have shown that SR of HCC metastases prolonged survival in selected patients[10,11,13,25]. Our results appear to be comparable with those reports which indicated that patients who received SR of extrahepatic metastases had better outcomes than patients who did not receive SR. In addition, in our study, patients undergoing SR of lung metastases had better survival rates than patients with bony and brain metastases, which is comparable with previous reports that surgical resection of pulmonary metastasis from HCC has favorable outcome. Although the small number of patients involved in this study might not reflect a real variation in outcomes due to metastatic location, we believe that our observations are valid. However, the difference in outcomes based on metastatic site may be related to the molecular biological pattern of HCC; therefore, further studies involving basic science and using a larger number of patients should be conducted to clarify the significance of our results.
Bony metastasis is one of the most frequent extrahepatic metastasis of HCC and occurs as multiple metastases in most patients[19,26,27]. In this study, the outcome of HCC patients with bony metastases was poor. Patients with metastases to bone had a median survival of 6.7 mo, which is similar to previous reports[28,29]. Data also indicate that the spine is the most common site of bony metastasis of HCC, and further studies suggest that surgical treatment for spinal metastatic HCC lesions usually improves quality of life instead of prolonging patient survival[30]. Nevertheless, prolongation of survival may still be achievable in selected patients, as observed in the current study. The longest survival of a patient who had undergone resection of metastasis at the left femoral shaft was 30 mo after the detection of extrahepatic metastasis.
Brain metastasis of HCC is relatively rare and is detected in less than 1% of HCC patients[20,31]. The overall prognosis of patients with brain metastases is extremely poor, and the most recent report showed that the median survival of these patients was only 6.8 wk after the detection of metastatic brain lesions[32]. Clinically, brain metastases are frequently associated with mass-producing hemorrhage, a condition requiring further surgical treatment. Although the surgical excision of brain metastases is considered a palliative treatment, several reports have also shown that a favorable outcome was achievable in patients with a single brain metastasis and good liver function and performance status[32,33]. Our study showed that patients who had undergone SR of brain metastases fared significantly better than NR patients, with a median survival of 6.1 mo vs 1.7 mo. Thus, SR of metastatic brain lesions of HCC should be considered in specific patients not only to improve quality of life but also to prolong life expectancy.
Nonetheless, one might argue that patients who are not suitable for surgical resection are naturally in poorer condition than patients who underwent SR for extrahepatic metastasis. Indeed, although HCC with extrahepatic metastasis is considered the terminal-stage of TNM staging, the outcome of patients with systemic multiple metastases theoretically might be poorer than those of one or two isolated extrahepatic metastases. However, the accumulated data and this study have shown that SR of extrahepatic metastases can effectively provide real benefits for patients with HCC. Although there is no standard treatment for extrahepatic metastases of HCC, aggressive treatment in patients with HCC should be considered, not only in cases of intrahepatic HCC, but also in the event of extrahepatic metastases. Taking into consideration that there are no effective nonsurgical treatment modalities for extrahepatic metastases of HCC, surgical treatment may be the only modality available for curing selected patients. As our study has shown, patients who have undergone HR prior to SR for extrahepatic metastases had the best outcome, indicating that patients should be closely followed up at regular intervals after initial treatment of primary HCC to ensure early detection of extrahepatic metastases at a resectable stage.
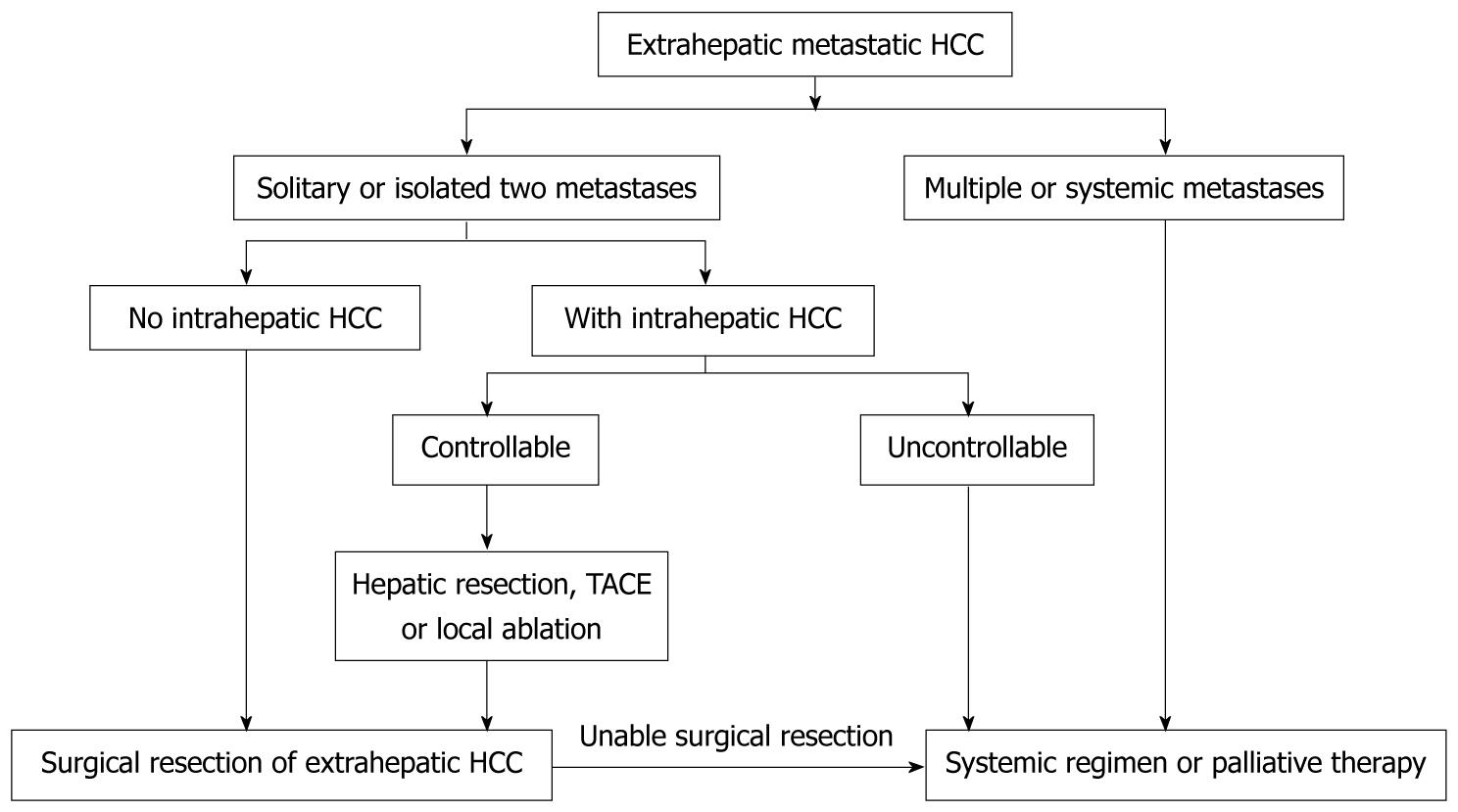
In conclusion, we herein propose the following algorithm for surgical management of extrahepatic metastases from HCC (Figure 5). Surgical excision of extrahepatic metastases should be considered in patients with one or two isolated extrahepatic metastases if the patient has otherwise good performance status, good hepatic functional reserve, and well-controlled intrahepatic HCC. Although the retrospective nature of the current study and the small number of patients with resectable extrahepatic metastases makes it hard to compare outcome of patients undergoing SR for isolated extrahepatic metastasis with those who do not receive SR, the marked differences we observed may prove helpful in the management of patients with HCC. Furthermore, to achieve better long-term outcomes of patients with HCC, as well as effective treatment of extrahepatic metastases, new promising treatments such as a novel systemic chemotherapy or targeting therapy should be developed.
Hepatocellular carcinoma (HCC) is a common malignant tumor, and ranked within the top five of cancer-related deaths worldwide. Patients with extrahepatic metastases of primary HCC are clinically considered at terminal-stage cancer, and there is still no effective treatment as well as no consensus on treatment strategies for these patients. Recently, several reports have shown benefit of surgical resection of extrahepatic metastases from HCC. However, the role of surgical resection in the treatment of extrahepatic metastasis of primary HCC remains uncertain.
The concept of treating metastatic cancer has been changing recently. Patients with several types of metastatic cancers such as colon cancer, gastrointestinal stromal tumor, neuroendocrine cancer, sarcoma, renal cell carcinoma, etc. are considered to gain benefit from surgical resection of metastatic lesions. Therefore, it seems reasonable to attempt aggressive surgical resection for HCC patients with extrahepatic metastases.
In the present study, the authors gathered their experience in the management of HCC, and further clarified the role of surgical resection in dealing with extrahepatic metastases from HCC. The results show that although the outcome of patients with extrahepatic metastases remains very poor, surgical resection could provide survival benefits for patients who had 1 or 2 isolated extrahepatic metastases under a prerequisite that patients has good performance status and good hepatic functional reserve, as well as successful treatment of intrahepatic HCC.
According to results from the study, the authors have proposed an algorithm for the management of extrahepatic metastases. Since there is no effective treatment for extrahepatic metastases in this era, surgical resection might be an applicable option and provide better outcome for well selected patients.
The authors reviewed their experience in dealing with primary HCC with extrahepatic metastasis, and proposed a treatment strategy suggesting that surgical resection might provide benefit to patients.
Peer reviewer: Osman C Ozdogan, Associate Professor, Department of Gastroenterology, Liver Unit, Marmara University School of Medicine, Istanbul 34662, Turkey
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70. |
| 2. | Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131:71-76. |
| 3. | Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676-691. |
| 4. | Ziparo V, Balducci G, Lucandri G, Mercantini P, Di Giacomo G, Fernandes E. Indications and results of resection for hepatocellular carcinoma. Eur J Surg Oncol. 2002;28:723-728. |
| 5. | Belghiti J, Regimbeau JM, Durand F, Kianmanesh AR, Dondero F, Terris B, Sauvanet A, Farges O, Degos F. Resection of hepatocellular carcinoma: a European experience on 328 cases. Hepatogastroenterology. 2002;49:41-46. |
| 6. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216-222. |
| 7. | Sato M, Watanabe Y, Ueda S, Iseki S, Abe Y, Sato N, Kimura S, Okubo K, Onji M. Microwave coagulation therapy for hepatocellular carcinoma. Gastroenterology. 1996;110:1507-1514. |
| 8. | Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy with combined angiography and computed tomography assistance for patients with hepatocellular carcinoma. Cancer. 2001;91:1342-1348. |
| 9. | Chan KM, Lee WC, Hung CF, Yu MC, Jan YY, Chen MF. Aggressive multimodality treatment for intra-hepatic recurrence of hepatocellular carcinoma following hepatic resection. Chang Gung Med J. 2005;28:543-550. |
| 10. | Tomimaru Y, Sasaki Y, Yamada T, Eguchi H, Takami K, Ohigashi H, Higashiyama M, Ishikawa O, Kodama K, Imaoka S. The significance of surgical resection for pulmonary metastasis from hepatocellular carcinoma. Am J Surg. 2006;192:46-51. |
| 11. | Lam CM, Lo CM, Yuen WK, Liu CL, Fan ST. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg. 1998;85:1198-1200. |
| 12. | Momoi H, Shimahara Y, Terajima H, Iimuro Y, Yamamoto N, Yamamoto Y, Ikai I, Yamaoka Y. Management of adrenal metastasis from hepatocellular carcinoma. Surg Today. 2002;32:1035-1041. |
| 13. | Yeh CN, Chen MF. Resection of peritoneal implantation of hepatocellular carcinoma after hepatic resection: risk factors and prognostic analysis. World J Surg. 2004;28:382-386. |
| 14. | Chen MF, Jeng LB. Partial hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S329-S334. |
| 15. | Chen MF, Hwang TL, Jeng LB, Jan YY, Wang CS, Chou FF. Hepatic resection in 120 patients with hepatocellular carcinoma. Arch Surg. 1989;124:1025-1028. |
| 16. | Lee PH, Lin WJ, Tsang YM, Hu RH, Sheu JC, Lai MY, Hsu HC, May W, Lee CS. Clinical management of recurrent hepatocellular carcinoma. Ann Surg. 1995;222:670-676. |
| 17. | Si MS, Amersi F, Golish SR, Ortiz JA, Zaky J, Finklestein D, Busuttil RW, Imagawa DK. Prevalence of metastases in hepatocellular carcinoma: risk factors and impact on survival. Am Surg. 2003;69:879-885. |
| 18. | Shuto T, Hirohashi K, Kubo S, Tanaka H, Yamamoto T, Higaki I, Takemura S, Kinoshita H. Treatment of adrenal metastases after hepatic resection of a hepatocellular carcinoma. Dig Surg. 2001;18:294-297. |
| 19. | Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781-1787. |
| 20. | Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698-703. |
| 21. | Fourquier P, Regnard JF, Rea S, Levi JF, Levasseur P. Lung metastases of renal cell carcinoma: results of surgical resection. Eur J Cardiothorac Surg. 1997;11:17-21. |
| 22. | Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-766. |
| 23. | Ollila DW. Complete metastasectomy in patients with stage IV metastatic melanoma. Lancet Oncol. 2006;7:919-924. |
| 24. | van Geel AN, Pastorino U, Jauch KW, Judson IR, van Coevorden F, Buesa JM, Nielsen OS, Boudinet A, Tursz T, Schmitz PI. Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer. 1996;77:675-682. |
| 25. | Chou HS, Lee KF, Yeh CN, Chen MF, Jeng LB. Long-term survival following resection of peritoneal implantation from hepatocellular carcinoma: a case report. Hepatogastroenterology. 2005;52:1221-1223. |
| 26. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. |
| 27. | Kuhlman JE, Fishman EK, Leichner PK, Magid D, Order SE, Siegelman SS. Skeletal metastases from hepatoma: frequency, distribution, and radiographic features. Radiology. 1986;160:175-178. |
| 28. | Liaw CC, Ng KT, Chen TJ, Liaw YF. Hepatocellular carcinoma presenting as bone metastasis. Cancer. 1989;64:1753-1757. |
| 29. | Okazaki N, Yoshino M, Yoshida T, Hirohashi S, Kishi K, Shimosato Y. Bone metastasis in hepatocellular carcinoma. Cancer. 1985;55:1991-1994. |
| 30. | Lin CC, Chen PQ, Chen WJ, Chen LH. Prognosis of operative treatment for metastatic hepatocellular carcinoma of the spine. Clin Orthop Relat Res. 2006;444:209-215. |
| 31. | Seinfeld J, Wagner AS, Kleinschmidt-DeMasters BK. Brain metastases from hepatocellular carcinoma in US patients. J Neurooncol. 2006;76:93-98. |
| 32. | Choi HJ, Cho BC, Sohn JH, Shin SJ, Kim SH, Kim JH, Yoo NC. Brain metastases from hepatocellular carcinoma: prognostic factors and outcome: brain metastasis from HCC. J Neurooncol. 2009;91:307-313. |
| 33. | Chang L, Chen YL, Kao MC. Intracranial metastasis of hepatocellular carcinoma: review of 45 cases. Surg Neurol. 2004;62:172-177. |