Published online Nov 21, 2009. doi: 10.3748/wjg.15.5425
Revised: September 23, 2009
Accepted: September 30, 2009
Published online: November 21, 2009
AIM: To investigate the expression and function of Wolfram syndrome 1 gene (WFS1) during the development of normal pancreas.
METHODS: Pancreas from Sprague-Dawley rat fetuses, embryos, young and adult animals were used in this study. Expression levels of WFS1 in pancreas of different development stages were detected by reverse transcription-polymerase chain reation (RT-PCR) and Western blotting. To identify the cell location of WFS1 in the developing rat pancreas, double-immunofluorescent staining was performed using antibodies to specific cell markers and WFS1, respectively.
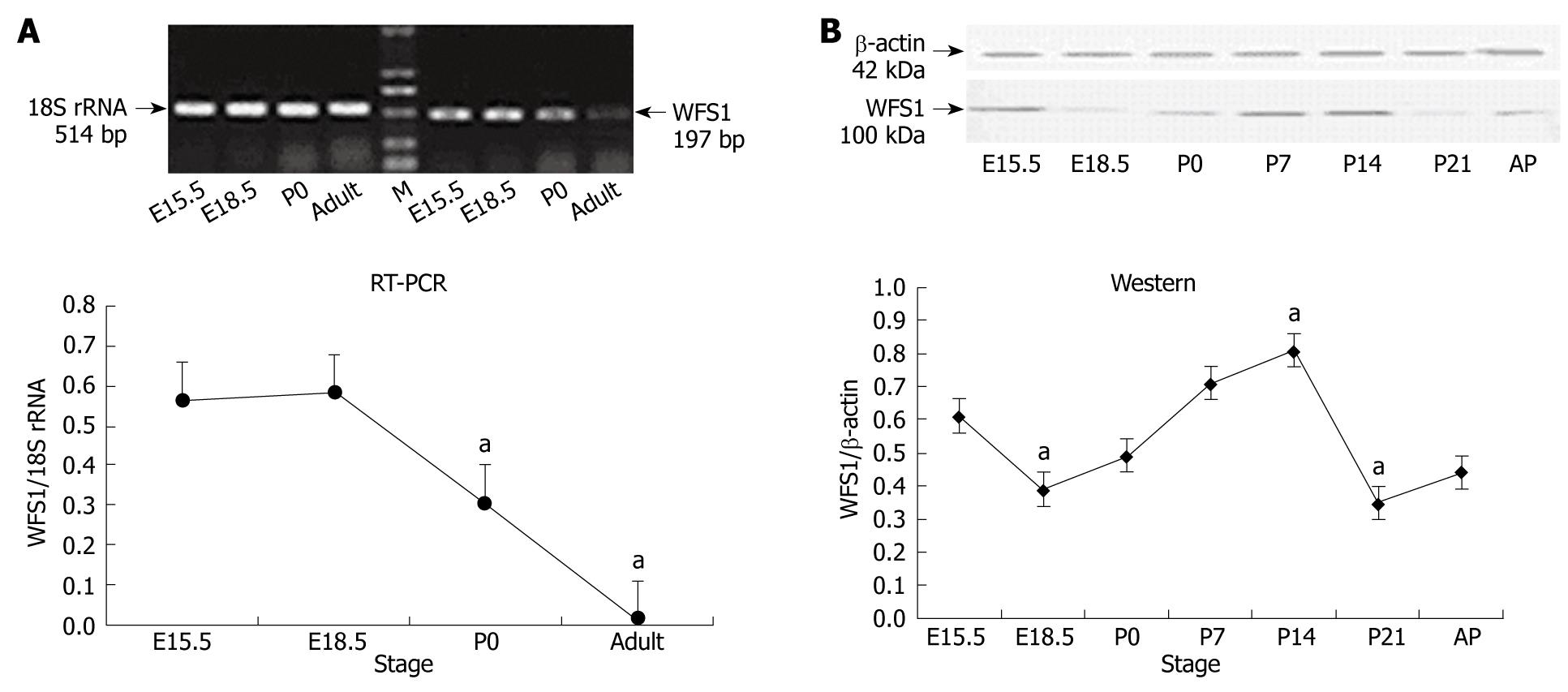
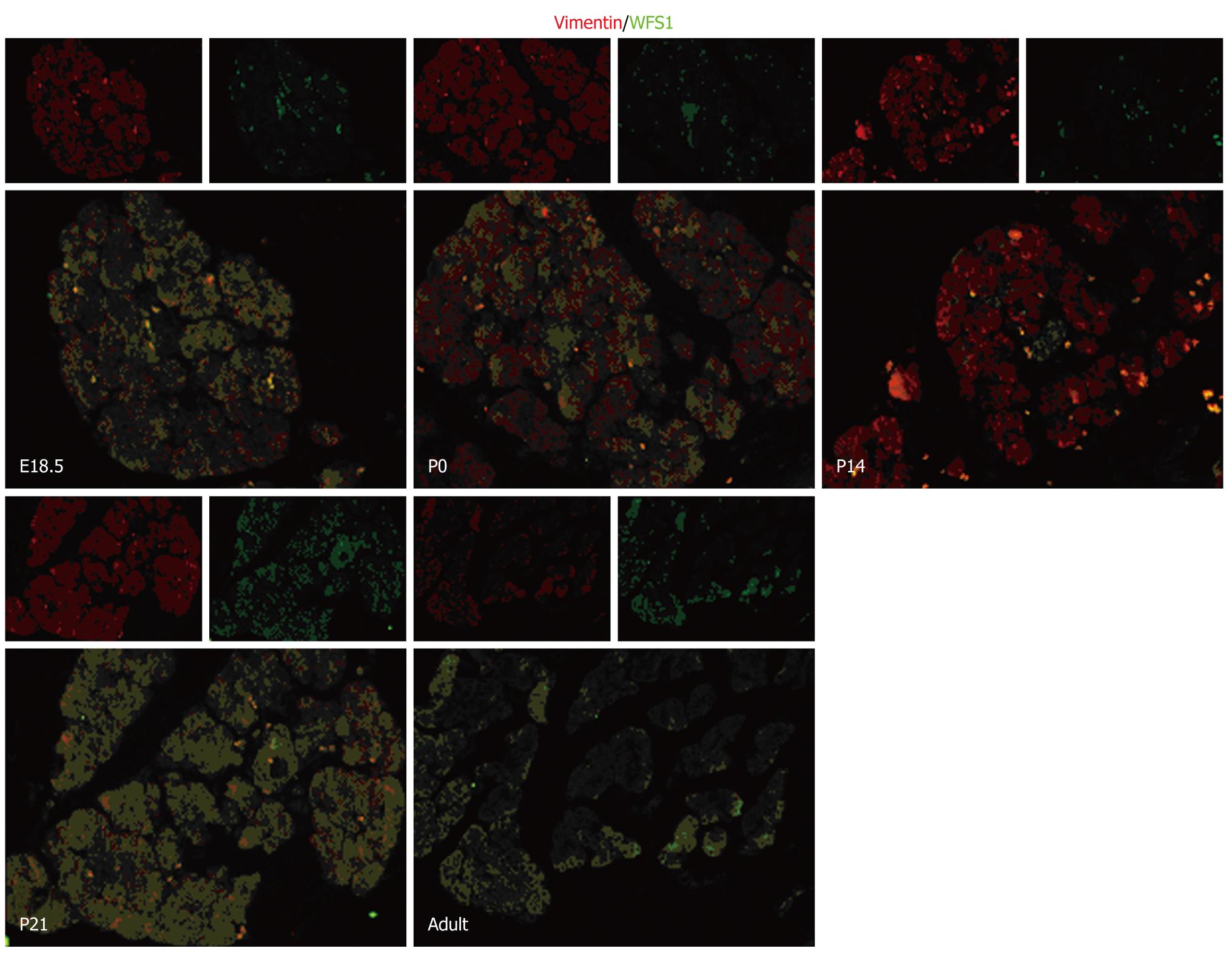
RESULTS: Compared to E15.5, the highest level of WFS1 mRNA was detected at E18.5, the level of WFS1 mRNA in E15.5 and P0 was less, and at a lowest at adult (P < 0.05 vs P0 and adult), respectively. Compare to E15.5, the highest level of WFS1 was at P14 and lowest at P21 (P < 0.05 vs P14 and P21), respectively. The WFS1 positive staining is expressed in the normal developing rat pancreas mainly in the islet beta-cells and mesenchyme at each stage tested.
CONCLUSION: These results indicate that WFS1 may be involved in some aspects of pancreatic development and further research on WFS1 may provide new evidences to prove the interactions between mesenchyma and epithelia at the same time.
-
Citation: Xu R, Xia B, Geng J, Shi J, Shi H, Yuan L, De W. Expression and localization of
Wolfram syndrome 1 gene in the developing rat pancreas. World J Gastroenterol 2009; 15(43): 5425-5431 - URL: https://www.wjgnet.com/1007-9327/full/v15/i43/5425.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5425
The pancreas is a complex organ composed of 2 different cell populations, exocrine and endocrine cells. The acini and ducts form the exocrine pancreas, which produces and transports digestive enzymes into the duodenum. The endocrine component contains 4 types of cells that secrete hormones to regulate glucose metabolism and other physiological processes[1]. In rats, the pancreas develops from the foregut endoderm in 3 major steps: endoderm formation [embryonic day (E) 7.5], pancreatic morphogenesis (E10.5), and differentiation of endocrine and exocrine cells (E13.5). At approximately E16, the islet progenitor cells leave the contiguous epithelium, migrate through the adjacent extracellular matrix into the surrounding mesenchyme, and aggregate to form the islets of Langerhans. The islets are not completely formed until shortly before birth on E18-E19[2-4] and undergo further remodeling and maturation for 2-3 wk after birth[5]. Thus, the developing pancreas presents a challenge for developmental biologists because of the complex morphogenetic processes underlying the development of this organ. Until now, the factors that control the progressive development of pancreatic architecture and function have remained unclear. To identify these functional factors we performed a gene array at E15.5, E18.5, birth and adult stages. The Wolfram syndrome 1 gene (WFS1), was one of the factors found to be abundantly expressed in islet cells at E15.5 and E18.5 (data not shown). Wolfram syndrome (WS) is an autosomal recessive, progressive, neurodegenerative disorder characterized by diabetes mellitus and optic atrophy[6]. This disorder is also associated with diabetes insipidus and deafness, hence the acronym DIDMOAD (diabetes insipidus, diabetes mellitus, optic atrophy, and deafness). Other manifestations such as atonic bladder, ataxia, nystagmus and predisposition to psychiatric illness may also be present[7,8]. WFS1, discovered in 1998, was mapped to chromosome 4p16.1 by positional cloning[9,10]. The gene is composed of 8 exons spanning 33.4 kb of genomic DNA. The 3.6 kb mRNA encodes an 890-amino acid polypeptide named wolframin[9,11] with 9 predicted transmembrane domains, and belongs to a novel gene family. Biochemical studies in cultured cells indicate that WFS1 protein is an integral, endoglycosidase H-sensitive membrane glycoprotein primarily localized in the endoplasmic reticulum[12]. Postmortem studies of the pancreas from subjects with WS have shown β-cell loss[10]. Mice with a disrupted WFS1 were also reported to exhibit impaired glucose homeostasis accompanied by a progressive reduction of β-cell mass[13]. Thus, WFS1 seems to play a role in the normal function of β cells, but little is known about its function during embryogenesis. To the best of our knowledge, no study has investigated the relationship between WFS1 expression and pancreas development, and the expression of WFS1 during pancreatic development in rats is poorly understood. Knowledge of the regional and temporal expression of WFS1 will be useful in understanding its potential roles in pancreatic development. Therefore, we further examined the expression patterns of WFS1 in rat pancreas during development.
Sprague-Dawley (SD) rats were purchased from the Animal Center of Nanjing Medical University (Nanjing, China). SD rats (2:1, male:female) were mated overnight. At noon the next day, if a vaginal plug was discovered, it was considered as Day 0.5 of gestation (E0.5). Embryos were removed at E15.5 and E18.5 from the uterus of pregnant rats, which were sacrificed by cervical dislocation. Pancreata from E15.5 and E18.5 rat embryos were isolated as previously described[14] under a stereomicroscope. Rat pancreata at postnatal (P) days 0, 7, 14, 21 and from adults, were directly isolated by the unaided eye. All experiments were conducted in accordance with the Chinese Law for Animal Protection and were approved by the local animal care committee. Five rats were used at each age stage. Dissected tissues were immediately rinsed 3 times with phosphate-buffered saline (PBS) to remove serum proteins, and fixed with 4% paraformaldehyde in PBS overnight for histology, or frozen in liquid nitrogen for RNA and protein isolation.
Total RNA was extracted from pancreata at each time point with TRIZOL reagent (Invitrogen Life Technologies, Burlington, Ontario, Canada), according to the manufacturer’s instructions. The quality of the RNA was verified by agarose gel electrophoresis using ethidium bromide staining. For each PCR, 2 μg of DNA-free total RNA with oligo (deoxythymidine) primers and reverse transcriptase were used. PCR was performed in 25 μL reactions containing 25 ng of cDNA, 0.2 nmol of each primer pair, and 0.3 μL of Taq DNA polymerase. PCR was carried out in a T-gradient Biometra PCR thermal cycler (Montreal Biotech Inc., Kirkland, Quebec, Canada) to determine the annealing temperature for WFS1 primers. We used the following primer pairs: WFS1, forward: 5'-CTGCTCTTTTGCTGGTTCT-3', reverse: 5'-GATGTCCTTGGTGATGTCG-3' (497 bp); and 18S rRNA, forward: 5'-ACGAACCAGAGCGAAAGC-3', reverse: 5'-GGACATCTAAGGGCATCACAG-3' (514 bp). PCR conditions were as follows: 2 min at 94°C, followed by up to 35 cycles of 94°C for 30 s, 54.3°C for 30 s, and 72°C for 1 min, with a final extension at 72°C for 5 min. To estimate the linear range of the nested reactions, we analyzed the PCR products for 10, 15, 20, 25, 30, and 35 cycles. The amplified products were analyzed on 1% agarose gels and visualized by ethidium bromide staining. The data were normalized for 18S rRNA expression.
The pancreata was homogenized in a detergent lysis buffer containing 8 mol/L urea, 2% CHAPS, 40 mmol/L Tris, 65 mmol/L DTT and 2% IPG buffer. The lysate was then centrifuged at 15 000 g for 1 h at 4ºC. The total protein concentration of each sample was analyzed using a modified Bradford assay. All samples were stored at -80°C prior to the electrophoresis.
An equal amount of protein samples (50 μg protein in 20 μL buffer) from each time point were boiled in 3 × loading buffer (10 mmol/L Tris-HCl, pH 6.8 including 3% SDS, 5% β-mercaptoethanol, 20% glycerol and 0.6% bromophenol blue) for 3 min and separated by 12.5% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). For blocking, membranes were incubated with 5% fat-free milk in Tris-buffered saline plus 0.05% Tween-20 (TBST) overnight at 4°C. The membranes were then incubated with the primary antibody (NB100-1918, rabbit polyclonal antibody to WFS1, diluted 1:1000, Novus Biologicals Littleton, CO, USA) (sc-47778 mouse polyclonal antibody to β-actin, diluted 1:1000, Santa Cruz Biotechnology Inc., USA) for 2 h at room temperature. After washing in TBST, the membranes were incubated with the peroxidase-linked goat anti-rabbit IgG conjugates (PIERCE, Prod 1858415) or peroxidase-linked goat anti-mouse IgG conjugates (sc-2055, Santa Cruz Biotechnology Inc. USA) for 1 h at room temperature. The membranes were then washed again in TBST, incubated in enhanced chemiluminescence reagents (ECL, Amersham Life Science, Cleveland, OH, USA) for 1 min, and exposed to VA 711 B Blue Sensitive X-ray films. Densitometric quantification of bands at sub-saturating levels was performed using the Syngenetool gel analysis software (Syngene, Cambridge, UK).
Tissues were fixed in 4% paraformaldehyde overnight at 4°C followed by a standard protocol of dehydration and paraffin embedding, and 5 μm sections were cut. The paraffin sections were deparaffinized in xylene and rehydrated in graded ethanol and distilled water. The non-specific binding sites were blocked in 1% bovine serum albumin for 30 min. For WFS1 protein and insulin, glucagon or vimentin double immunofluorescence, the rabbit anti-WFS1 primary polyclonal antibody was applied and revealed using FITC-labeled Rabbit anti-goat IgG (1:400, sc-2777, Santa Cruz Biotechnology Inc., USA). Mouse anti-insulin primary polyclonal antibody (1:1000, Santa Cruz Biotechnology Inc., USA) or mouse anti-vimentin primary monoclonal antibody (1:1000, Chemicon Temecula, CA, USA) was then applied and revealed by cy3-labeled anti-mouse IgG (1:400, Chemicon Temecula, CA, USA). Sections were placed in Gel Mount Aqueous Mounting Medium (G0918, Sigma, USA) with a cover glass, and were examined under an Olympus BX51 microscope (Olympus Optical, Tokyo, Japan). Images were taken at a magnification × 400.
Analysis of the experimental data was performed using PD Quest 7.0 software and the paired Student t-test. P < 0.05 was considered statistically significant. Data are presented as the mean ± SD.
The mRNA expression levels of WFS1 in rat pancreas during its development were examined by RT-PCR. 18S RNA as used as an internal control and assessed under the same conditions. As shown in Figure 1A, RT-PCR for the WFS1 specific region yielded a band of the expected size (197 bp) in rat pancreas tissue at different developmental stages. The mRNA expression of WFS1 was high at E15.5 and E18.5, started to decrease at birth, and continued to decline to adulthood.
We performed Western blotting using total protein samples extracted from pancreata obtained at E15.5, E18.5, P0, P7, P14 and P21 and from adult rats (Figure 1B). β-actin (42 kDa) was used as an internal control under the same conditions. Analysis of the blots showed that WFS1 (100 kDa) protein was expressed in the pancreas. Densitometric quantification of each band showed that the expression of WFS1 protein was high at E15.5, and then decreased until birth (P0). Subsequently, the level of WFS1 protein increased gradually and peaked at P14. The expression of WFS1 protein was lowest at P21. The level of WFS1 protein was relatively high in the adult rat.
To determine the localization of WFS1 protein during pancreatic development, serial pancreas sections (obtained at E18.5, P0, P14, P21 and in adulthood) underwent double immunofluorescence. We found that WFS1 protein was co-expressed with insulin and could not be detected in α-cells. As shown in Figure 2, there was a consistent overlap between the WFS1 protein-positive cells and cells labeled with insulin at each stage tested. This means that, during pancreatic development and remodeling, the WFS1 protein is continually expressed in β cells. As shown in Figure 3, there was little overlap between the WFS1 protein-positive cells and cells labeled with glucagon at any of the stages tested. This indicates that WFS1 protein is not expressed in α-cells during development. Interestingly, we also found that WFS1-positive cells were scattered throughout the pancreas. Therefore, we performed further double immunofluorescence, which confirmed that WFS1 protein was co-expressed with vimentin in mesenchymal cells (Figure 4).
Organogenesis involves 2 processes: morphogenesis, which is the shaping of an organ, and cytodifferentiation, which is the acquisition and expression of specialized cellular functions within that organ[15]. During pancreatic development, specialized cell types are generated from progenitor cell populations and are precisely organized into the elaborate structure of the adult organ. Previously, most researchers focused on deriving functional islets from stem cells or other cell types. Gu et al[16] generated transcriptional profiles of enriched cells isolated from pancreata at 4 biologically significant stages of endocrine pancreas development (endoderm before pancreas specification, early pancreatic progenitor cells, endocrine progenitor cells and adult islets of Langerhans) to identify new endocrine regulatory genes and markers. With temporal and spatial analysis of genes expressed in embryonic endocrine cells, a database of potential progenitor cell markers was generated. In the present study, using a gene array, WFS1 was found to be one of the genes that were specifically and highly expressed in islet cells at E15.5 and E18.5. Then, we used RT-PCR and Western blotting to analyze the expression of WFS1 at 6 biologically significant stages of rat pancreas development: E15.5, E18.5, P0, P14, P21 and in adult rats. During the later embryonic period, the pancreatic cells exhibit dramatic growth, differentiation and proliferation. Accordingly, it may not just be a concomitant phenomenon that the expression of WFS1 protein is greater during the later embryonic period. The present study confirmed the changes in WFS1 mRNA levels in the rat pancreas during development by RT-PCR.
The architecture of the islet is completely formed shortly before birth; however, the function of the islet is not fully developed at this stage. It has been reported that the pancreas of neonatal rat undergoes further remodeling and maturation for 2-3 wk after birth[17]. The remodeling process includes a decrease in β-cell mass and enhanced islet neogenesis. The number of β-cells is mainly regulated by apoptosis and cell replication[18]. The results of Western blotting revealed that the WFS1 protein level was highest during the postneonatal period, particularly P14. Although the function of wolframin is still not completely known, recent studies have revealed its localization to the endoplasmic reticulum and that it may play a important role in endoplasmic reticulum (ER) homeostasis by regulating Ca2+[11,19]. During islet development, particularly in the ER at E15.5 and P14, secretory and transmembrane proteins involved in development regulation fold into their native conformation and undergo posttranslational modifications important for their activity and structure. Consequently, ER homeostasis and the WFS1 protein may be important in islet development. It is well known that WFS1 protein induces cation channel activity in ER membranes[11] and regulates calcium levels in the ER[19]. WFS1 protein also plays a role in stimulus-secretion coupling for insulin exocytosis in pancreatic β cells[13,16,19,20]. Therefore, enhanced expression of WFS1 protein in adults suggests that WFS1 may retain its function in adult islets.
Surprisingly, the pattern of WFS1 protein expression was not consistent with the pattern of WFS1 mRNA expression, as described above. This difference may result from post-transcriptional control, although the mechanisms need further investigation. RNA binding protein controls the various steps and the rate of transcription of microRNA, which regulates the destruction of RNA and changes in chromatin structure may be involved in the regulation of WFS1 expression.
During remodeling in the perinatal period, apoptosis is an important mechanism that decreases β-cell mass. Apoptosis of β-cells is significantly increased in neonates compared with adult rats, and peaks at 2 wk of age[18], while the highest level of WFS1 protein was detected at the same time. It has been reported that ER stress induces WFS1 expression in pancreatic β-cells[21] and the expression of WFS1 is regulated by inositol requiring 1α (Ire1α) and PKR-like ER kinase (PERK), central regulators of the unfolded protein response[22]. It has also been reported that WFS1-deficient pancreatic β-cells exhibit increased phosphorylation of RNA-dependent protein kinase-like ER kinase, chaperone gene expression and active XBP1 protein levels, indicating an enhanced ER stress response. Furthermore, the increased ER stress response was accompanied by impaired β-cell proliferation and increased caspase-3 cleavage[21]. Since the WFS1 protein attenuates ER stress, maintains cell cycle progression and represses the apoptotic pathway, specifically in pancreatic β-cells, it may play an important role in the maintenance of β-cell mass by balancing β-cell growth (differentiation and proliferation) and β-cell death (apoptosis) during pancreatic remodeling.
During organogenesis, specialized cell types are generated from progenitor cell populations and are precisely organized into the elaborate structure of the adult organ. Numerous cell-cell communications and the initiation of complex inter-regulating genetic networks are involved to ensure the fidelity of organogenesis. Several studies have reported the role of mesenchymal and/or epithelial interactions in pancreatic development and suggested that multiple diffusible mesenchyme-derived factors may act on the developing epithelia to influence cell division, cell fate, differentiation, and to determine the proportion of endocrine vs exocrine tissue[23-26]. Examination of the ontogeny of WFS1 in the developing rat pancreas revealed an intimate relationship between WFS1 protein and the mesenchymal and/or epithelial interactions[27] in pancreatic development. Vimentin is a marker for mesenchymal cells[28,29]. In the present study, the results of double immunofluorescence revealed that WFS1 protein was co-expressed with vimentin. This indicates that WFS1 protein is localized to the pancreatic mesenchyme shortly before birth on E18.5 and after birth. Previous studies revealed that the late embryonic stage is a key phase in late pancreatic organogenesis, in which a dramatic increase in the number of endocrine and exocrine cells with high levels of insulin and exocrine enzymes are observed. The islets are fully formed shortly before birth on E18.5 and undergo further remodeling and maturation after birth[26]. Considering the previous reports of WFS1 protein function and the results of our experiments, we suggest that WFS1 protein plays an important role in many aspects of pancreas development. However, the underlying mechanism is not fully understood and needs further research, which may also provide new evidence to confirm the interactions between mesenchymal and epithelial cells.
In summary, our present study has, for the first time, demonstrated that WFS1 protein is localized to the mesenchyme in the rat pancreas by immunofluorescence. The dynamic expression of WFS1 in the various developmental stages of the pancreas indicates that WFS1 may be involved in many aspects of pancreatic development.
Wolfram syndrome (WS), caused by the mutant of the Wolfram syndrome 1 gene (WFS1), is an autosomal recessive, progressive, neurodegenerative disorder accompanied by diabetes mellitus and optic atrophy. Although it has been reported that WFS1 protein is found to be enriched in endocrine progenitors vs non-endocrine progenitor cells at embryonic day (E) 13.5, its expression and function during the development of normal pancreas remain unknown.
It is thought that the pancreatic endocrine cells including beta cells arise from duct epithelial cells, however much research has been done to explore a different source. Novel strategies would obviously benefit from the use of beta stem cells/progenitor cells.
WFS1 expression has never been assessed in the developing pancreas and it is highly expressed in P14 on which the apoptosis rate of beta cells is at a high point. WFS1 protein has been proved to be a downstream factor in the endoplasmic reticulum stress signal pathway.
This study reveals the possible mechanism of apoptosis in pancreas remodeling and may lead to new possibilities for diabetes therapy.
This is a very interesting study that provides novel information that will be useful for the understanding of the role WSF1 in pancreatic development as well as disease.
Peer reviewers: Minoti V Apte, Associate Professor, Pancreatic Research Group, South Western Sydney Clinical School, The University of New South Wales, Liverpool, NSW 2170, Australia; Martin E. Fernandez-Zapico, Assistant Professor, Mayo Clinic, 200 First St. SW, Rochester, United States
S- Editor Tian L L- Editor Cant MR E- Editor Lin YP
| 1. | Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025-1034. |
| 2. | Jørgensen MC, Ahnfelt-Rønne J, Hald J, Madsen OD, Serup P, Hecksher-Sørensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685-705. |
| 3. | Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29:436-467. |
| 4. | Spooner BS, Walther BT, Rutter WJ. The development of the dorsal and ventral mammalian pancreas in vivo and in vitro. J Cell Biol. 1970;47:235-246. |
| 6. | Domenech E, Gomez-Zaera M, Nunes V. Wolfram/DIDMOAD syndrome, a heterogenic and molecularly complex neurodegenerative disease. Pediatr Endocrinol Rev. 2006;3:249-257. |
| 7. | Barrett TG, Bundey SE. Wolfram (DIDMOAD) syndrome. J Med Genet. 1997;34:838-841. |
| 8. | Rando TA, Horton JC, Layzer RB. Wolfram syndrome: evidence of a diffuse neurodegenerative disease by magnetic resonance imaging. Neurology. 1992;42:1220-1224. |
| 9. | Strom TM, Hörtnagel K, Hofmann S, Gekeler F, Scharfe C, Rabl W, Gerbitz KD, Meitinger T. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum Mol Genet. 1998;7:2021-2028. |
| 10. | Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, Mueckler M, Marshall H, Donis-Keller H, Crock P. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet. 1998;20:143-148. |
| 11. | Osman AA, Saito M, Makepeace C, Permutt MA, Schlesinger P, Mueckler M. Wolframin expression induces novel ion channel activity in endoplasmic reticulum membranes and increases intracellular calcium. J Biol Chem. 2003;278:52755-52762. |
| 12. | Takeda K, Inoue H, Tanizawa Y, Matsuzaki Y, Oba J, Watanabe Y, Shinoda K, Oka Y. WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum Mol Genet. 2001;10:477-484. |
| 13. | Ishihara H, Takeda S, Tamura A, Takahashi R, Yamaguchi S, Takei D, Yamada T, Inoue H, Soga H, Katagiri H. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet. 2004;13:1159-1170. |
| 14. | Shi J, Ni XF, Chen Y. Patterning biomolecules with a water-soluble release and protection interlayer. Langmuir. 2007;23:11377-11380. |
| 15. | Crisera CA, Kadison AS, Breslow GD, Maldonado TS, Longaker MT, Gittes GK. Expression and role of laminin-1 in mouse pancreatic organogenesis. Diabetes. 2000;49:936-944. |
| 16. | Gu G, Wells JM, Dombkowski D, Preffer F, Aronow B, Melton DA. Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development. 2004;131:165-179. |
| 17. | Montanya E, Téllez N. Pancreatic remodeling: beta-cell apoptosis, proliferation and neogenesis, and the measurement of beta-cell mass and of individual beta-cell size. Methods Mol Biol. 2009;560:137-158. |
| 18. | Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736-1741. |
| 19. | Takei D, Ishihara H, Yamaguchi S, Yamada T, Tamura A, Katagiri H, Maruyama Y, Oka Y. WFS1 protein modulates the free Ca(2+) concentration in the endoplasmic reticulum. FEBS Lett. 2006;580:5635-5640. |
| 20. | Kato T, Ishiwata M, Yamada K, Kasahara T, Kakiuchi C, Iwamoto K, Kawamura K, Ishihara H, Oka Y. Behavioral and gene expression analyses of Wfs1 knockout mice as a possible animal model of mood disorder. Neurosci Res. 2008;61:143-158. |
| 21. | Ueda K, Kawano J, Takeda K, Yujiri T, Tanabe K, Anno T, Akiyama M, Nozaki J, Yoshinaga T, Koizumi A. Endoplasmic reticulum stress induces Wfs1 gene expression in pancreatic beta-cells via transcriptional activation. Eur J Endocrinol. 2005;153:167-176. |
| 22. | Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y, Urano F. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem. 2005;280:39609-39615. |
| 23. | Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:111-127. |
| 24. | Miralles F, Battelino T, Czernichow P, Scharfmann R. TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J Cell Biol. 1998;143:827-836. |
| 25. | Duvillié B, Attali M, Bounacer A, Ravassard P, Basmaciogullari A, Scharfmann R. The mesenchyme controls the timing of pancreatic beta-cell differentiation. Diabetes. 2006;55:582-589. |
| 26. | Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569-1580. |
| 27. | Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261-2264. |
| 28. | Hou LQ, Wang YH, Liu LJ, Guo J, Teng LP, Cao LH, Shi H, Yuan L, De W. Expression and localization of mesothelin in developing rat pancreas. Dev Growth Differ. 2008;50:531-541. |
| 29. | Liu XY, Dong D, Sun P, Du J, Gu L, Ge YB. Expression and location of alpha-fetoprotein during rat colon development. World J Gastroenterol. 2009;15:1738-1743. |